Bioavailability of Iron and the Influence of Vitamin a in Biofortified Foods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Preparation of Raw Materials
2.3. Determination of Food Composition
2.4. Preparation of Diets
2.5. Bioavailability
2.5.1. Animal Assay
2.5.2. Depletion/Repletion Method
2.5.3. Hemoglobin
2.5.4. Efficiency in Hemoglobin Regeneration (HRE)
2.6. Biomolecular Analysis
2.6.1. Extraction of Total Ribonucleic acid-RNA of Duodenal Mucosa and Liver
2.6.2. Complementary DNA (cDNA) Synthesis
2.6.3. Quantification of Transcripts by Reverse Transcription Followed by Real-time polymerase chain reaction RT-PCR
2.7. Statistical Analysis
3. Results
3.1. Animal Assay
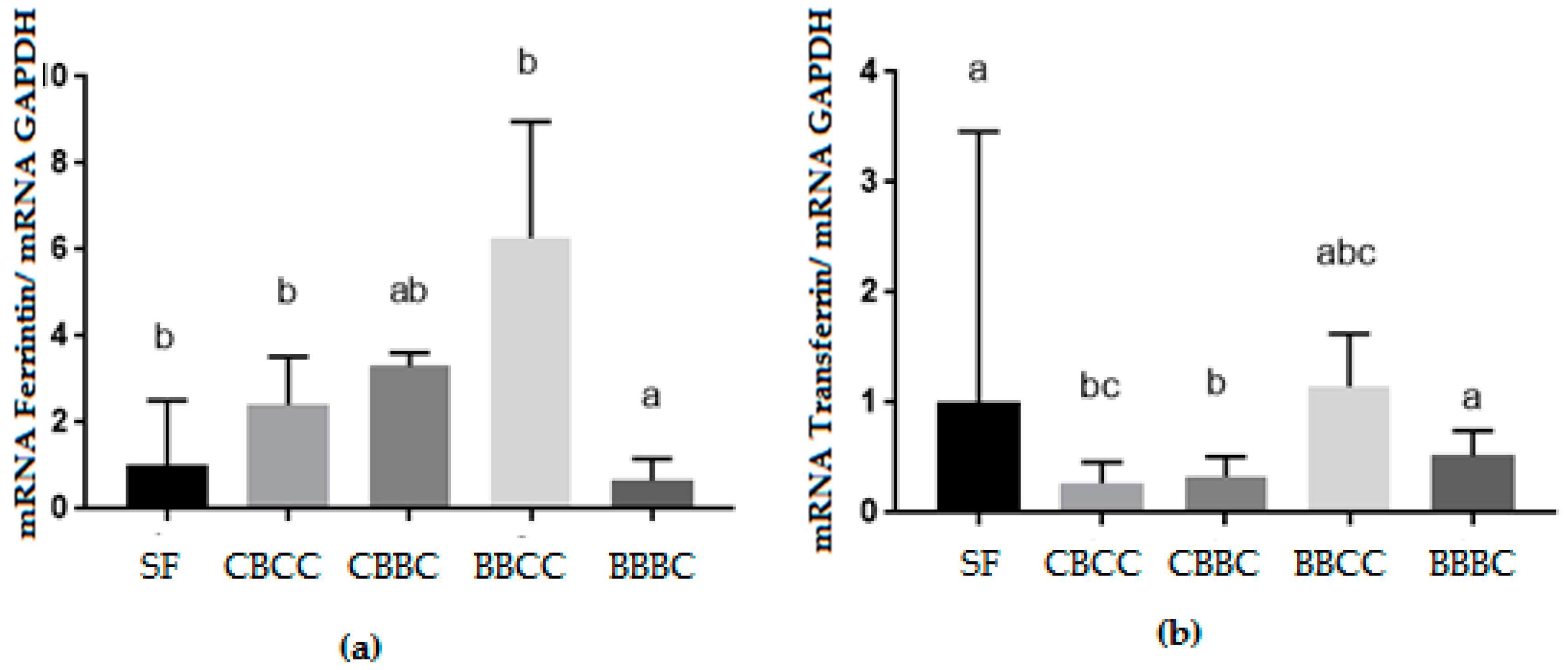
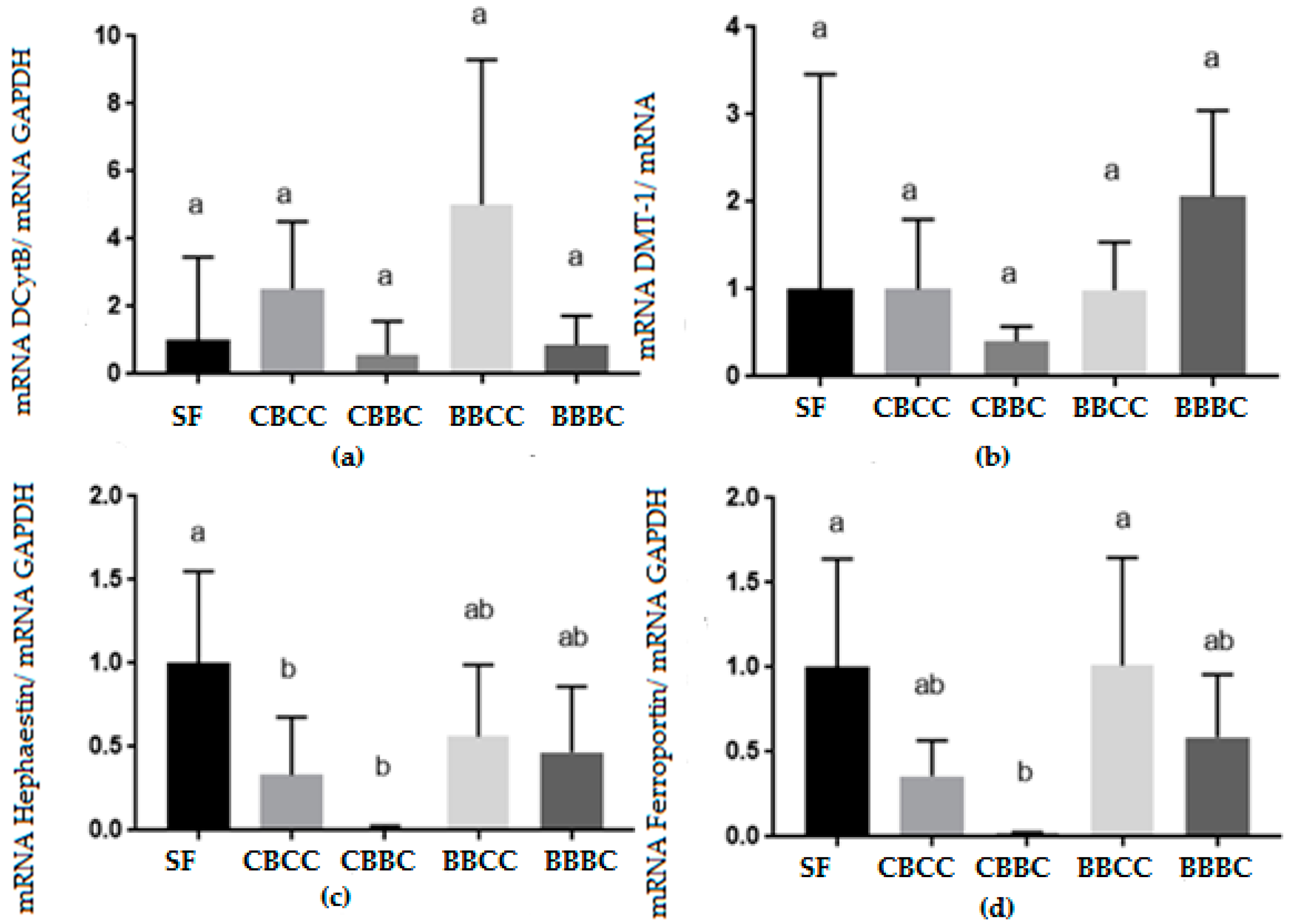
3.2. Biomolecular Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brazil Ministry of Health. Notebooks of Basic Attention-n°20 Series, A. In Norms and Technical Manuals; Brazil Ministry of Health: Brasília, Brazil, 2007. [Google Scholar]
- Sida-Arreola, J.P.; Sanchez, E.; Avila-Quezada, G.D.; Acosta-Muniz, C.H.; Zamudio-Flores, P.B. Biofortificación con micronutrientes en cultivos agrícolas y su impacto en la nutrición y salud humana. Tecnociencia Chihuah. 2015, 9, 67–74. [Google Scholar]
- Gonçalves, A.S.F.; Gonçalvez, W.M.; de Silva, K.M.J.; Oliveira, R.M. Use of plant biofortification: A review. J. Univ. Cent. Patos Minas 2015, 6, 75–87. [Google Scholar]
- Netto, M.P.; Priore, S.E.; Framceschini, S.C.C. Interaction between vitamin A and iron in different population groups. Braz. J. Matern. Child Health 2007, 7, 15–22. [Google Scholar]
- Ceballos, H.; Luna, J.; Escobar, A.F.; Ortiz, D.; Perez, J.C.; Sanchez, T.; Pachon, H.; Dufour, D. Spatial distribution of dry matter in yellow fleshed cassava roots and its influence on carotenoid retention upon boiling. Food Res. Int. 2012, 45, 52–59. [Google Scholar] [CrossRef]
- De Carvalho, J.L.V.; Nutti, M. Biofortification of agricultural products for human nutrition. In Proceedings of the 64th Annual Meeting of SBPC, São Luís, MA, USA, 25 July 2012. [Google Scholar]
- Phorbee, O.O.; Olayiwola, I.O.; Sanni, S.A. Bioavailability of Beta Carotene in Traditional Fermented, Roasted Granules, Gari from Bio-Fortified Cassava Roots. Food Nutr. Sci. 2013, 4, 1247–1254. [Google Scholar]
- Oliveira, D.G. Simultaneous Selection for Production, Biofortification and Cooking in Segregating Populations of Cowpea. Master’s Thesis, University Federal do Piauí, Teresina, Brazil, 2014. [Google Scholar]
- Empresa brasileira de Pesquisa Agropecuária – EMBRAPA.Cultivate Cowpea Beans with Olive Grains and Rich in Iron and Zinc; EMBRAPA: Teresina, Brazil, 2009.
- Silva, A.G.M. Quality Evaluation of Biofortified Foods Produced in Agroecological Systems in the Humid Tropics. Ph.D. Thesis, Paulista State University, São José do Rio Preto, Brazil, 2016. [Google Scholar]
- Parada, J.; Aguilera, J.M. Food Microstructure Affects the Bioavailability of Several Nutrients. J. Food Sci. 2007, 72, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Casgrain, A.; Collings, R.; Harvey, L.J.; Boza, J.J.; Fairweather-Tait, S.J. Micronutrient bioavailability research priorities. Am. J. Clin. Nutr. 2010, 91, 1423–1429. [Google Scholar] [CrossRef]
- Arruda, L.F. Influence of Dietary Iron Content on the Expression of Iron Metabolism, Oxidative Stress and Aging Markers in Adult Mice. Ph.D. Thesis, Brasília University, Brasília, Brazil, 2013. [Google Scholar]
- Cunha, M.S.B. Vitamin A Deficiency Modulates Iron Metabolism via Ineffective Erythropoiesis Independently of the Inflammatory Response. Master’s Thesis, University of Brasília, Brasília, Brazil, 2013. [Google Scholar]
- Grotto, H.Z.W. Iron physiology and metabolism. Braz. J. Hematol. Hemother. 2010, 32, 8–17. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1998. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1997; Volume 2. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1992; Volume 2. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventors, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. J. 1999, 299, 152–178. [Google Scholar]
- He, X.T.; Logan, T.J.; Traina, S.J. Physical and chemical characteristics of selected U.S. municipal solid waste composts. J. Environ. Qual. 1995, 24, 543–552. [Google Scholar] [CrossRef]
- Latta, M.; Eskin, J.J. A simple and rapid colorimetric method for phytate determination. Agric. Food Chem. 1980, 28, 1313–1315. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Kimura, M. HaverstPlus Handbook for Carotenoid Analysis; HarvestPlus: Washington, DC, USA, 2004. [Google Scholar]
- Pinheiro-Sant’Ana, H.M.; Stringheta, P.C.; Brandão, S.C.C.; Azeredo, R.M.C. Carotenoid retention and vitamin A value in carrot (Daucuscarota, L.) prepared by food service. Food Chem. 1998, 61, 145–151. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, J.R.G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition Ad Hoc Writing Committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 14th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1984. [Google Scholar]
- Haro-Vicente, J.; Pérez-Conesa, D.; Rincón, F.; Ros, G.; Martinéz-Graciá, C.; Vidal, M. Does ascorbic acid supplementation affect iron bioavailability in rats fed micronized dispersible ferric pyrophophate fortified fruit juice? Eur. J. Nutr. 2008, 47, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgent, T.D. Analysis of relative gene expression Data using real time quantitative PCR and the 2-2DDCT Method. Ideal Libr. 2001, 25, 402–408. [Google Scholar]
- Brigide, P. Availability of Iron in Grains of Common bean (Phaseolus vulgaris L.) Iradiados. Master’s Thesis, São Paulo University, Piracicaba, Brazil, 2002. [Google Scholar]
- Bigonha, S.M. Protein Quality and Bioavailability of Ferro-Zinc in Biofortified Beans. Ph.D. Thesis, Viçosa Federal University, Viçosa, Brazil, 2013. [Google Scholar]
- Vaz-Tostes, M.; Verediano, T.A.; Mejia, E.G.; Costa, N.M.B. Evaluation of iron and zinc bioavailability of beans targeted for biofortification using in vitro and in vivo models and their effect on the nutritional status of preschool children. J. Sci. Food Agric 2015. [Google Scholar] [CrossRef]
- Marinho, J.T.S.; Pereira, R.C.A.; Costa, J.G. Characterization of cowpea cultivars (Vigna unguiculata (L.) Walp) in plantations in Acre. EMBRAPA ACRE. Res. Bull. 2001, 31. [Google Scholar]
- Germano, R.M.A. Availability of Iron in the Presence of β-Carotene and the Effect of Interferents on Food Combinations. Master’s Thesis, São Paulo University, Piracicaba, Brazil, 2002. [Google Scholar]
- Feniman, C.M. Characterization of Manioc rRoots (Manihot Esculenta Crantz) of Cultivar IAC 576-70 Regarding Cooking, Chemical Composition and Starch Properties at Two Harvest Times. Master’s Thesis, São Paulo University, Piracicaba, Brazil, 2004. [Google Scholar]
- Dias, L.T.; Leonel, M. Physical-chemical characterization of cassava flour from different localities in Brazil. Sci. Agrotechnol. 2006, 30, 692–700. [Google Scholar]
- Tako, E.; Glahn, R.P.; Laparra, J.M.; Welch, R.M.; Lei, X.; Kelly, J.D.; Rutzke, M.A.; Miller, D.D. Iron and Zinc Bioavailabilities to Pigs from Red and White Beans (Phaseolus vulgaris L.) Are Similar. J. Agric. Food Chem. 2009, 57, 3134–3140. [Google Scholar] [CrossRef]
- Correa, S.R. Bioavailability of Iron in Vitro and in Vivo of Biofortified Mixed Cowpea and Sweet Potato Beans. Master’s Thesis, Federal University of Espirito Santo, Espirito Santo, Brazil, 2017. [Google Scholar]
- Santos, M.M.A. Evaluation of bioavailability of Cu, Fe and Zn in fava beans (Phaseolus lunatus L.). Master’s Thesis, Piauí Federal University, Teresina, Brazil, 2016. [Google Scholar]
- Pachon, H.; Ortiz, D.A.; Araujo, C.; Blair, M.W.; Restrepo, J. Iron, Zinc, and Protein Bioavailability Proxy Measures of Meals Prepared with Nutritionally Enhanced Beans and Maize. J. Food Sci. 2009, 74, 147–154. [Google Scholar] [CrossRef]
- Mezette, T.F.; Carvalho, C.R.L.; Morgano, M.A.; Silva, M.G.; Parra, E.S.B.; Galera, J.M.S.V.; Valle, T.L. Selection of elite clones of table cassava for agronomic characteristics. EMBRAPA-Brazilian Agricultural Research Corporation. Reg. Adapt. 2009, 68, 601–609. [Google Scholar]
- Barrueto-Gonzalez, N.B. Bioavailability of minerals from legume sources. Simbio-Logias 2008, 1, 1. [Google Scholar]
- Tako, E.; Reed, S.; Anandaraman, A.; Beebe, S.E.; Hart, J.J.; Glahn, R.P. Studies of Cream Seeded Carioca Beans(Phaseolus vulgaris L.) from a Rwandan Efficacy Trial: In Vitro and In Vivo Screening Tools Reflect Human Studies and Predict Beneficial Results from Iron Biofortified Beans. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Hallberg, L.; Brune, M.; Rossander, L. Iron absorption in man: Ascorbic acid and dose-dependent inhibition by phytate. Am. J. Clin. Nutr. 1989, 49, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Afonso, S.M.E. Physical-Chemical Characterization and Antioxidant Activity of New Bean Varieties (Phaseolus vulgaris L.). Master’s Thesis, Bragança Agrarian High School, Bragança, Portugal, 2010. [Google Scholar]
- Hunt, J.R. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. A. J. Clin. Nutr. 2003, 78, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Bernaude, F.S.; Rodrigues, T.C. Food fiber-Adequate intake and effect on metabolism health. Braz. Arch. Endocrinol. Metab. 2013, 57, 397–405. [Google Scholar]
- Fukuda, W.M.G.; Carvalho, H.W.L.; Oliveira, L.A. BRS Jari: New Cassava Variety for Table with High Beta Carotene Content in the Roots; Embrapa Mandioca and Tropical Fruit Growing: Cruz das Almas, BA, Brazil; Embrapa Tabuleiros Costeiros: Aracaju, Sergipe, Brazil, 2009. [Google Scholar]
- Berni, P.R.A. Bioavailability of β-carotene in biofortified cassava casseroles and sweet potatoes: Study of the effects of genotypes and processing. Master’s Thesis, São Paulo University, Piracicaba, Brazil, 2014. [Google Scholar]
- Silva, R.M.G.; Figueiredo, P.A.; de Peixoto, E.C.T.M.; Silva, L.P. Antioxidant activity and determination of total phenols, carotenoids, beta carotenes, lycopene and zinc in white, yellow and pink varieties of Manihot esculenta Crantz. Biosci. J. 2014, 30, 556–564. [Google Scholar]
- Nascimento, P. Evaluation of the Retention of Carotenoids of Squash, Manioc and Sweet Potatoes. Master’s Thesis, Paulista State University, São José do Rio preto, Brazil, 2006. [Google Scholar]
- Toaiari, S.D.A.; Yuyama, L.K.O.; Aguiar, J.P.L.; Souza, R.F.S. Bioavailability of açaí iron (Euterpe oleracea Mart.) And manioc flour fortified with iron in rats. Rev. Nutr. 2005, 18, 291–299. [Google Scholar] [CrossRef]
- Barbosa, C.Z. Selection of elite strains of cowpea (Vigna unguiculata (L.) Walp.) For biofortification of iron and zinc. Master’s Thesis, Federal University of Piauí, Piauí, Brazil, 2015. [Google Scholar]
- Salerno, P.S.V. Effect of cafeteria diet on rats submitted to Chronic Stress Varied: Analysis of biochemical and behavioral parameters. Master’s Thesis, Pelotas Federal University, Pelotas, Brazil, 2014. [Google Scholar]
- Cardenas, L.L.A.R. Bioavailability of zinc and iron, nutritional and functional value of different cultivars of common bean submitted to domestic treatments. Bachelor’s Thesis, Viçosa University, Viçosa, Brazil, 2006. [Google Scholar]
- Murray-Kob, L.E.; Wenger, M.J.; Scott, S.P.; Rhoten, S.E.; Lung’Aho, M.G.; Haas, J.D. Consumption of Iron-Biofortified Beans Positively Affects Cognitive Performance in 18- to 27-Year-Old Rwandan Female College Students in an 18-Week Randomized Controlled Efficacy Trial. J. Nutr. 2017, 54, 2109–2117. [Google Scholar]
- Martini, F.C.C. Comparison between the Bioavailability of Iron in the Presence of Vitamin A and Beta-Carotene in Foods and Medicines. Master’s Thesis, São Paulo University, Piracicaba, Brazil, 2002. [Google Scholar]
- Dias, D.M.; Moreira, C.M.E.; Gomes, M.J.; Toledo, L.R.C.; Nutti, M.R.; Santana, P.H.M.; Martino, H.S. Rice and Bean Targets for Biofortification Combined with High Carotenoid Content Crops Regulate Transcriptional Mechanisms Increasing Iron Bioavailability. Nutrients 2015, 7, 9683–9696. [Google Scholar] [CrossRef]
- Layrisse, M.; Garcia-Casal, M.N.; Solano, L.; Baron, M.A.; Arguello, F.; Llovera, D.; Ramírez, J.; Leets, I.; Tropper, E. The role of vitamin A on the inhibitors of non heme iron absorption: Preliminary results. J. Nutr. Biochem. 1997, 8, 61–67. [Google Scholar] [CrossRef]
- Lynch, S.R. Interaction of iron with other nutrients. Nutr. Rev. 1997, 55, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Sales, M.C. Biochemical Markers of the Nutritional Status of Iron, Vitamin A and Zinc: Interaction and Influence of Subclinical Infectious Clinical Processes. Master’s Thesis, Paraíba State University, Campina Grande, Brazil, 2012. [Google Scholar]
- Moreira, D. Vitamin A Deficiency and Transcript Levels of Iron Transporters in the Rat Intestine. Master’s Thesis, Brasília University, Brasília, Brazil, 2009. [Google Scholar]
| REPLETION(g/1000 g) | ||||||
|---|---|---|---|---|---|---|
| FOOD | DEPLETION | SF 1 | CBCC 2 | CBBC 3 | BBCC 4 | BBBC 5 |
| Ferrous sulfate | 0 | 0.1495 | 0 | 0 | 0 | 0 |
| Conventional beans | 0 | 0 | 253 | 253 | 0 | 0 |
| Biofortified Beans | 0 | 0 | 0 | 0 | 216 | 216 |
| Rice | 0 | 0 | 214 | 214 | 182 | 182 |
| Biofortified Cassava | 0 | 0 | 0 | 96 | 0 | 82 |
| Conventional cassava | 0 | 0 | 96 | 0 | 82 | 0 |
| Albumin | 200 | 200 | 80 | 80 | 87 | 87 |
| Maltodextrin | 132 | 132 | 132 | 132 | 132 | 132 |
| Sucrose | 100 | 100 | 100 | 100 | 100 | 100 |
| Soybean oil | 70 | 70 | 70 | 70 | 70 | 70 |
| Cellulose | 50 | 50 | 0 | 0 | 0 | 0 |
| Mineral mix 0% Iron | 35 | 35 | 35 | 35 | 35 | 35 |
| Vitamin mix | 10 | 10 | 10 | 10 | 10 | 10 |
| L-Cystine | 3 | 3 | 3 | 3 | 3 | 3 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Starch | 397.5 | 397.5 | 4.5 | 4.5 | 80.5 | 80.5 |
| Name | Primer 1 (5’–3’) | Primer 2 (3’–5’) |
|---|---|---|
| GAPDH1 | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
| Ferritin | CAGCCGCCTTACAAGTCTCT | ATGGAGCTAACCGCGAAGAC |
| Transferrin | AGCTGCCACCTGAGAACATC | CGCACGCCCTTTATTCATGG |
| Ferroportin | TTCCGCACTTTTCGAGATGG | TACAGTCGAAGCCCAGGACCGT |
| DMT-12 | CTGATTTACAGTCTGGAGCAG | CACTTCAGCAAGGTGCAA |
| Hephaestin | GGCACAGTTACAGGGCAGAT | AGTAACGTGGCAGTGCATCA |
| DcytB3 | TGCAGACGCAGAGTTAAGCA | CCGTGAAGTATACCGGCTCC |
| n = 3 | CB 1 | BB 2 | CC 3 | BC 4 | Rice 5 |
|---|---|---|---|---|---|
| Energy (kcal) | 294.40 ± 0.70 | 269.52 ± 0.89 | 315.50 ± 1.12 | 231.72 ± 0.26 | 371.11 ± 1.48 |
| Carbohydrate (g) | 44.62 ± 0.82 | 34.48 ± 0.43 | 75.48 ± 0.37 | 53.23 ± 0.29 | 81.23 ± 0.70 |
| Protein (g) | 24.80 ± 0.86 | 28.64 ± 0.53 | 3.13 ± 0.01 | 4.03 ± 0.03 | 11.48 ± 0.40 |
| Lipid (g) | 2.00 ± 0.05 | 1.90 ± 0.17 | 0.12 ± 0.28 | 0.30 ± 0.11 | 0.03 ± 0.05 |
| Ashes (g) | 3.27 ± 0.08 | 3.51 ± 0.13 | 2.23 ± 0.05 | 2.45 ± 0.07 | 0.37 ± 0.08 |
| Moisture (g) | 7.54 ± 0.07 | 6.14 ± 0.05 | 10.19 ± 0.09 | 10.00 ± 0.20 | 6.54 ± 0.45 |
| n = 3 | CB 1 | BB 2 | CC 3 | BC 4 | Rice 5 |
|---|---|---|---|---|---|
| PHYTATE (g/100g) | 0.21 ± 0.18 | 0.29 ± 0.13 | 0.18 ± 0.05 | 0.06 ± 0.02 | 0.02 ± 0.00 |
| IRON (mg/kg) | 43.83 | 52.41 | 5.96 | 12.33 | 1.94 |
| PHYTATE/IRON MOLAR RATIO | 4.11 | 4.73 | 25.41 | 4.15 | 6.98 |
| β-CAROTENE (μg/g) | - | - | 0.9 | 7.6 | - |
| PHENOLIC COMPOUNDS (mg gallic acid/100g) | 0.04 ± 0.00 | 1.42 ± 0.05 | 0 ± 0.00 | 0.41 ± 0.1 | 0 ± 0.00 |
| TOTAL FIBER (g/100g) | 18.09 | 25.34 | - | - | 0.36 |
| INSOLUBLE FIBER (g/100g) | 15.13 | 19.91 | - | - | 0.00 |
| SOLUBLE FIBER (g/100g) | 2.96 | 5.43 | - | - | 0.36 |
| EXPERIMENTAL GROUPS (n = 8) | Weight Gain (g) | Food Consumption (g/day) | FER 6 |
|---|---|---|---|
| SF 1 | 45.41 ± 12.89 b | 16.17 ± 1.43 a | 0.20 ± 0.04 b |
| CBCC 2 | 59.66 ± 13.74 b | 16.18 ± 2.15 a | 0.26 ± 0.04 a |
| CBBC 3 | 57.90 ± 15.95 b | 16.91 ± 2.46 a | 0.24 ± 0.05 ab |
| BBCC 4 | 57.66 ± 8.13 b | 16.61 ± 1.81 a | 0.25 ± 0.02 ab |
| BBBC 5 | 79.54 ± 14.92 a | 17.63 ± 2.05 a | 0.28 ± 0.05 a |
| EXPERIMENTAL GROUPS (n = 8) | Initial Hb 6 (g/dL) | Final Hb 6 (g/dL) | Hb 6 Gain (g/dL) |
|---|---|---|---|
| SF 1 | 6.25 ± 0.56 | 8.04 ± 0.84 | 1.79 ± 1.04 |
| CBCC 2 | 6.25 ± 0.52 | 7.98 ± 1.04 | 1.73 ± 1.13 |
| CBBC 3 | 6.24 ± 0.53 | 7.99 ± 0.70 | 1.75 ± 0.74 |
| BBCC 4 | 6.26 ± 0.47 | 8.10 ± 1.18 | 1.83 ± 1.14 |
| BBBC 5 | 6.29 ± 0.46 | 8.00 ± 0.93 | 1.72 ± 0.78 |
| DIETS (n = 8) | Fe 6 Consumed (mg) | Hb 7 Gain (g/dL)/Fe Consumed (g) | HRE 8 (%) | RBV 9 of HRE (%) |
|---|---|---|---|---|
| SF 1 | 2.72 ± 0.24 | 0.65 ± 0.36 | 51.02 ± 19.34 | 100.00 |
| CBCC 2 | 2.72 ± 0.36 | 0.67 ± 0.48 | 57.96 ± 14.25 | 113.60 |
| CBBC 3 | 2.84 ± 0.41 | 0.61 ± 0.25 | 57.17 ± 13.10 | 112.05 |
| BBCC 4 | 2.79 ± 0.30 | 0.69 ± 0.47 | 60.54 ± 17.27 | 118.67 |
| BBBC 5 | 2.96 ± 0.34 | 0.57 ± 0.25 | 63.97 ± 18.17 | 125.39 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares Antunes, P.; Vaz-Tostes, M.d.G.; Tomáz Sant’Ana, C.; Araújo de Faria, R.; Lopes Toledo, R.C.; Brunoro Costa, N.M. Bioavailability of Iron and the Influence of Vitamin a in Biofortified Foods. Agronomy 2019, 9, 777. https://doi.org/10.3390/agronomy9120777
Tavares Antunes P, Vaz-Tostes MdG, Tomáz Sant’Ana C, Araújo de Faria R, Lopes Toledo RC, Brunoro Costa NM. Bioavailability of Iron and the Influence of Vitamin a in Biofortified Foods. Agronomy. 2019; 9(12):777. https://doi.org/10.3390/agronomy9120777
Chicago/Turabian StyleTavares Antunes, Paula, Maria das Graças Vaz-Tostes, Cíntia Tomáz Sant’Ana, Renata Araújo de Faria, Renata Celi Lopes Toledo, and Neuza Maria Brunoro Costa. 2019. "Bioavailability of Iron and the Influence of Vitamin a in Biofortified Foods" Agronomy 9, no. 12: 777. https://doi.org/10.3390/agronomy9120777
APA StyleTavares Antunes, P., Vaz-Tostes, M. d. G., Tomáz Sant’Ana, C., Araújo de Faria, R., Lopes Toledo, R. C., & Brunoro Costa, N. M. (2019). Bioavailability of Iron and the Influence of Vitamin a in Biofortified Foods. Agronomy, 9(12), 777. https://doi.org/10.3390/agronomy9120777





