1. Introduction
In Sub-Saharan Africa, maize is the most important cereal crop farmed on over 25 million ha, mainly by smallholder farmers. These smallholder maize farms yield beyond 38 million metric tons of grain, mainly for subsistence purposes [
1]. The production of maize has however largely been stalled by pests and diseases that affect maize crops during the growing, post-harvesting processing, and storage stages.
Gray Leaf Spot (GLS) is one of the major diseases that adversely affects maize crop production. The disease is caused by a
Cercospora zeae-maydis fungus, which affects maize crops across all the phenological stages. According to Dhami, et al. [
2],
Cercospora zeae-maydis is a polycyclic facultative pathogen or fungi which survives as mycelium in the residues of infected maize crops after harvesting. The conidia infect the maize crops through the stomatal openings of leaves. Approximately two to three weeks after colonizing maize leaf tissues, gray-to-tan lesions grow between the leaf veins, giving them a narrow rectangular appearance, which is 5–70 mm long by 2–4 mm wide [
2]. These lesions ultimately sporulate, spread, and coalesce, covering the entire leaf surface area. Subsequently, these lesions excessively reduce the level of sugar, causing stalk lodging, which ultimately results in premature death or extreme yield losses of up to 100% [
2]. In this regard, despite the fact that it can be managed through the utilization of resistant hybrids, crop rotations, tillage practices, and, if need be, the use of foliar-applied fungicides, the GLS is widely recognized as the most significant yield-limiting disease in maize crop production, [
3,
4,
5]. Therefore, efficient novel techniques for forecasting pandemics similar to GLS are necessary to foster food security, especially in the developing countries in Sub-Saharan Africa, where maize farming is the mainstay of millions of livelihoods.
In developing countries, it is currently a common practice for farmers to rely on field surveys to monitor the health of agricultural crops such as maize [
6]. Furthermore, the administration of agrochemicals to treat fungal pathogen disease, such as GLS, is also currently conducted in an indiscriminate manner, which results in exorbitant economic losses associated with purchasing and applying fungicides. Such techniques of administering agrochemicals also result in excessive pollution of proximal stream and underground water sources [
7]. Furthermore, the estimation of damages associated with such epidemic crop diseases, including their spatial extent, as well as their real-time spatial distribution, have largely been contingent on physical surveys. However, techniques such as physical surveys have proved to be highly subjective, tedious, relatively time-consuming, and unreliable at landscape scales, especially when preparing for the next cropping season [
6]. Subsequently, there is a need for cheap, fast, and consistent methods for agricultural-crop-disease monitoring, as well as forecasting of related food-crop diseases, for fostering food security while reducing the associated economic losses, as well as environmental pollution from the indiscriminate administration of pesticides. Above all, such efficient techniques for agricultural-crop-disease monitoring will provide the critically necessary information for decision making, particularly in determining the need for timely administration of agrochemicals, to reduce yield losses.
To date, earth observation techniques have been widely proven to be an effective noninvasive, nondestructive, and autonomous tool for detecting and monitoring biotic and abiotic stress in agricultural crops at local to regional scales and different phenological stages [
7,
8,
9,
10]. The success of remotely sensed data in monitoring and forecasting crop diseases such as GLS stems from its synoptic views, which offer timely acquired, spatially explicit, continuous, and abundant wavebands, with the ability to detect subtle biochemical and physical changes of crop plants. Earth observation sensors record the spectral reflectance of plant canopies of both infected and healthy crops. Subsequently, the spectral signature of infected crops tends to be different from that of healthy crops due to the biochemical and physiological alterations caused by crop diseases such as GLS [
11,
12,
13,
14,
15,
16]. The infection of crop plants results in the alteration of spectral reflectance, mainly due to the perpetual diminution of chlorophyll content, as well as the modification of internal tissue structure, which, in turn, results in physiological changes in the canopy of the crops [
11]. When a plant is infected, the disease-stress reduces the chlorophyll concentration, which results in increased reflectance in the red region of the electromagnetic spectrum (EMS) [
11]. Comparatively, healthy crop plants are associated with a high reflectance in the green and near-infrared spectrums and low reflectance in the red and blue spectrums [
11]. Furthermore, the increase in the magnitude of infection specifically alters numerous leaf optical properties, such as the leaf angle distribution (LAD), leaf area index (LAI), and the biomass of a plant, which directly influence and determine the target-energy interactions (i.e., energy absorption, reflectance, and transmission) in remote-sensing vegetation. Subsequently, maize crop farming could benefit from the timeous, spatially explicit wall-to-wall detection and monitoring system offered by remote-sensing techniques, which reduces costs and ensures food security [
13,
17].
Hyperspectral sensors have been proven to be the most robust and accurate sources of remotely sensed data in vegetation-mapping studies, particularly agricultural crops [
17,
18,
19,
20,
21,
22]. For instance, Calderón, Navas-Cortés, and Zarco-Tejada [
22] illustrated that hyperspectral remotely sensed data could accurately discriminate Verticillium Wilt diseases on olive crops, with optimal overall accuracies of 71.4% and 75.0% at the initial and severe stages of infection. The high discrimination accuracies in crops at different levels of infection associated with hyperspectral data are attributed to its narrow spectral channels, which have the ability to capture minute spectral variations induced by crop diseases at various stages, which are otherwise masked by broad multispectral sensors. However, hyperspectral data are often costly and have high data dimensionality, while being limited to local-scale applications. Hence, the rapid development of sensor technologies and the emergence of freely available broadband sensors have raised a lot of interest in favor of sensor missions, such as Landsat. Specifically, the recently launched Landsat Operational Land imager (OLI) has been of significant interest in agricultural crops mapping studies. Although Landsat data perform satisfactorily in mapping agricultural crop traits at landscape scales, their broad wavebands does not adequately cover portions of the EMS that are critical in vegetation mapping, such as the red edge. This limitation resulted in the emergence of Sentinel-2 multispectral imager (MSI), as well as the Vegetation and Environment monitoring on a New MicroSatellite (VENµS), as better alternatives in the remote sensing of crop diseases, such as GSL. Furthermore, the earth observation community is eagerly awaiting hyperspectral sensors which will offer free data at relatively large spatial scales, such as the Indian Space Research Organization’s Geostationary Hyperspectral Imager Satellite (GISAT), Italy/Israel’s Shalom, Germany’s Environmental Mapping and Analysis Program (EnMap), and the National Aeronautics and Space Administration’s (NASA) Hyperspectral InfraRed Imager (HyspIRI) [
21].
The HyspIRI sensor will be characterized by two instruments with an intelligent payload module: Visible/near infrared/Shortwave InfraRed (VSWIR) imaging spectrometer and a thermal infrared (TIR) multispectral imager. The sensor will offer global coverage data at 5 and 16 days and temporal resolutions of 30 and 60 m spatial resolution [
23,
24,
25,
26]. Such a temporal resolution is optimal for monitoring diseases with relatively longer latent periods, such as GLS, which can take as long as 14 to 28 days after infection for the lesions to sporulate [
2,
27,
28]. Above all, the VSWIR instrument will offer 7 nm narrow contiguous wavebands perceived to be sensitive to the detection and mapping of crop diseases to increase yields and surpass food insecurities. Hyperspectral sensors such as HyspIRI offer specific narrow spectral channels, which are suitable for targeted monitoring of specific optical biochemical and physical plant parameters, before and after infection by diseases, at relatively low costs [
25,
29]. Nonetheless, there is still a highly significant knowledge gap in the full exploitation of hyperspectral remotely sensed data, especially in the crop farming. Since the inception of NASA’s HyspIRI mission, there has been a call for comparative studies to assess the performance of HyspIRI’s spectral settings and other hyper and multispectral sensors so as to advance our knowledge and models in vegetation-health characterization [
21]. It is against this background that this study seeks to assess the strength and the practicality of simulated HyspIRI’s spectral configuration in detecting and discriminating maize crops at various levels of Maize Gray Leaf Spot (MGLS) diseases infection levels in relation to Sentinel-2MSI, VENµS, and Landsat OLI.
3. Results
The ANOVA results showed that there were significant differences (
p = 0.005) between the healthy, intermediately infected, and severely infected maize crops, based on data resampled to the spectral settings of HyspIRI, Sentinel-2MSI, VENµS, and Landsat OLI.
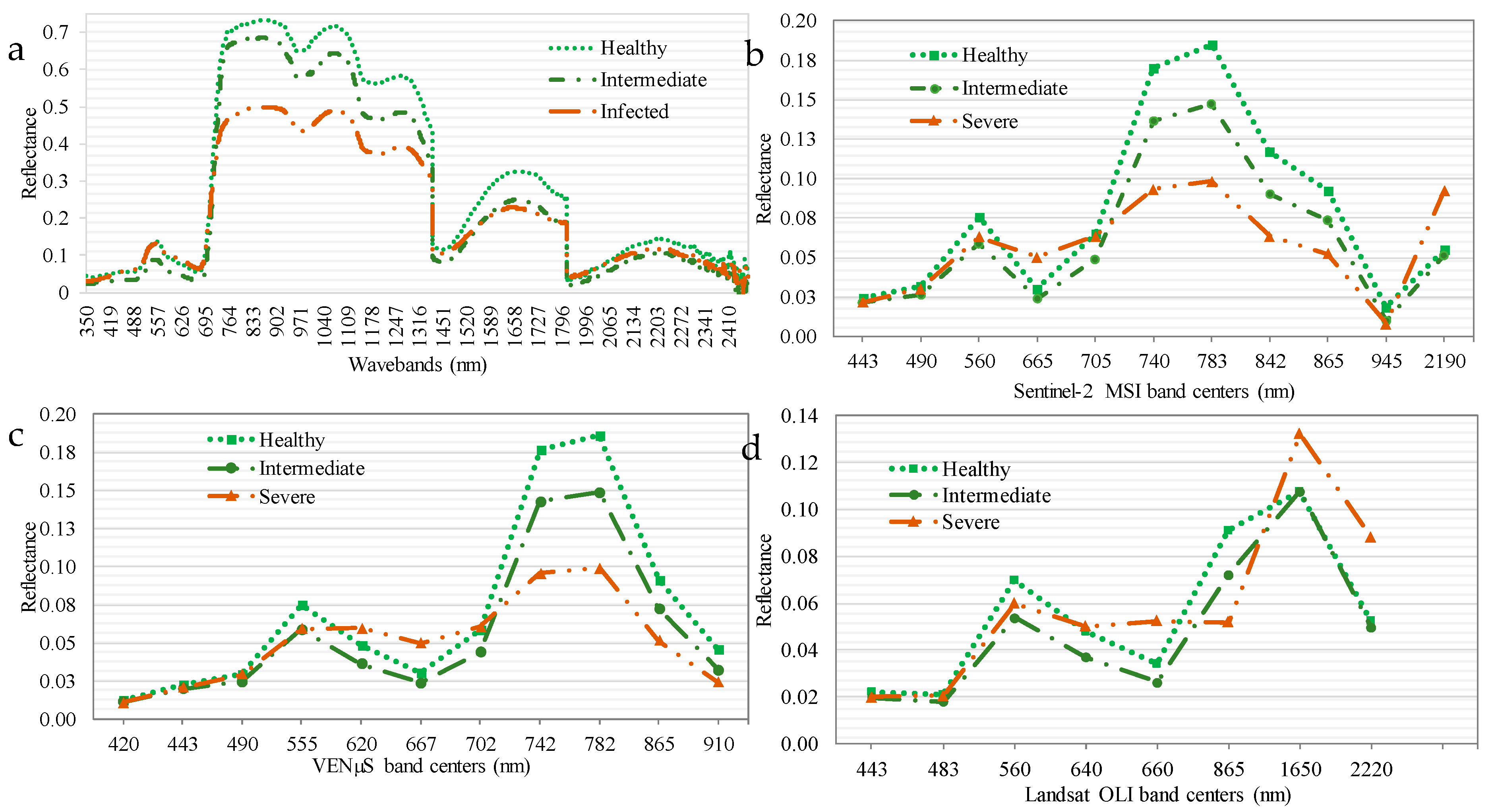
Figure 3 illustrates the differences in the means of healthy, intermediately infected, and severely infected maize crops. Windows of spectral separability between the reflectance of healthy, intermediately infected, and severely infected maize crops were exhibited in the red-edge, as well as in the near-infrared sections of the EMS, based on the spectral settings of all the sensors (
Figure 3). There were less windows of spectral separability where healthy maize could be spectrally discriminated from that which was intermediately and highly infected in the visible section of the EMS cover by the four sensors (
Figure 3).
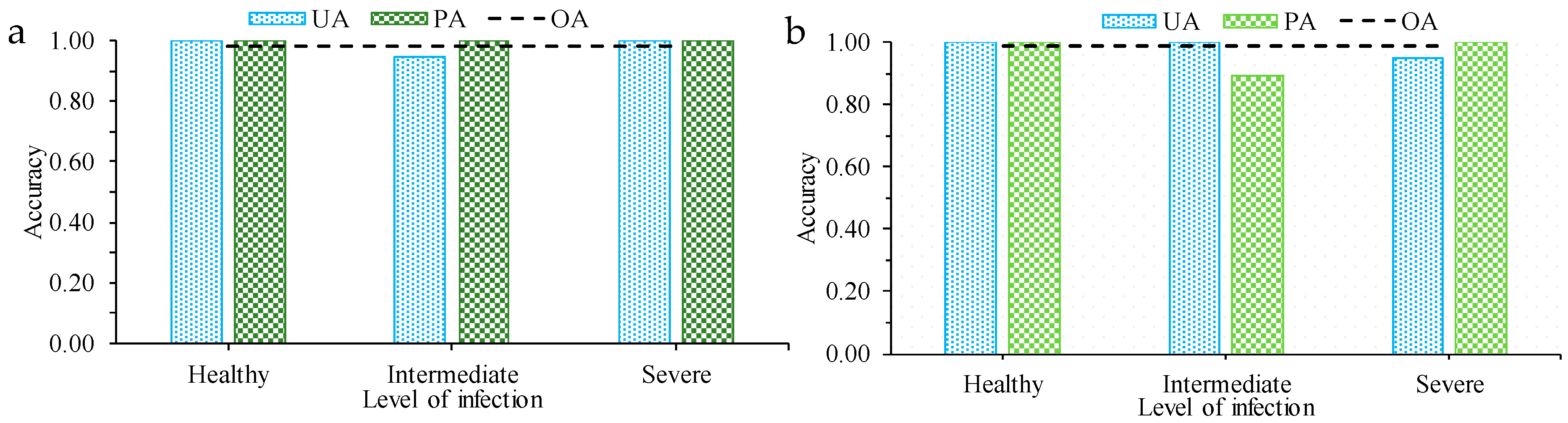
In discriminating between the different stages of GSL infection on maize, using PLS–DA, our results indicated that HyspIRI exhibited higher classification accuracies in relation to the other sensors considered in this study (
Figure 4). Specifically, HyspIRI exhibited an overall accuracy of 0.99 and a producer and user accuracy of 99% for the healthy and severely infected maize crops. The user accuracy of the intermediately infected crops exhibited a slightly lower user accuracy of 0.94, in relation to the other two infection levels. When using Sentinel-2 MSI spectral settings, accuracies comparable to those of HyspIRI were obtained (overall accuracy 0.95). Only the user accuracies for the intermediately and severely infected were below 0.95 when Sentinel 2 MSI spectral settings were used. A similar trend was observed when using the spectral settings of VENµS sensor. However, the user accuracies for the intermediately and severely infected further decreased to less than 0.9 when VENµS spectral settings were used. The VENµS spectral setting yielded an overall classification accuracy of 0.94. A different trend was observed when using the Landsat 8 OLI spectral settings. High user and producer accuracies of 1.0 were exhibited by the Landsat OLI spectral settings for the severely infected maize crops, whereas those for the healthy and the intermediately infected maize crops were relatively lower than those exhibited by the HyspIRI, Sentinel-2 MSI, and VENµS spectral settings. The overall accuracy exhibited by Landsat 8 OLI was 0.89.
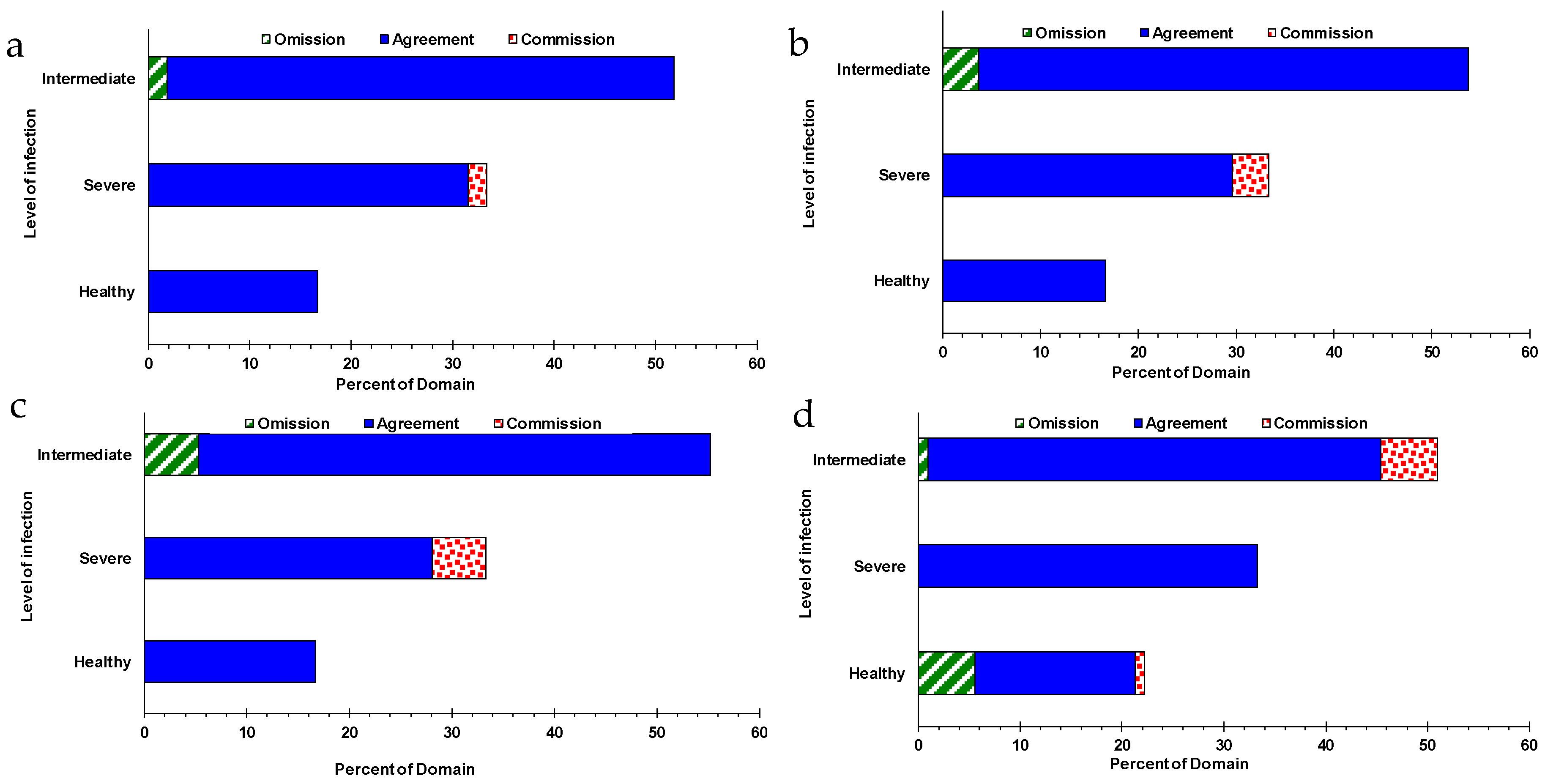
When assessing the accuracy and strength of HyspIRI spectral configurations in relation to those of Sentinel-2MSI, VENµS, and Landsat OLI, based on the allocation of agreement commission and omission, results of this study showed that HyspIRI was stronger and more accurate in discriminating different GLS levels in maize crops (
Figure 5). Specifically, HyspIRI exhibited less than 5% allocations of disagreement (commission) for the infected only and optimal allocations of agreement for all the GLS infection levels (
Figure 5). Again, a similar trend was of a considerable magnitude of the allocation of disagreement (omission) was observed based on the Sentinel-2 MSI and VENµS sensor settings for the severely ‘infected’ maize crops. When using Sentinel-2 MSI sensor’s settings, the omission increased to above 5% and to about 8%, when using the spectral setup of VENµS sensor. When Landsat OLI’s spectral setup was used, the magnitude of omission and commission for the healthy and intermediately infected maize crops increased significant by a magnitude above 10% combined.
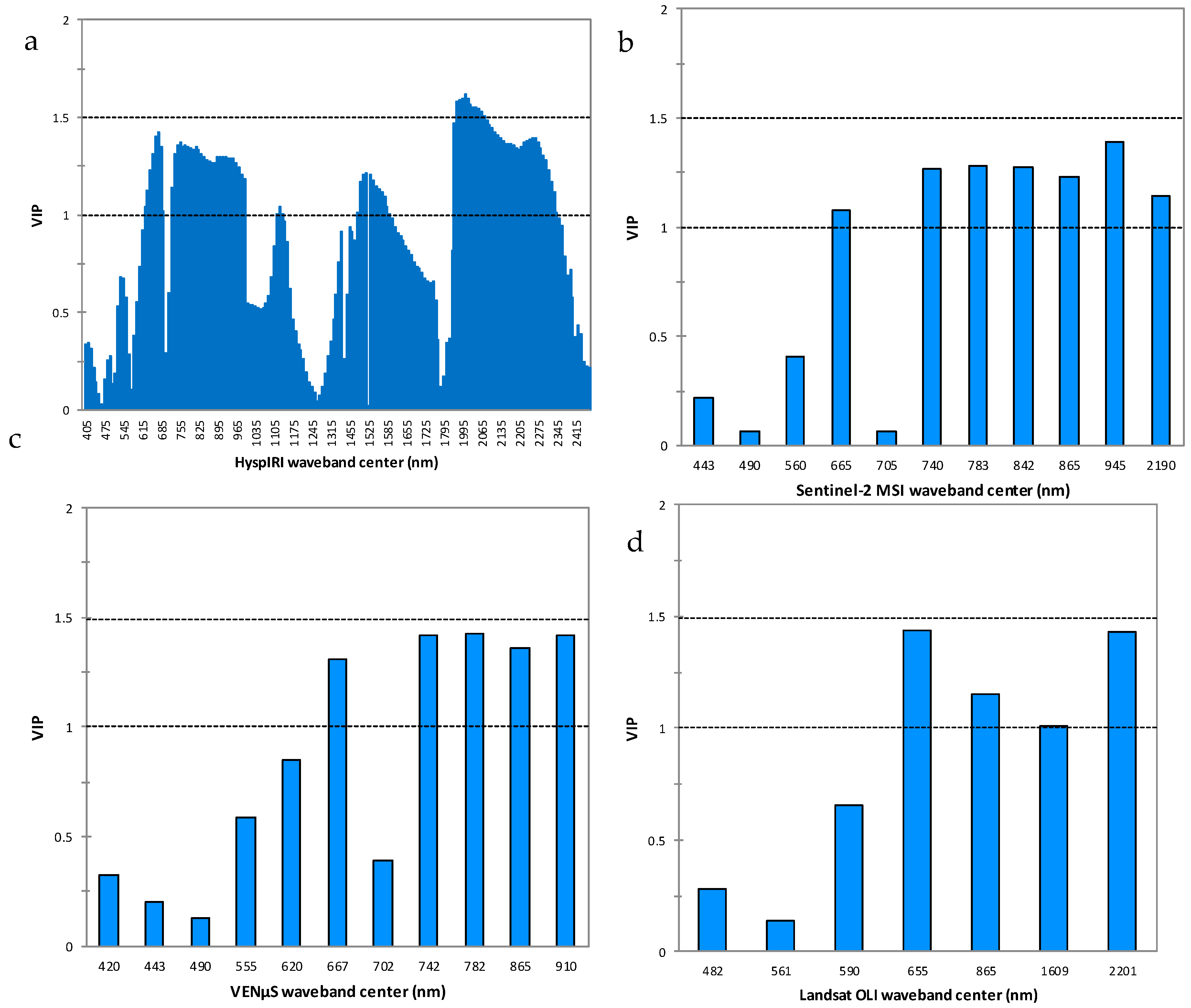
The most optimal wavebands that were instrumental and influential in making HyspIRI stronger and better in discriminating between different levels of GLS infections in maize were from the red, red-edge, mid, and far infrared spectral regions (
Figure 6a). Specifically, the NIR and FIR wavebands between 750 and 800 nm, as well as between 1995 and 2065 nm, followed by the red-edge section, were the highest in terms of variable importance in projection scores when using HyspIRI’s spectral settings, as illustrated on
Figure 6a. When Sentinel-2 MSI spectral settings were used, the NIR band centered at 945 nm and the red-edge band centered at 740 nm, as well as the NIR bands centered at 783, 842, and 865 nm, were the most influential variables (
Figure 6b). Again, VENµS’s wavebands from the red-edge and NIR region centered at 945, 740, 842, and 783 nm, in order of importance, were the most influential variables in discriminating between different levels of GLS infections on maize crops (
Figure 6c). Meanwhile, Landsat OLI’s bands 7, 4, 5, and 6 were the most influential variables, as illustrated in
Figure 6d.
4. Discussion
The spectral setup of HyspIRI was more robust and accurate in classifying healthy maize crops from those that were intermediately, as well as severely, infected by GLS, in relation to the spectral settings of Sentinel-2MSI, VENµS, and Landsat OLI. The literature underscores the narrow widths of hyperspectral wavebands in vegetation discrimination as the reason for their optimal performance when compared with broad wavebands, which tend to mask critical fine-grained information that is instrumental in discriminating between infected crops and healthy ones [
18,
19,
35]. This explains the optimal performance of HyspIRI in relation to the other three sensors.
Specifically, Sentinel-2MSI, VENµS, and Landsat OLI have broader wavebands characterized by widths which range from 15 to 115 nm. These wavebands tend to be insensitive to the optical traits that are altered by diseases such as the GLS, resulting in their being insensitive to the infected crop’s optical canopy traits. This explains the less accuracies exhibited by sensors such as Landsat OLI in this study. In a similar study, Dhau, et al. [
5] illustrated the utility of hyperspectral data in discriminating maize crops that were under different levels of maize-streak virus infections. Specifically, they attained an overall accuracy of 95.83%, which is similar to that demonstrated by HyspIRI in this study.
Results in this study showed that the performance of resampled Sentinel-2 MSI was comparable to that of HyspIRI, but was not significantly different from that of VENµS. However, Sentinel-2 MSI performed better than Landsat OLI, which exhibited the least classification accuracies. Although Sentinel-2 MSI does not have spectral channels that are as narrow as those of HyspIRI, it covers the red-edge section, which are not covered by Landsat OLI sensor. This could explain the optimal performance of Sentinel-2 when compared to Landsat OLI sensor’s spectral setup. When comparing the spectral setup of Sentinel-2 MSI, there were slightly less variations in terms of the EMS regions covered by both these sensors, hence their almost-similar performance in discriminating between different levels of GSL disease in this study. Above all, the band widths of Landsat OLI, when compared to those of Sentinel-2 MSI, are slightly broad, masking even more information that is critical in discriminating maize crops at various GLS-infection levels. Results of this study validate the theoretical discourse put forth by Marshall and Thenkabail [
19] which emphasizes the importance of high spectral resolution as opposed to high spatial resolution, particularly on sensors that cover the red-edge section of the EMS when HypIRI outperformed Sentinel-2MSI, VENµS, and Landsat OLI.
Resampled HyspIRI’s NIR and red-edge bands were more influential variables in discriminating between maize crops under different levels of GLS infections. The optimal performance of HyspIRI’s spectral settings could be explained by its contiguous 10 nm narrow wavebands, which are sensitive in detecting the subtle changes in the biochemical and physiological changes induced by the GLS on maize crops. The GSL disease manifests as black spots, which attack the stomatal activity and then later on develop into long rectangular-shaped grey-to-tan-colored lesions. These changes alter the optical foliar traits, particularly the pigment concentrations and chlorophyll content, into rectangular-shaped grey-to-tan lesions. When chlorophyll and pigment concentrations are reduced, infected maize crops become very discriminable from those that are healthy, as well as those under the initial and intermediate infection stages, particularly in the NIR and the red-edge regions [
11,
12]. Healthy plants with high chlorophyll concentrations tend to reflect highly on the NIR, whereas the chlorophyll concentrations shift the red-edge position toward the longer wavebands, facilitating an optimal discrimination based on the narrow spectra channels. Meanwhile, the GLS lesions in infected maize crop leaves reduce chlorophyll concentration, which then shifts the red-edge position toward the short wavebands, facilitating optimal discriminations based on the red-edge section of the EMS, especially when using narrower spectral channels in relation to broad wavebands. In a similar study, Dhau et al. [
5] showed that the highly influential wavebands in discriminating maize crops under various stages of maize-streak virus infections were from the NIR (881 and 2338 nm) and visible (552 and 603 nm) spectral sections of the EMS, using proximal sensed data. Al-Ahmadi, et al. [
36], in a related study, demonstrated that the narrow spectral channels of hyperspectral data, particularly the NIR 1940 band, optimally detected
Macrophomina phaseolina toxin effects in soybeans, as was the case in this study. Results of these studies illustrate and concur with those of the present study, which noted that the visible (red-edge) and the NIR sections of the EMS are critical for characterizing different levels of crop infections by diseases such as GLS.
In assessing the spectral separability of the three different levels of GLS infections based on the resampled HyspIRI, Sentinel-2MSI, VENµS, and Landsat OLI data, results showed that there were less windows for spectrally discriminating between healthy maize and intermediately and highly infected maze in the visible section of the EMS by the four sensors. This could be attributed to the fact that the visible section of the EMS is highly susceptible to atmospheric influences. Atmospheric influences result in the attenuation and dilution of the signal, making the spectral discrimination of different levels of GLS-infected maize futile, based on the bands from the visible section of the EMS covered by resampled HyspIRI, Sentinel-2MSI, VENµS, and Landsat OLI data in this study.
Implications of Utilizing Simulated HyspIRI Spectral Settings
Despite the optimal performance and success exhibited by HyspIRI in discriminating maize crops grown under different levels of GLS infection in relation to Sentinel-2MSI, VENµS, and Landsat OLI based on simulated data, there is still a need to assess this sensor’s satellite-borne measured data. Considering the fact that this study intended to only assess the spectral configuration of these sensors, there is a need to assess the effect of their radiometric and spatial resolutions in a similar application setup. Largely, the satellite-borne reflectance remotely sensed data tend to be affected by numerous factors associated with the sensor’s platform (i.e., the zenith angle of the sensor and speed of the platform) and atmospheric-related influences (i.e., presence of atmospheric impurities, haze, and sun irradiance). Spectrometric-proximally sensed data tends to be less affected by atmospheric related influences and has a different sun angle (i.e., a beam divergence is of ±60°); hence, it offers a robust dataset that is suitable for testing the usability and strength of forthcoming sensor missions, such as HyspIRI’s spectral band settings. Meanwhile, HyspIRI will have a lower signal-to-noise ratio of 700:1 at 600 nm and 500:1 at 2200 nm when compared to ASD FieldSpec, which ranges between 700:1 and 2100:1 for 350–2500 nm [
23]. Although the ground sampling distance of 30 m could be regarded as moderate, the literature illustrates that it is optimal for characterizing crop physiology [
37,
38,
39]. For example, Mfuka et al. [
37] were able to characterize white mold pathogen on soybeans to optimal overall accuracies of 95%–99%, based on the ground sampling distance of 30 m characteristic of Landsat OLI data. In this regard, the 30 m ground sampling distance is deemed effective for agronomical management of the diseases such as GLS. However, the performance of these sensors’, namely, HyspIRI, Sentinel-2MSI, VENµS, and Landsat OLI spatial and spectral settings derived using satellite platforms, still needs to be evaluated in a similar environment.
5. Conclusions
We tested the strength and practicality of HyspIRI’s spectral configuration in detecting and discriminating between maize crops at various stages of GLS-disease infection in relation to that of Sentinel-2MSI, VENµS, and Landsat OLI. Grounded on the findings of this study, we conclude that.
HyspIRI’s spectral setup is more robust and effective in characterizing maize crops growing under different levels of GLS-disease infection when compared to Sentinel-2MSI, VENµS, and Landsat OLI-2.
Sentinel-2 MSI could be a better alternative to HyspIRI, considering the fact that its spectral setup performed comparable to that of HyspIRI in discriminating maize crops with different levels of GLS infections.
This study illustrates that HyspIRI hyperspectral remotely sensed data could be a panacea to the lack of spatially explicit spectral resolution data as a better alternative for accurate and effective monitoring and forecasting of agricultural-crop diseases similar to the GSL, which are severe yield-reduction agents. Furthermore, results of this work are a significant step toward achieving urgently required techniques for comprehensively assessing, monitoring, and forecasting food-crop disease epidemics at relatively larger scales. These techniques effectively build on initiatives for improving food-crop production, while addressing the global village’s mandate of achieving food security, especially for developing nations, which are more susceptible to frequent droughts, limited resources, and poverty. Notwithstanding the soundness of this study’s findings, there is still need to assess the spectral settings of these sensors on different crops and physical settings. Above all, there is still a need to test the actual remotely sensed products of these sensors in effectively monitoring and forecasting agricultural-crop diseases. There is also need to assess the performance of vegetation indices of these sensors in discriminating maize crops at various stages of GLS diseases infection.