Investigating the Impact of Biostimulants on the Row Crops Corn and Soybean Using High-Efficiency Phenotyping and Next Generation Sequencing
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Non-Destructive Measurements
2.2.1. Digital Biovolume Assessment
2.2.2. Color Classification
2.2.3. Stress Index
2.3. RNA-Seq Analysis
2.4. qRT-PCR Analysis
2.5. Statistical Analysis of Data
3. Results and Discussion
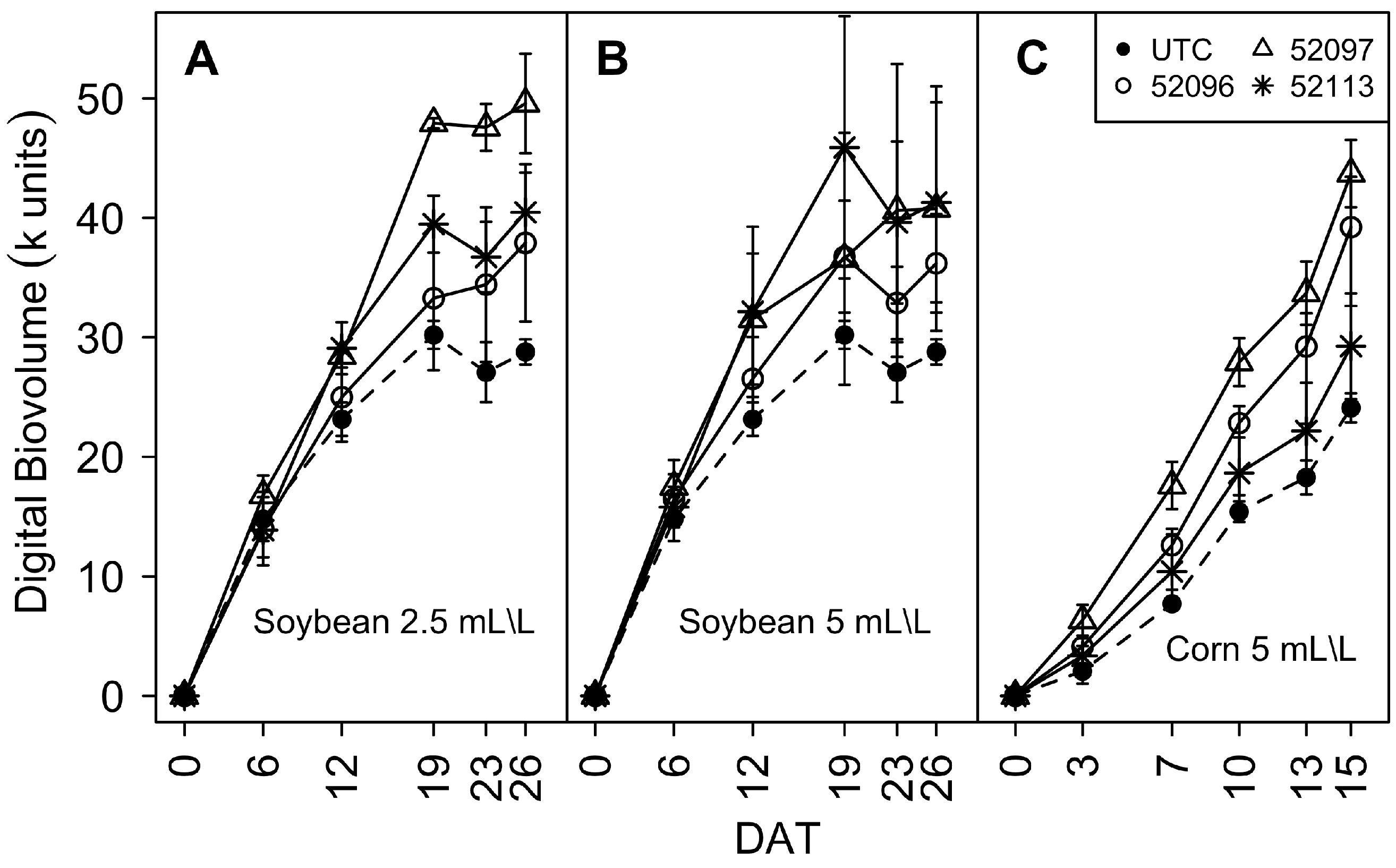
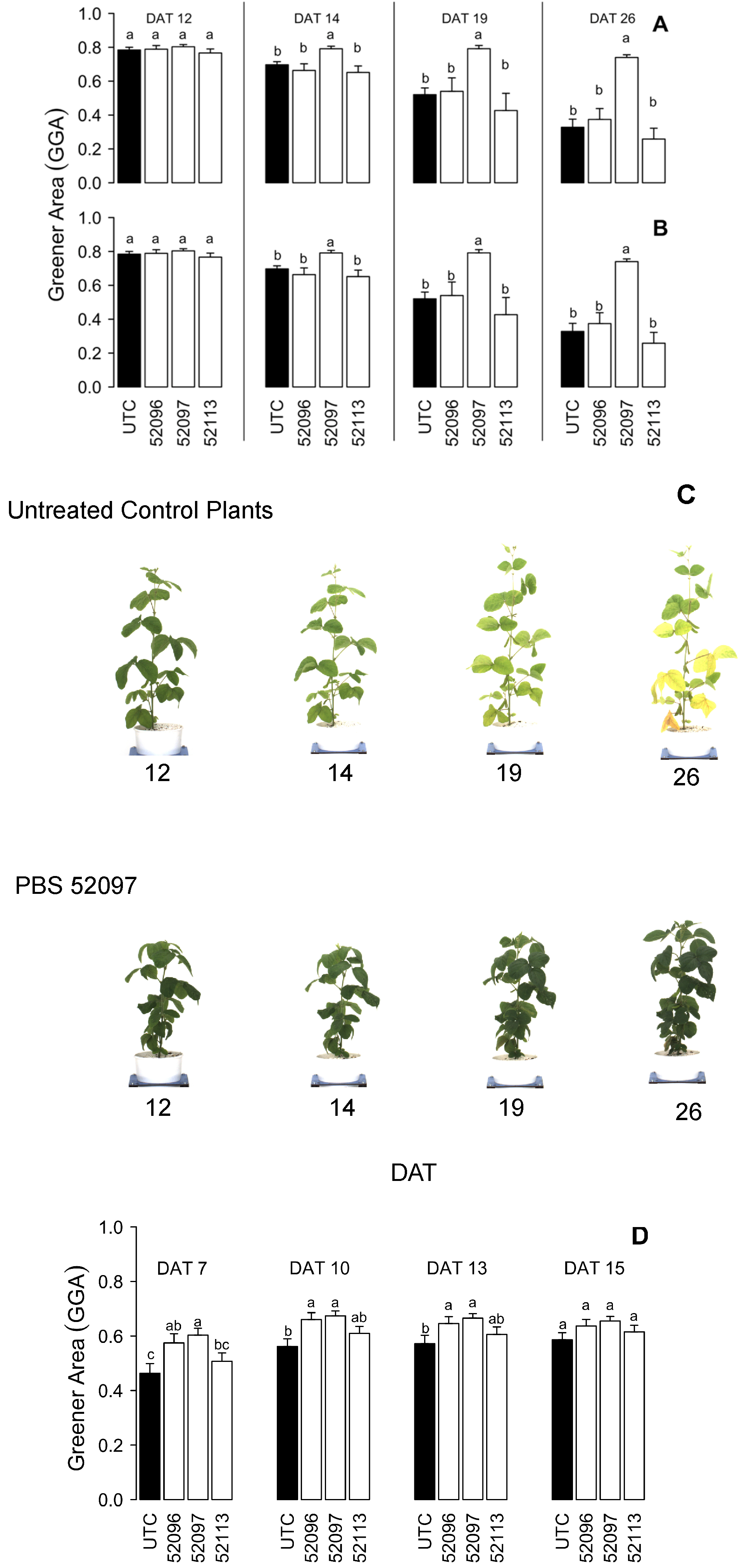
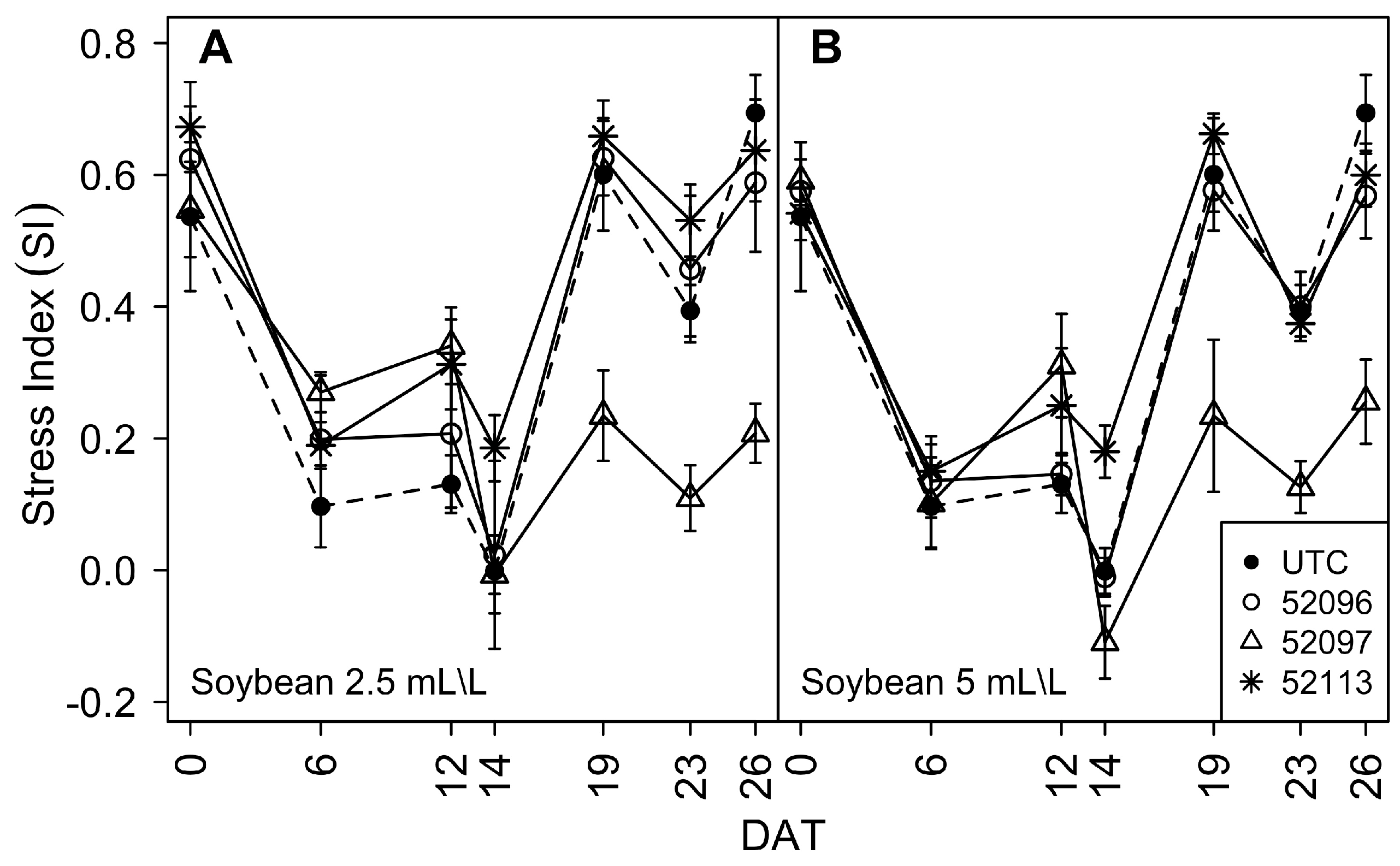
3.1. Phenomic Parameters
3.1.1. Digital Biovolume
3.1.2. Greener Area
3.1.3. Stress Index
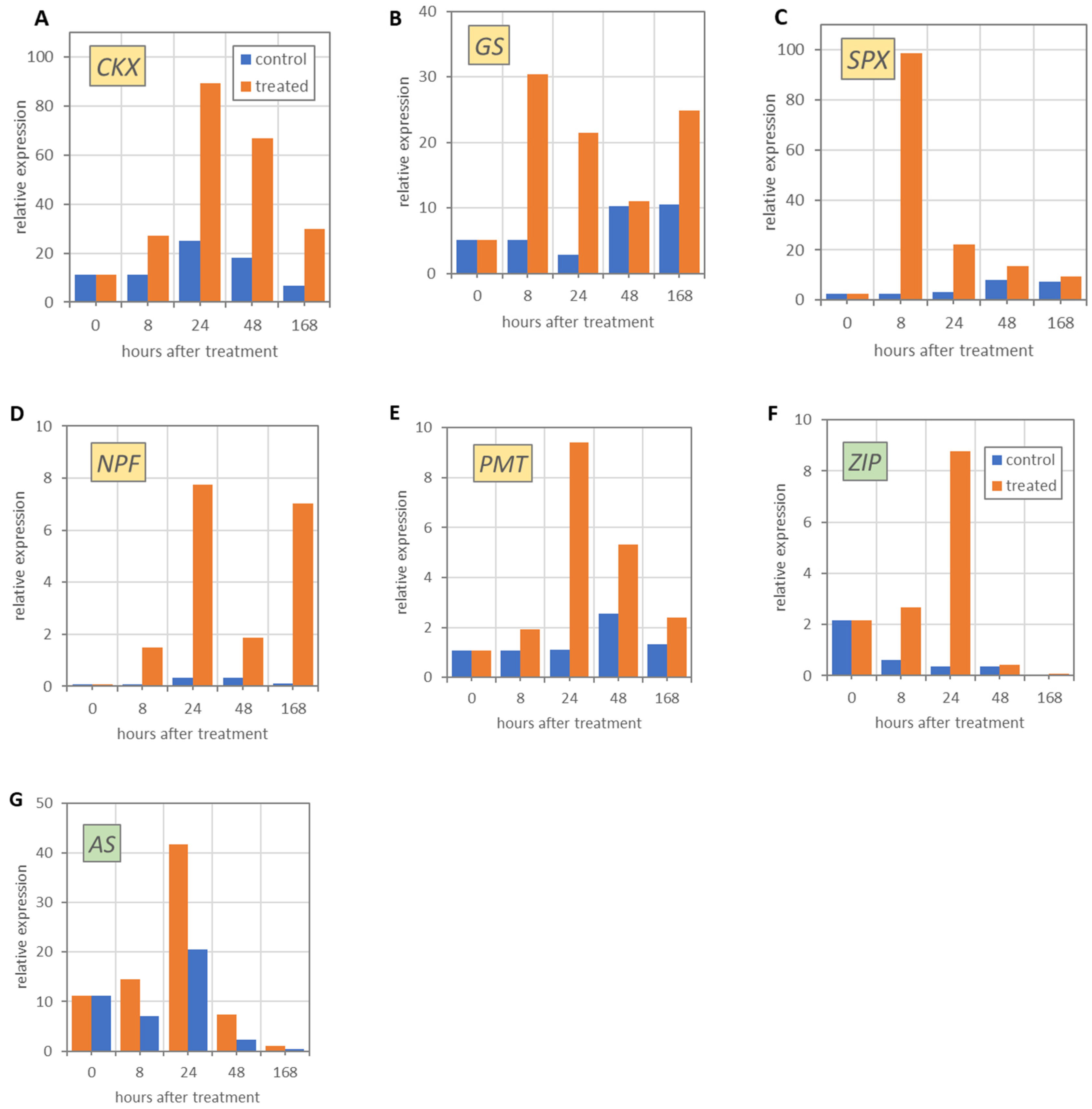
3.2. Molecular Analyses
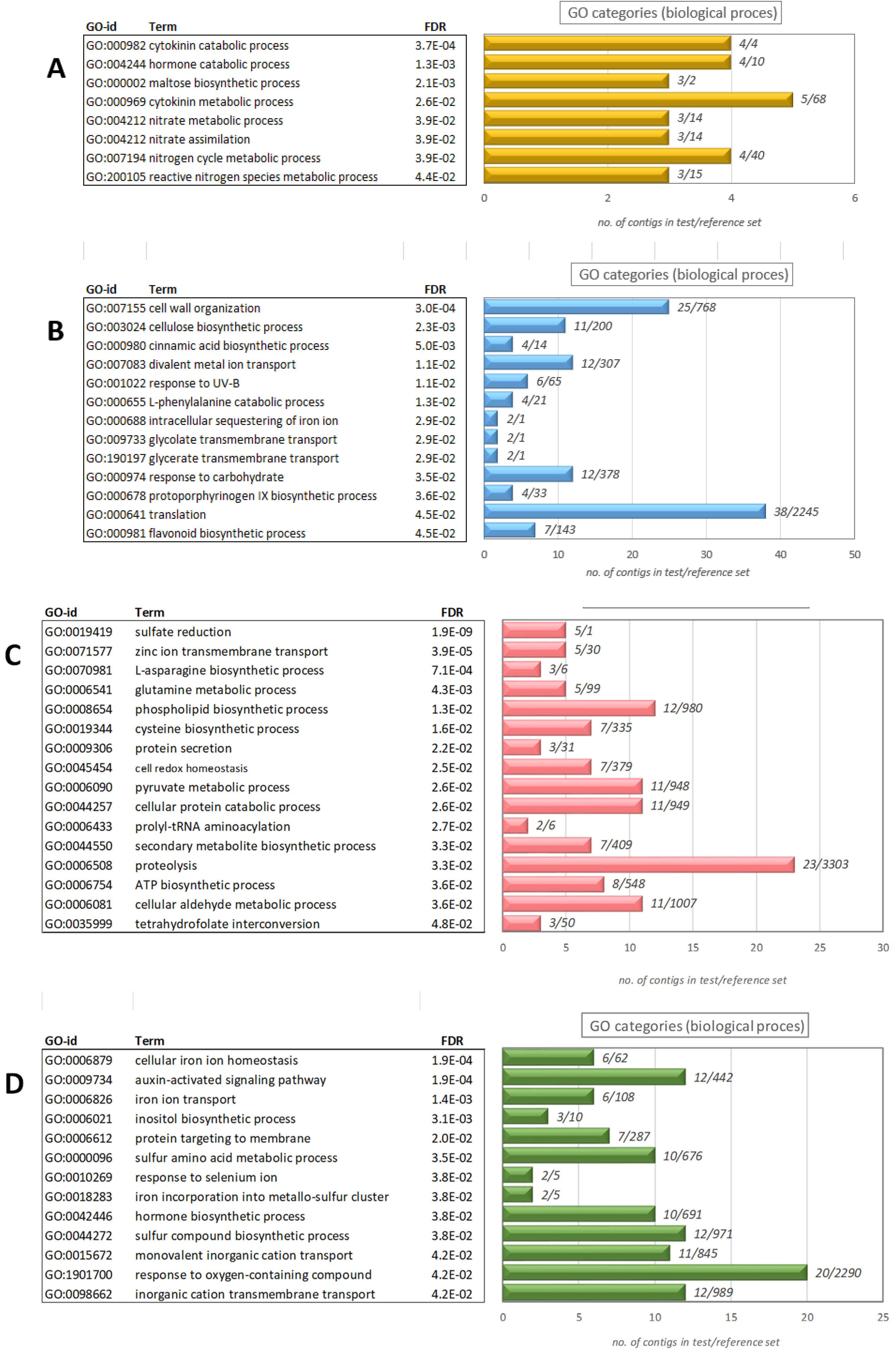
3.3. Functional Annotation Using Gene Ontology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takeda, S.; Matsuoka, M. Genetic approaches to crop improvement: Responding to environmental and population changes. Nat. Rev. Genet. 2008, 9, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Khan, S.; Ma, X. Climate change impacts on crop yield, crop water productivity and food security—A review. Prog. Nat. Sci. 2009, 19, 1665–1674. [Google Scholar] [CrossRef]
- Eckardt, N.A.; Cominelli, E.; Galbiati, M.; Tonelli, C. The future of science: Food and water for life (Meeting Report). Plant Cell 2009, 21, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations [FAO]. Towards the Future we Want. End Hunger and Make the Transition to Sustainable Agricultural and Food Systems. Rome: FAO at Rio+20. 2012. Available online: http://www.fao.org/docrep/015/an894e/an894e00.pdf (accessed on 15 November 2019).
- Carvalho, S.; Vasconcelos, M.W. Producing More with Less: Strategies and novel technologies for plant-based food biofortification. Food Res. Int. 2013, 54, 961–971. [Google Scholar] [CrossRef]
- International Food Policy Research Institute [IFPRI]. Producing More With Less? 2014. Available online: http://www.ifpri.org/blog/producing-more-less (accessed on 15 November 2019).
- Montanaro, G.; Xiloyannis, C.; Nuzzo, V.; Dichio, B. Orchard management, soil organic carbon and ecosystem services in Mediterranean fruit tree crops. Sci. Hort. 2017, 217, 92–101. [Google Scholar] [CrossRef]
- Pearson, C.J. Regenerative, Semiclosed Systems: A Priority for Twenty-First-Century Agriculture. BioScience 2007, 57, 409–418. [Google Scholar] [CrossRef]
- Kremen, C.; Iles, A.; Bacon, C. Diversified farming systems: An agroecological, systems-based alternative to modern industrial agriculture. Environ. Stud. Sci. 2012, 17, 44. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hort. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- European Biostimulant Industry Council (EBIC). 2018. Available online: http://www.biostimulants.eu/ (accessed on 15 November 2019).
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Povero, G.; Mejia, J.F.; Di Tommaso, D.; Piaggesi, A.; Warrior, P. A Systematic Approach to Discover and Characterize Natural Plant Biostimulants. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Parađiković, N.; Vinković, T.; Vinković Vrček, I.; Žuntar, I.; Bojić, M.; Medić-Šarić, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Parađiković, N.; Vinković, T.; Vinković Vrček, I.; Tkalec, M. Natural biostimulants reduce the incidence of BER in sweet yellow pepper plants (Capsicum annuum L.). Agric. Food Sci. 2013, 22, 307–317. [Google Scholar] [CrossRef]
- Bulgari, R.; Morgutti, S.; Cocetta, G.; Negrini, N.; Farris, S.; Calcante, A.; Spinardi, A.; Ferrari, E.; Mignani, I.; Oberti, R.; et al. Evaluation of Borage Extracts As Potential Biostimulant Using a Phenomic, Agronomic, Physiological, and Biochemical Approach. Front. Plant Sci. 2017, 8, 935. [Google Scholar] [CrossRef]
- Saa, S.; Olivos-Del Rio, A.; Castro, S.; Brown, P.H. Foliar application of microbial and plant based biostimulants increases growth and potassium uptake in almond (Prunus dulcis [Mill.] D.A. Webb). Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Adani, F.; Genevini, P.; Zaccheo, P.; Zocchi, G. The effect of commercial humic acid on tomato plant growth and mineral nutrition. J. Plant. Nutr. 1998, 21, 561–575. [Google Scholar] [CrossRef]
- Parrado, J.; Bautista, J.; Romero, E.J.; García-Martínez, A.M.; Friaza, V.; Tejada, M. Production of a carob enzymatic extract: Potential use as a biofertilizer. Bioresour. Technol. 2008, 99, 2312–2318. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Reynaud, H.; Canaguier, R.; Trtílek, M.; Panzarová, K.; et al. Understanding the Biostimulant Action of Vegetal-Derived Protein Hydrolysates by High-Throughput Plant Phenotyping and Metabolomics: A Case Study on Tomato. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Petrozza, A.; Santaniello, A.; Summerer, S.; Di Tommaso, G.; Di Tommaso, D.; Paparelli, E.; Piaggesi, A.; Perata, P.; Cellini, F. Physiological responses to Megafol® treatments in tomato plants under drought stress: A phenomic and molecular approach. Sci. Hort. 2014, 174, 185–192. [Google Scholar] [CrossRef]
- Alam, M.Z.; Braun, G.; Norrie, J.; Hodges, D.M. Ascophyllum extract application can promote plant growth and root yield in carrot associated with increased root-zone soil microbial activity. Can. J. Plant Sci. 2014, 94, 337–348. [Google Scholar] [CrossRef]
- Vernieri, P.; Borghesi, E.; Tognoni, F.; Serra, G.; Ferrante, A.; Piagessi, A. Use of biostimulants for reducing nutrient solution concentration in floating system. Acta Hortic. 2006, 718, 477–484. [Google Scholar] [CrossRef]
- Danzi, D.; Briglia, N.; Petrozza, A.; Summerer, S.; Povero, G.; Stivaletta, A.; Cellini, F.; Pignone, D.; De Paola, D.; Janni, M. Can high throughput phenotyping help food security in the Mediterranean area? Front. Plant Sci. 2019. [Google Scholar] [CrossRef]
- Leff, B.; Ramankutty, N.; Foley, J.A. Geographic distribution of major crops across the world. Glob. Biogeochem. Cycles 2004. [Google Scholar] [CrossRef]
- Santaniello, A.; Giorgi, F.M.; Di Tommaso, D.; Di Tommaso, G.; Piaggesi, A.; Perata, P. Genomic approaches to unveil the physiological pathways activated in Arabidopsis treated with plant-derived raw extracts. Acta Hortic. 2013, 1009, 161–174. [Google Scholar] [CrossRef]
- Hoeberichts, A.; Povero, G.; Ibañez, M.; Strijker, A.; Pezzolato, D.; Mills, R.; Piaggesi, A. Next Generation Sequencing to characterise the breaking of bud dormancy using a natural biostimulant in kiwifruit (Actinidia deliciosa). Sci. Hort. 2017, 225, 252–263. [Google Scholar] [CrossRef]
- Trevisan, S.; Manoli, A.; Ravazzolo, L.; Franceschi, C.; Quaggiotti, S. mRNA-Sequencing Analysis Reveals Transcriptional Changes in Root of Corn Seedlings Treated with Two Increasing Concentrations of a New Biostimulant. J. Agric. Food Chem. 2017, 65, 9956–9969. [Google Scholar] [CrossRef]
- Furbank, R.T.; Tester, M. Phenomics-technologies to relieve the phenotyping bottleneck. Trends Plant Sci. 2011, 16, 635–644. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. A Combined Phenotypic and Metabolomic Approach for Elucidating the Biostimulant Action of a Plant-Derived Protein Hydrolysate on Tomato Grown Under Limited Water Availability. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Summerer, S.; Petrozza, A.; Cellini, F. High throughput plant phenotyping: A new and objective method to detect and analyse the biostimulant properties of different products. Acta Hortic. 2013, 1009, 143–148. [Google Scholar] [CrossRef]
- Ugena, L.; Hýlová, A.; Podlešáková, K.; Humplík, J.F.; Doležal, K.; De Diego, N.; Spíchal, L. Characterization of Biostimulant Mode of Action Using Novel Multi-Trait High-Throughput Screening of Arabidopsis Germination and Rosette Growth. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Spíchal, L.; Panzarová, K.; Casa, R.; Colla, G. High-Throughput Plant Phenotyping for Developing Novel Biostimulants: From Lab to Field or From Field to Lab? Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Briglia, N.; Montanaro, G.; Petrozza, A.; Summerer, S.; Cellini, F.; Nuzzo, V. Drought phenotyping in Vitis vinifera using RGB and NIR imaging. Sci. Hort. 2019, 256, 108555. [Google Scholar] [CrossRef]
- Eberius, M.; Lima-Guerra, J. High-throughput plant phenotyping-data acquisition, transformation, and analysis. In Bioinformatics; Edwards, D., Stajich, J., Hansen, D., Eds.; Springer: New York, NY, USA, 2013; pp. 259–278. [Google Scholar] [CrossRef]
- Casadesús, J.; Kaya, Y.; Bort, J.; Nachit, M.M.; Araus, J.L.; Amor, S.; Ferrazzano, G.; Maalouf, F.; Maccaferri, M.; Martos, V. Using vegetation indices derived from conventional digital cameras as selection criteria for wheat breeding in water-limited environments. Ann. Appl. Biol. 2007, 150, 227–236. [Google Scholar] [CrossRef]
- Chang, S.; Puryear, J.; Cairney, J. A simple and efficient method to isolate RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 114–117. [Google Scholar] [CrossRef]
- Parkhomchuk, D.; Borodina, T.; Amstislavskiy, V.; Banaru, M.; Hallen, L.; Krobitsch, S.; Lehrach, H.; Soldatov, A. Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucl. Acids Res. 2009, 37, 123. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.Z.; Yassour, M.; Adiconis, X.; Nusbaum, C.; Thompson, D.A.; Friedman, N.; Gnirke, A.; Regev, A. Comprehensive comparative analysis of strand-specific RNA sequencing methods. Nat. Methods 2010, 7, 709–715. [Google Scholar] [CrossRef]
- Schmutz, J.; Cannon, S.B.; Schlueter, J. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Smart, C.M. Crops that stay green. Ann. Appl. Biol. 1993, 123, 193–219. [Google Scholar] [CrossRef]
- Jameson, P.E.; Song, J. Cytokinin: A key driver of seed yield. J. Exp. Bot. 2016, 67, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.M.; Habash, D.Z. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol. 2009, 182, 608–620. [Google Scholar] [CrossRef]
- Thomsen, H.C.; Eriksson, D.; Møller, I.S.; Schjoerring, J.K. Cytosolic glutamine synthetase: A target for improvement of crop nitrogen use efficiency? Trends Plant Sci. 2014, 19, 656–663. [Google Scholar] [CrossRef]
- Secco, D.; Wang, C.; Arpat, B.A.; Wang, Z.; Poirier, Y.; Tyerman, S.D. The emerging importance of the SPX domain-containing proteins in phosphate homeostasis. New Phytol. 2012, 193, 842–851. [Google Scholar] [CrossRef]
- Léran, S.; Varala, K.; Boyer, J.C.; Chiurazzi, M.; Crawford, N.; Daniel-Vedele, F. A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends Plant Sci. 2014, 19, 5–9. [Google Scholar] [CrossRef]
- Slewinski, T. Diverse transporters and their homologs in vascular plants: A physiological perspective. Mol. Plant. 2011, 4, 641–662. [Google Scholar] [CrossRef]
- Hall, J.L.; Williams, L.E. Transition metal transporters in plants. J. Exp. Bot. 2003, 54, 2601–2613. [Google Scholar] [CrossRef]
- Gaufichon, L.; Reisdorf-Cren, M.; Rothstein, S.J.; Chardon, F.; Suzuki, A. Biological functions of asparagine synthetase in plants. Plant Sci. 2010, 179, 141–153. [Google Scholar] [CrossRef]
| FC | >2 | >3 | >4 | >6 | >9 | |
|---|---|---|---|---|---|---|
| up | 877 | 331 | 173 | 69 | 32 | Maize |
| down | 1672 | 593 | 315 | 142 | 67 | |
| total | 2549 | 924 | 488 | 211 | 99 | |
| up | 278 | 65 | 22 | 6 | 2 | Soybean |
| down | 321 | 59 | 17 | 1 | 1 | |
| total | 599 | 124 | 39 | 7 | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briglia, N.; Petrozza, A.; Hoeberichts, F.A.; Verhoef, N.; Povero, G. Investigating the Impact of Biostimulants on the Row Crops Corn and Soybean Using High-Efficiency Phenotyping and Next Generation Sequencing. Agronomy 2019, 9, 761. https://doi.org/10.3390/agronomy9110761
Briglia N, Petrozza A, Hoeberichts FA, Verhoef N, Povero G. Investigating the Impact of Biostimulants on the Row Crops Corn and Soybean Using High-Efficiency Phenotyping and Next Generation Sequencing. Agronomy. 2019; 9(11):761. https://doi.org/10.3390/agronomy9110761
Chicago/Turabian StyleBriglia, Nunzio, Angelo Petrozza, Frank A. Hoeberichts, Nathalie Verhoef, and Giovanni Povero. 2019. "Investigating the Impact of Biostimulants on the Row Crops Corn and Soybean Using High-Efficiency Phenotyping and Next Generation Sequencing" Agronomy 9, no. 11: 761. https://doi.org/10.3390/agronomy9110761
APA StyleBriglia, N., Petrozza, A., Hoeberichts, F. A., Verhoef, N., & Povero, G. (2019). Investigating the Impact of Biostimulants on the Row Crops Corn and Soybean Using High-Efficiency Phenotyping and Next Generation Sequencing. Agronomy, 9(11), 761. https://doi.org/10.3390/agronomy9110761





