1. Introduction
There are some important abiotic stresses that adversely impact the global food security of a fast growing populace, which is anticipated to reach 9.6 billion by 2050 [
1]. Salt-affected soil is one such abiotic stress that not only hinders the growth and productivity of plants but also degrades the soil productivity in arable croplands worldwide and particularly in arid and semi-arid regions [
2]. Soils with high amounts of soluble salts (saline soils) or exchangeable sodium (sodic soils) or both (saline-sodic) adversely affect the growth of most crop plants and are collectively called salt-affected soils [
3]. Salt-affected soils are described as having an electrical conductivity (EC) in the saturated soil extract of ≥4 dS m
−1, and sodic soils as having an exchangeable-sodium percentage (ESP) ≥15% [
4]. Around 450 Mha, 7% of the worldwide land area, is categorized as salt-affected soils with an estimated yearly increment of 1%–2% [
1]. Loss of crop yield is predicted to accelerate if no action is taken to avoid land degradation due to salinity. Salt-affected soils undergo adverse changes in their osmotic and ionic properties, which can decrease water absorption and microbial activity, which, in turn, limits the uptake of essential plant nutrients (K, Ca, Mg, and P) and reduces crop yields [
5]. In order to address the challenges of worldwide food security caused by salt-affected soils, it is imperative to minimize the effects of soil salinity on crops either by eliminating soluble salts and/or exchangeable Na
+ or by curtailing the negative effects of salts on plants [
6]. Several approaches have been used to minimize the effects of soil salinity on crops: breeding salt tolerant cultivars, applying plant growth regulators, and seed inoculation with halotolerant plant growth-promoting rhizobacteria [
6]. Another crucial practice to eliminate the impact of soil salinity on plants is the addition of soil conditioners, which can both mitigate and improve the fertility of salt-affected soils [
7].
The soil is also exposed to another problem due to climate changes, which cause low crop growth and productivity, such as a water deficit that might increase in the future [
8]. Water constitutes between 80% to 90% of plants, it is the central molecule in all physiological processes of plants, and it is the principal means of transport for metabolites and nutrients [
9]. Water deficit is one of the major sources of plant stress which has an adverse effect on plant growth and crop productivity by inhibiting leaf expansion and stomatal aperture and thus leading to lower photosynthetic rates [
2]. Drought situations diminish the water potential and turgor of plants, which creates difficulties in implementing normal physiological functions [
10].
Sustainable crop production relies on the interaction between salinity and soil moisture conditions. Therefore, combating water deficit and salt-affected soil simultaneously is a challenging task for attaining global food security.
Plant growth-promoting rhizobacteria (PGPR) have the potential to improve crop growth under abiotic stress by several direct and indirect mechanisms [
11]. Inoculation of plants with stress-tolerant PGPR protects them from the negative impacts of stress and minimizes plant growth suppression caused by the particular stress. Exopolysaccharides (EPS) produced by bacteria improve nutrient uptake and indirectly affect N
2 fixation and phosphate solubilization by binding free phosphorus in non-legumes [
6]. PGPR can augment crop growth under water deficit and salt-affected soils by alleviating the negative effects of stress-induced ethylene through their 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity, production of exopolysaccharides that bind Na
+ and reduce its uptake in plants, and the up- or down-regulation of stress-responsive genes and accumulation of osmolytes [
12]. If PGPR are used in association with organic amendments, they could not only increase crop production but also improve the physicochemical and biological characteristics of the soil [
5].
Biochar is an activated-carbon (C) soil conditioner that is developed from the combustion of biomass in anaerobic conditions at temperatures below 1000 °C through the process of pyrolysis or dry carbonization [
13]. Biochar has attracted considerable attention to ameliorating soil health, soil cation exchange capacity (CEC), soil moisture content, and augment crop productivity in rice [
14]; it is typically high in ash, pH, and surface area [
6]. Nowadays, waste biomass is used extensively for the production of biochar because of its cost-effectiveness and food security advantages [
15]. The direct mechanism of biochar is the enhanced availability of essential nutrients in the soil, such as K
+ and the lessening of Na
+ absorption [
16]. The indirect mechanism involves the enrichment of soil physicochemical properties, biological properties, and soil enzymatic activity, all of which increase the plant water status. [
16,
17] reported that biochar greatly increased the water holding capacity of soil, chlorophyll content, stomatal conductance, photosynthetic rate, and relative water content under water deficient conditions. Biochar application improved the leaf relative water content and osmotic potential under water deficient conditions in maize as compared to the control [
18]. Likewise, biochar improved the water use efficiency of quinoa under water deficit [
19]. This indicated that biochar response under water deficit could differ with soil and biochar type. Reports have indicated that biochar could mitigate water deficit in cereal crops when added with PGPR [
20]. Inoculation of biochar with “
Azosprillum sp.” further improved the growth, yield, nitrogen (N), and phosphorus (P) uptake under water deficient conditions compared to microbial inoculation only [
21].
As a major staple crop, rice (
Oryza sativa L.) is an important cereal, which supports more than three billion people worldwide by comprising 50% to 80% of their daily calorie intake [
22]. Rice also requires a larger amount of water throughout its life cycle as compared to other crops. In Egypt, about 0.5 Mha are cultivated annually, giving a total production of 6.1 Mt [
22]. Rice production is decreasing rapidly due to the negative impacts of various environmental stresses, such as water deficit and soil salinity [
8]. In addition to rice yields, water deficit and salt-affected soils also influence the morphological, physiological, and biochemical characteristics of rice [
23]. Therefore, it is very important to develop cost-effective technologies to ameliorate the deleterious effects of water and salt stresses on rice plants to assist with the upcoming problem of food security [
24].
There is little information about the synergistic use of PGPR and biochar, particularly under water deficit and saline soil conditions in the field. Consequently, the current investigation was assumed with an intent to assess the synergistic use of biochar and PGPR for alleviating water deficit stress and saline soil by utilizing rice as a test plant. It is projected that the study results will be beneficial for formulating novel management strategies for improving growth and rice productivity under water deficit and salt-affected soil in field conditions.
4. Discussion
Cropping plants are continuously exposed to different environmental perturbations, and more data is needed to understand how to manage these different types of stress. Therefore, the present study was conducted with the intent to test two new strategies that could minimize the negative impacts of water deficit and saline soil on rice. The independent and synergistic uses of biochar and PGPR were tested and found to be potential strategies for ameliorating the adverse effects on drought stress and soil salinity for rice production. It was found that all investigated parameters decreased significantly under different irrigation intervals (every 6 (I
1), 8 (I
2), and 10 (I
3) days) in salt-affected soil due to osmotic stress and nutritional disorders. The findings exhibited that the synergistic use of biochar and PGPR decreased the harmful effect of water deficit stress and salt-affected soil on rice growth [
49], improved soil physicochemical properties [
50], soil moisture constants, nutrient uptake, physiological characteristics, yield, and its components.
Among the tested strains,
P. koreensis and
B. coagulans produced a higher amount of IAA and solubilizing phosphate under stressed conditions (salinity and drought) [
8]. In our study, high NaCl and PEG 6000 concentrations reduced the FGP and VI of rice seeds of the germinant. Among the tested strains,
P. koreensis and
B. coagulans improved FGP and led to a decrease of mean germination time at different stress concentrations. To overcome the elevated NaCl and PEG 6000 concentrations on the germination of rice, seeds were treated with the two different strains of PGPRs. Those strains demonstrated a great ability to induce the germination of rice seeds under increasing NaCl and PEG 6000 than the untreated seeds at the same treatment level. Therefore, the PGPRs
P. koreensis and
B. coagulans likely improve the uptake of water and mitigate the poisonous effects of increased soil salinity and drought on rice germination, which may encourage their use as bioinoculum in actual farming conditions [
12].
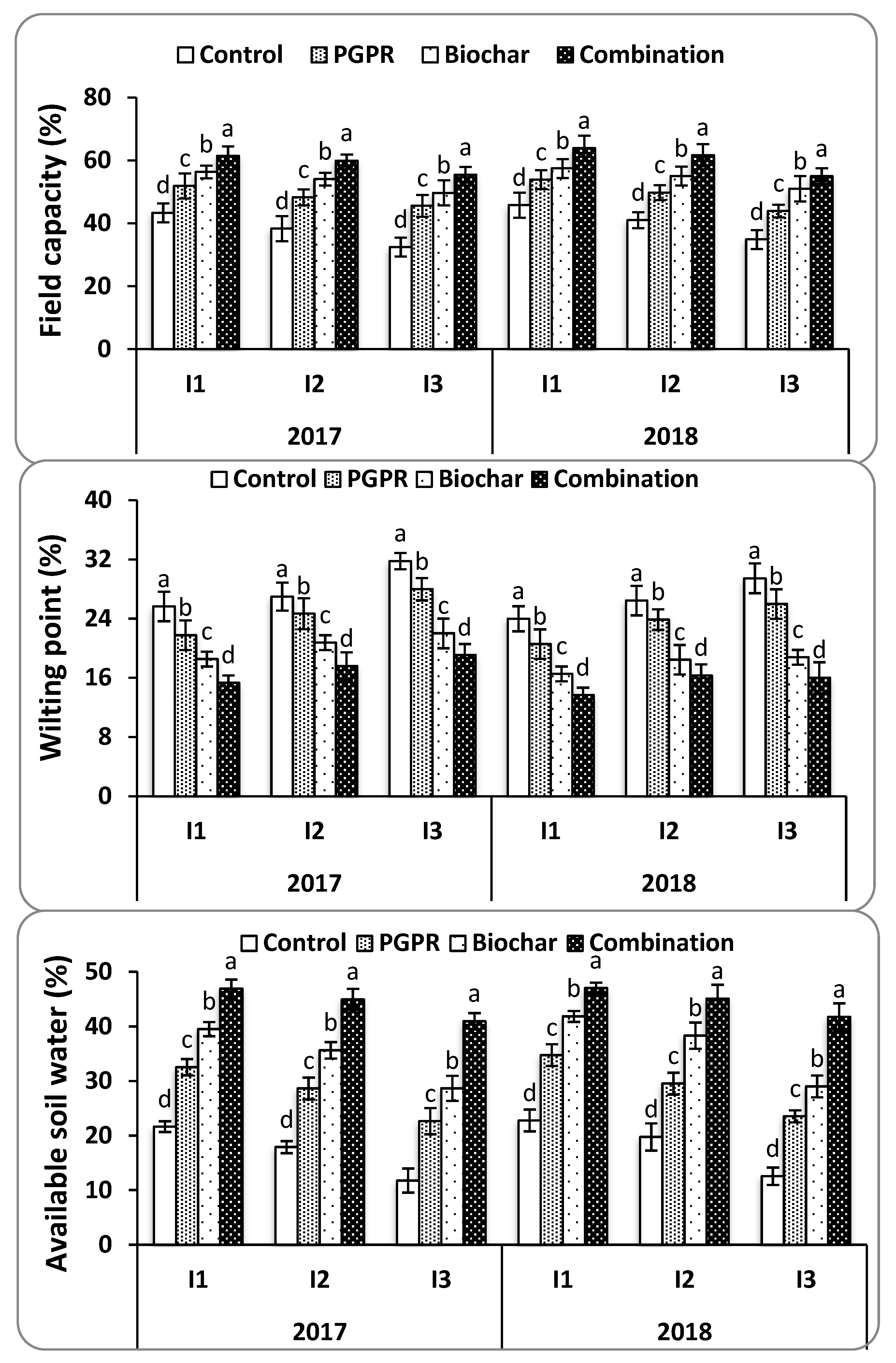
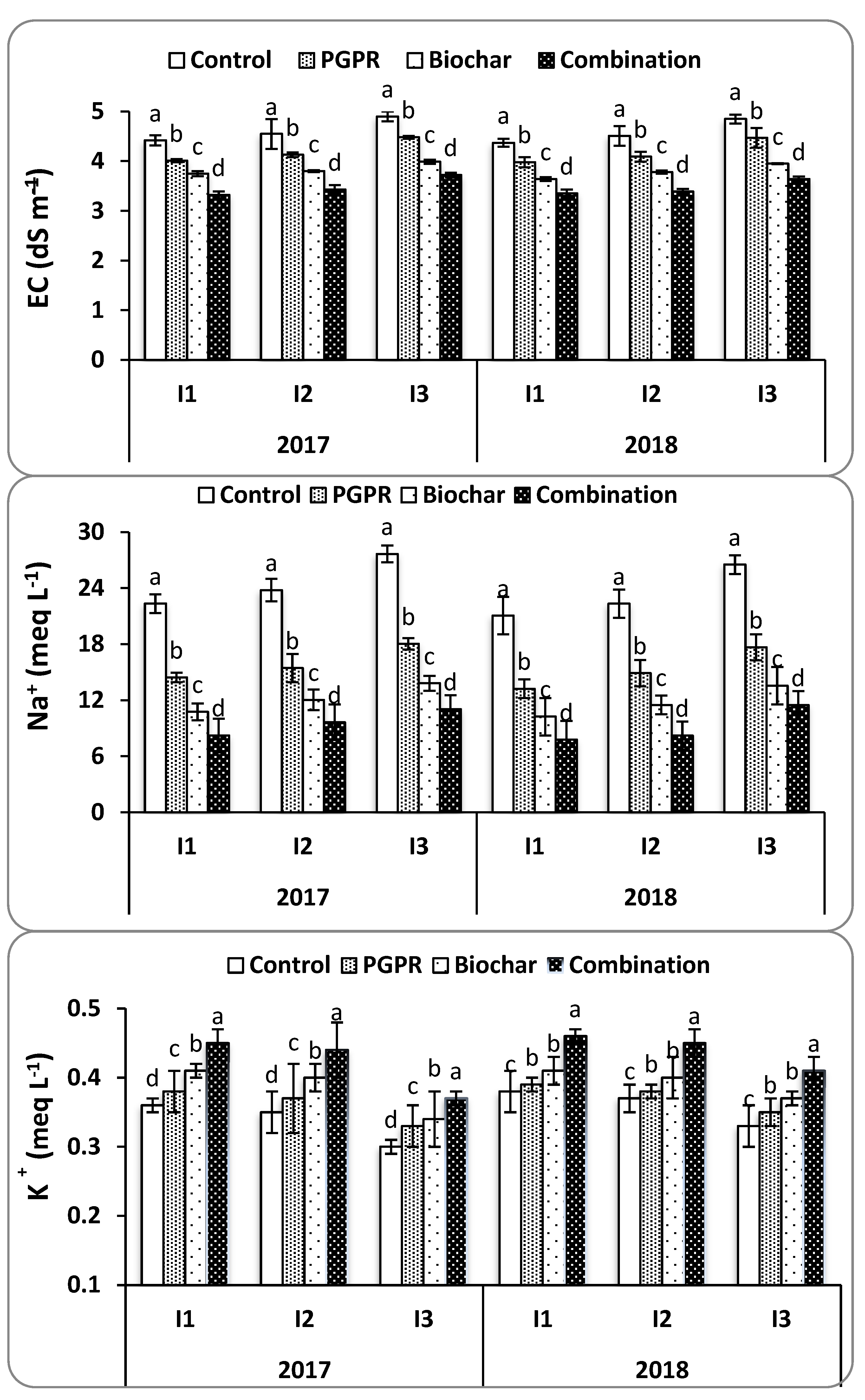
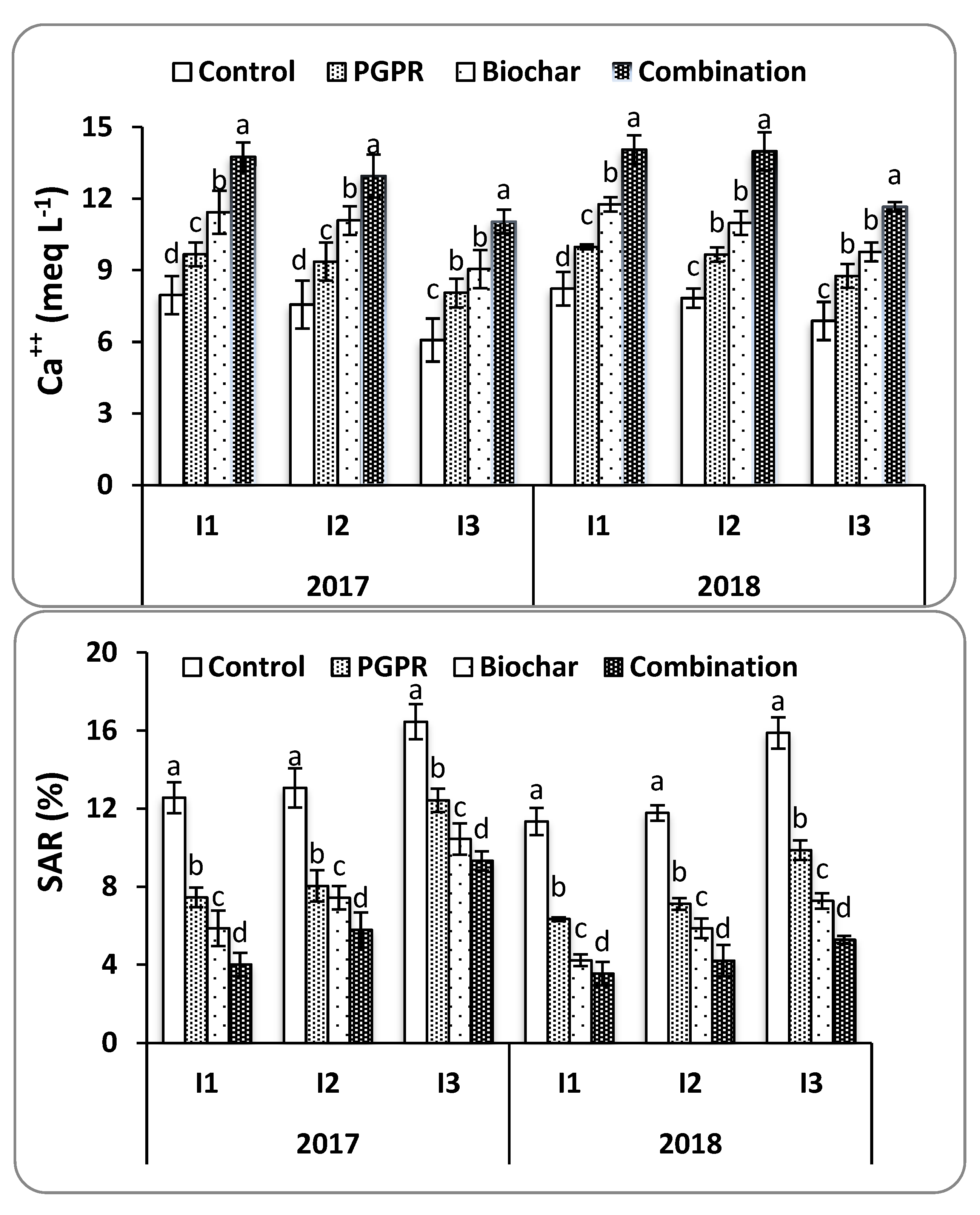
4.1. Soil Physicochemical Properties
SAR was measured to evaluate the potential for PGPRs and biochar to mitigate the adverse effects of Na
+ on soil structure under water deficit conditions. It was found that the combination of biochar and PGPR reduced soil SAR and EC at harvest in both seasons. This resulted in increased Ca
++ and K
+ concentrations in soil solution by placing Na
+ onto exchange sites and releasing other nutrients into the soil solution. Soil moisture content was improved by increasing field capacity, soil available water, and decreasing soil salt content, all of which resulted in improved soil structure and consequently allowed for the enhanced growth of rice roots. These findings are consistent with those noted by [
51].
The improved growth, nutrient absorption, and physiology of rice in response to PGPR inoculation at each irrigation interval and in saline soil could be ascribed to the biosynthesis of phytohormones such as IAA. IAA may be related to the total biomass of the plant [
52] and augmented availability of nutrients [
53]. It may also influence the production of ACC deaminase, EPS, and osmolytes [
54,
55]. As declared in the findings, biochar-amended soil resulted in greater improvements of rice growth and physiology compared with PGPR. Inoculation by PGPR was influential for soil quality and plant growth. However, its lower impact might be attributed to improved soil physicochemical and root proliferation [
56].
4.2. Soil Moisture Constants
Biochar increased the soil moisture constants for both field capacity and available soil water whilst decreasing the wilting point [
16]. Maintaining high soil moisture content in the root environment enables plants to grow better without facing stress problems [
15]. Furthermore, biochar improved soil nutrient cycling for N, P, and K. Improved plant uptake likely occurred due to biochar’s porous structure, large surface area, and negative surface charge [
50]. Biochar increased the soil’s cation exchange capacity and allowed for the retention of nutrients, which enhanced plant N, P, and K tissue concentrations. As explained above, the addition of biochar in association with PGPR resulted in optimal moisture content for longer periods compared with the individual treatments and the control. This was mainly due to augmented water-holding capacity and reduced soil bulk density [
49], which resulted in enhanced available mineral nutrients [
57]. The synergistic effect of PGPR and biochar further improved the total bacterial abundance owing to its biostimulation impact [
58] and augmented the microbial activities in the rhizosphere. This ultimately resulted in better growth of rice under the stress conditions.
4.3. Physiological Characteristics
It was found that increasing Na
+ in soil solution reduces the absorption of K
+, Ca
2+, N, and other essential nutrients in saline soils [
11]. In this study, the combination of biochar and PGPR resulted in reduced Na
+ and improved absorption of K
+, as described in
Figure 2 and
Figure 3. This was attributed to the higher Na
+ adsorption capacity of biochar, which has been recently stated by [
59]. Consequently, leaf Na
+ content was reduced with integrated use of biochar along with PGPR as compared to the control treatment. Previous reports indicated that biochar helped maintain the nutrient balance in soil solution by releasing mineral nutrients, particularly K
+, Ca
2+, and Mg
2+, and thereby reducing Na
+ uptake [
16]. The effect of biochar, therefore, ultimately increased the ratio of K
+ to Na
+. This can be clearly seen in the current study, where increased leaf K
+ content and decreased leaf Na
+ content resulted in an increased K
+/Na
+ ratio under each irrigation interval and saline soil. Additionally, PGPR could also reduce plant Na
+ uptake by the excretion of IAA and bacterial exopolysaccharide, which could bind Na
+ and prevent its uptake in plants [
60].
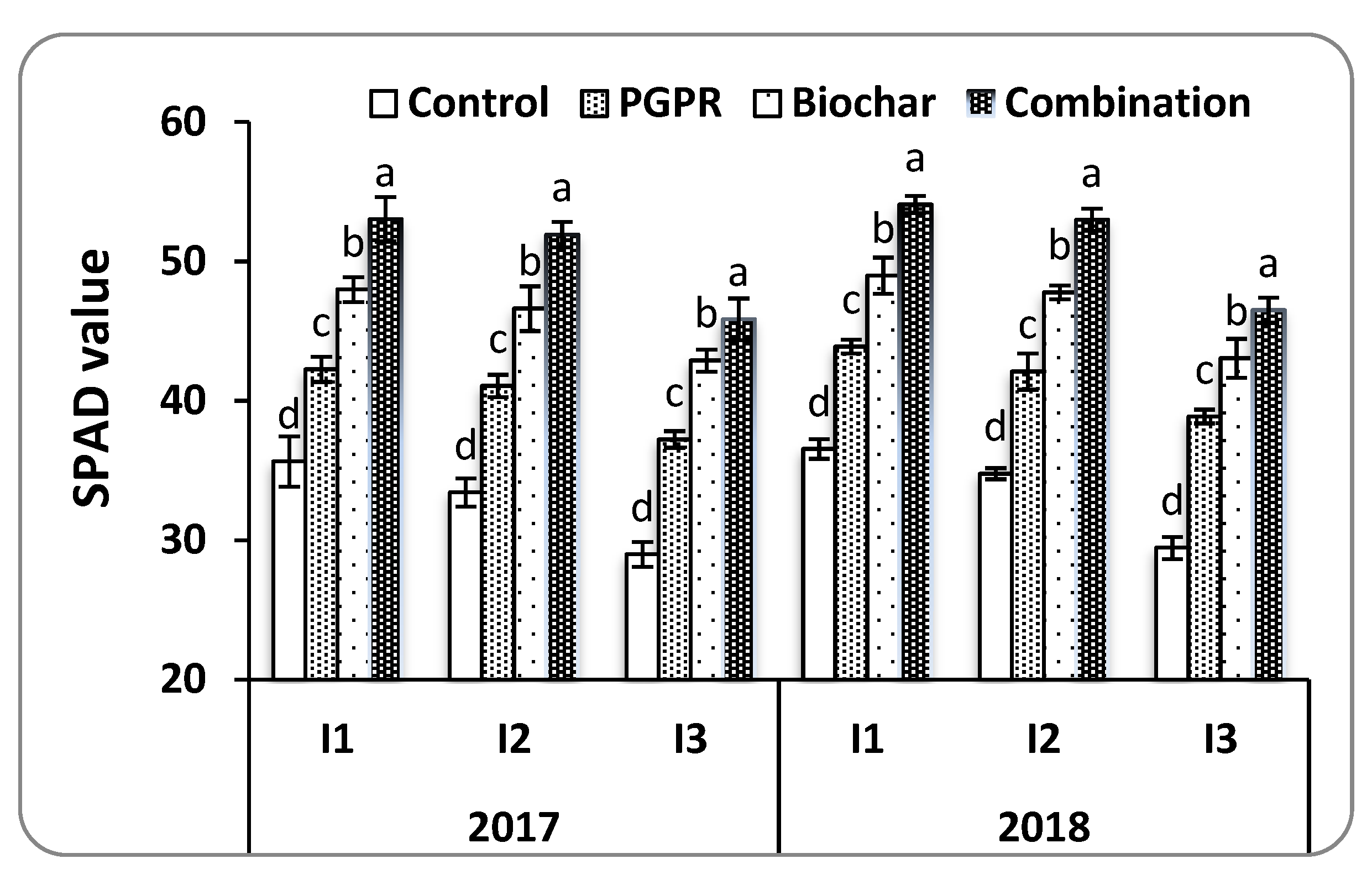
Chlorophyll content has been considered as a suitable parameter for the physiological evaluation under environmental stressors like drought and salinity [
13]. The decline of SPAD meter under water and salinity stressors is primarily caused by chloroplast damages, which contribute to important physiological changes during plant growth resulting in decrease crop productivity [
61]. The integrated use of biochar and PGPR recorded the highest SPAD values under water deficit and salt-affected soil. This is directly linked to the nitrogen content in the leaf, which is the macronutrient necessary for chlorophyll development [
6]. These findings could elucidate the increased leaf N uptake by the combining of biochar and PGPR in this study, which is positively reflected on yield and its components (1000-grain weight, number of grains panicle
−1, and number of panicles m
−2).
Relative water content and stomatal conductance are crucial parameters that are directly linked to soil water status and productivity [
62]. It was found that the lower relative water content and reduced stomatal conductance caused by drought stress and saline conditions led to increased proline content and reduced photosynthesis, nutritional imbalance, and grain yield [
14]. Notably, the stimulatory impacts of PGPR can be further improved by the application of biochar [
6]. This is perhaps owing to the additional water holding capacity of biochar [
13]. As described in the results, the synergistic use of biochar with PGPR increased soil moisture. This may have caused a dilution effect in the soil solution [
14]. Thereby reducing osmotic stress and preventing plants from losing turgor under water deficit and saline soil, leading to increase relative water content and improved stomatal conductance [
13,
63]. A strong positive relationship was observed between leaf proline content and leaf Na
+ concentration likely as a plant response to the abiotic stress; these results are in conformity with [
64]. Furthermore, it has been affirmed that proline content is negatively correlated with stomatal conductance and relative water content under water deficit and saline conditions [
14].
4.4. Yield and Its Components
In this study, the highest harvest index and grain yields were obtained by plants with the shortest irrigation interval (every six days, I
1). It has been stated that rice yields largely relate to traits such as 1000-grain weight, the number of grains per panicle, and the number of panicles m
−2. Water availability contributes to plant growth and development, and water deficit reduces plant productivity and thus decreases yield-related traits [
65]. Under the longer irrigation intervals (every 8 and 10 days), losses due to water deficit stress and saline soil could be attributed to the closure of stomata, decrease relative water content, and chlorophyll content, which ultimately lead to a reduction of photosynthesis and minimized grain yield [
13]. The combination of biochar and PGPR had a highly positive impact on water relations, nutrient cycles, and the availability and uptake of nutrients when compared to the individual applications and the control treatment. The synergistic use of biochar and PGPR had the ability to increment yield when applied to the plants irrigated every eight days (I
2), which was on par with those irrigated every six days (I
1) and under saline soil conditions.
4.5. Nutrient Uptake
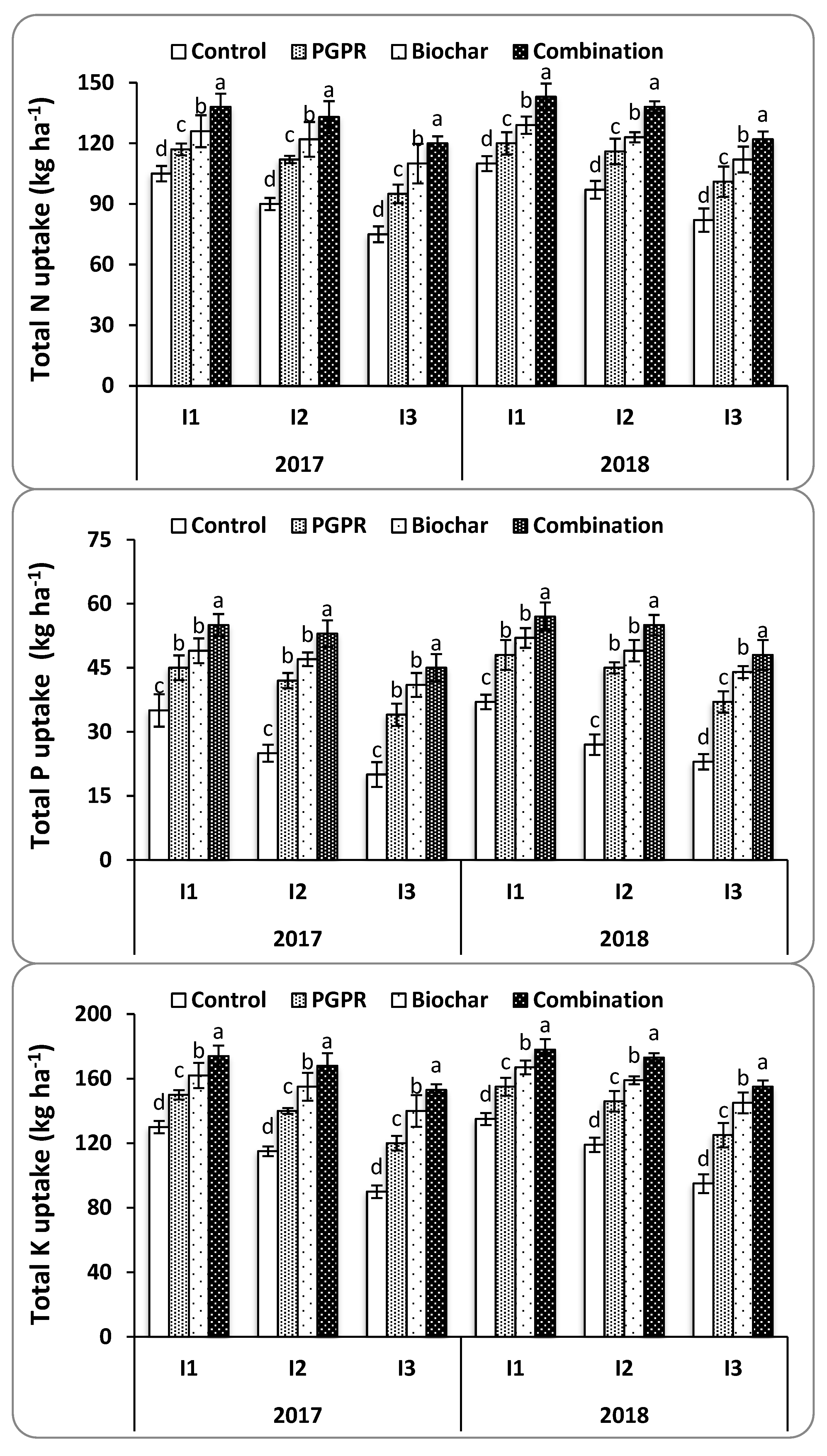
In this study, the highest N, P, and K uptakes were attained from rice plants treated with the combination of biochar and PGPR under each of the three irrigation intervals and in saline soil as opposed to the sole applications and the control treatment. Among irrigation intervals, plants irrigated every eight days (I2) were on par with those irrigated every six days (I1) under saline soil conditions. Both of these were higher than plants irrigated every 10 days (I3) when treated with the combination of biochar and PGPR in both seasons. The findings of the current study clearly demonstrate that the combination of biochar and PGPR further improved N, P, and K uptake significantly more than the application of either one alone. It appears that biochar and PGPR have a pivotal effect in the formation and stabilization of soil particulates under water deficit and saline conditions, and this can help with enhanced nutrient uptake.