Expression Levels of the γ-Glutamyl Hydrolase I Gene Predict Vitamin B9 Content in Potato Tubers
Abstract
1. Introduction
2. Materials and Methods
2.1. Potato Tuber Material
2.2. Folate Analysis by Microbiological Assay
2.3. Folate Analysis by HPLC
2.4. RNA Isolation
2.5. RNA Sequencing
2.6. Real Time Quantitative Reverse Transcription PCR
3. Results
3.1. Folate Content and Profile in S. boliviense PI 597736 Individuals
3.2. Expression of Folate Related Genes in Fol Lines as Determined by RNA-Seq Analysis
3.3. GGH1 Expression in Various Low and High Folate Germplasm as Determined by Real-Time Quantitative RT-PCR Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blancquaert, D.; De Steur, H.; Gellynck, X.; Van Der Straeten, D. Present and future of folate biofortification of crop plants. J. Exp. Bot. 2014, 65, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Storozhenko, S.; Ravanel, S.; Zhang, G.F.; Rébeillé, F.; Lambert, W.E.; Van Der Straeten, D. Folate enhancement in staple crops by metabolic engineering. Trends Food Sci. Technol. 2005, 16, 271–281. [Google Scholar] [CrossRef]
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Bentley, T.G.K.; Willett, W.C.; Weinstein, M.C.; Kuntz, K.M. Population-level changes in folate intake by age, gender, and race/ethnicity after folic acid fortification. Am. J. Public Health 2006, 96, 2040–2047. [Google Scholar] [CrossRef] [PubMed]
- Appling, D.R. Compartmentation of folate-mediated one-carbon metabolism in eukaryotes. FASEB J. 1991, 5, 2645–2651. [Google Scholar] [CrossRef] [PubMed]
- Shane, B.; Stokstad, E.L.R. Vitamin B12-folate interrelationships. Annu. Rev. Nutr. 1985, 5, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.T.; Stover, P.J. Folate-mediated one-carbon metabolism. Vitam. Horm. 2008, 79, 1–44. [Google Scholar]
- Krista, S.C.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar]
- Roje, S. S-Adenosyl-L-methionine: Beyond the universal methyl group donor. Phytochemistry 2006, 67, 1686–1698. [Google Scholar] [CrossRef]
- Hanson, A.D.; Roje, S. One-carbon metabolism in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 119–137. [Google Scholar] [CrossRef]
- Zhou, H.R.; Zhang, F.F.; Ma, Z.Y.; Huang, H.W.; Jiang, L.; Cai, T.; Zhu, J.K.; Zhang, C.; He, X.J. Folate polyglutamylation is involved in chromatin silencing by maintaining global DNA methylation and histone H3K9 dimethylation in Arabidopsis. Plant Cell 2013, 25, 2545–2559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, X.; Miki, D.; Cutler, S.; La, H.; Hou, Y.J.; Oh, J.E.; Zhu, J.K. Sulfamethazine suppresses epigenetic silencing in arabidopsis by impairing folate synthesis. Plant Cell 2012, 24, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Ye, J.; Kamphorst, J.J.; Shlomi, T.; Thompson, C.B.; Rabinowitz, J.D. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 2014, 510, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Gorelova, V.; De Lepeleire, J.; Van Daele, J.; Pluim, D.; Mei, C.; Cuypers, A.; Leroux, O.; Rebeille, F.; Schellens, J.H.M.; Blancquaert, D.; et al. Dihydrofolate reductase/thymidylate synthase fine-tunes the folate status and controls redox homeostasis in plants. Plant Cell 2017, 29, 2831–2853. [Google Scholar] [CrossRef]
- Meng, H.; Jiang, L.; Xu, B.; Guo, W.; Li, J.; Zhu, X.; Qi, X.; Duan, L.; Meng, X.; Fan, Y.; et al. Arabidopsis plastidial folylpolyglutamate synthetase is required for seed reserve accumulation and seedling establishment in darkness. PLoS ONE 2014, 9, e101905. [Google Scholar] [CrossRef]
- Van Wilder, V.; De Brouwer, V.; Loizeau, K.; Gambonnet, B.; Albrieux, C.; Van Der Straeten, D.; Lambert, W.E.; Douce, R.; Block, M.A.; Rebeille, F.; et al. C1 metabolism and chlorophyll synthesis: The Mg-protoporphyrin IX methyltransferase activity is dependent on the folate status. New Phytol. 2009, 182, 137–145. [Google Scholar] [CrossRef]
- Webb, M.E.; Smith, A.G. Chlorophyll and folate: Intimate link revealed by drug treatment. New Phytol. 2009, 182, 3–5. [Google Scholar] [CrossRef]
- Hanson, A.D.; Gregory, J.F. Folate biosynthesis, turnover, and transport in plants. In Annual Review of Plant Biology; Merchant, S.S., Briggs, W.R., Ort, D., Eds.; Annual Reviews: Palo Alto, CA, USA, 2011; Volume 62, pp. 105–125. [Google Scholar]
- Orsomando, G.; Bozzo, G.G.; de la Garza, R.D.; Basset, G.J.; Quinlivan, E.P.; Naponelli, V.; Rebeille, F.; Ravanel, S.; Gregory, J.F., 3rd; Hanson, A.D. Evidence for folate-salvage reactions in plants. Plant J. 2006, 46, 426–435. [Google Scholar] [CrossRef]
- Noiriel, A.; Naponelli, V.; Bozzo, G.G.; Gregory, J.F., 3rd; Hanson, A.D. Folate salvage in plants: Pterin aldehyde reduction is mediated by multiple non-specific aldehyde reductases. Plant J. 2007, 51, 378–389. [Google Scholar] [CrossRef]
- Basset, G.; Quinlivan, E.P.; Ziemak, M.J.; Diaz De La Garza, R.; Fischer, M.; Schiffmann, S.; Bacher, A.; Gregory, J.F., 3rd; Hanson, A.D. Folate synthesis in plants: The first step of the pterin branch is mediated by a unique bimodular GTP cyclohydrolase I. Proc. Natl. Acad. Sci. USA 2002, 99, 12489–12494. [Google Scholar] [CrossRef]
- Goyer, A.; Illarionova, V.; Roje, S.; Fischer, M.; Bacher, A.; Hanson, A.D. Folate biosynthesis in higher plants. cDNA cloning, heterologous expression, and characterization of dihydroneopterin aldolases. Plant Physiol. 2004, 135, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Basset, G.J.; Quinlivan, E.P.; Ravanel, S.; Rebeille, F.; Nichols, B.P.; Shinozaki, K.; Seki, M.; Adams-Phillips, L.C.; Giovannoni, J.J.; Gregory, J.F., 3rd; et al. Folate synthesis in plants: The p-aminobenzoate branch is initiated by a bifunctional PabA-PabB protein that is targeted to plastids. Proc. Natl. Acad. Sci. USA 2004, 101, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Basset, G.J.; Ravanel, S.; Quinlivan, E.P.; White, R.; Giovannoni, J.J.; Rebeille, F.; Nichols, B.P.; Shinozaki, K.; Seki, M.; Gregory, J.F., 3rd; et al. Folate synthesis in plants: The last step of the p-aminobenzoate branch is catalyzed by a plastidial aminodeoxychorismate lyase. Plant J. 2004, 40, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Rebeille, F.; Macherel, D.; Mouillon, J.M.; Garin, J.; Douce, R. Folate biosynthesis in higher plants: Purification and molecular cloning of a bifunctional 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase/7,8-dihydropteroate synthase localized in mitochondria. EMBO J. 1997, 16, 947–957. [Google Scholar] [CrossRef]
- Ravanel, S.; Cherest, H.; Jabrin, S.; Grunwald, D.; Surdin-Kerjan, Y.; Douce, R.; Rebeille, F. Tetrahydrofolate biosynthesis in plants: Molecular and functional characterization of dihydrofolate synthetase and three isoforms of folylpolyglutamate synthetase in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2001, 98, 15360–15365. [Google Scholar] [CrossRef]
- Neuburger, M.; Rebeille, F.; Jourdain, A.; Nakamura, S.; Douce, R. Mitochondria are a major site for folate and thymidylate synthesis in plants. J. Biol. Chem. 1996, 271, 9466–9472. [Google Scholar] [CrossRef]
- Orsomando, G.; de la Garza, R.D.; Green, B.J.; Peng, M.; Rea, P.A.; Ryan, T.J.; Gregory, J.F., 3rd; Hanson, A.D. Plant gamma-glutamyl hydrolases and folate polyglutamates: Characterization, compartmentation, and co-occurrence in vacuoles. J. Biol. Chem. 2005, 280, 28877–28884. [Google Scholar] [CrossRef]
- Eudes, A.; Bozzo, G.G.; Waller, J.C.; Naponelli, V.; Lim, E.K.; Bowles, D.J.; Gregory, J.F., 3rd; Hanson, A.D. Metabolism of the folate precursor p-aminobenzoate in plants: Glucose ester formation and vacuolar storage. J. Biol. Chem. 2008, 283, 15451–15459. [Google Scholar] [CrossRef]
- Konings, E.J.M.; Roomans, H.H.S.; Dorant, E.; Goldbohm, R.A.; Saris, W.H.M.; van den Brandt, P.A. Folate intake of the dutch population according to newly established liquid chromatography data for foods. Am. J. Clin. Nutr. 2001, 765–776. [Google Scholar] [CrossRef]
- Goyer, A.; Navarre, D.A. Determination of folate concentrations in diverse potato germplasm using a trienzyme extraction and a microbiological assay. J. Agric. Food Chem. 2007, 55, 3523–3528. [Google Scholar] [CrossRef]
- Goyer, A.; Sweek, K. Genetic diversity of thiamin and folate in primitive cultivated and wild potato (Solanum) species. J. Agric. Food Chem. 2011, 59, 13072–13080. [Google Scholar] [CrossRef] [PubMed]
- Jabrin, S.; Ravanel, S.; Gambonnet, B.; Douce, R.; Rebeille, F. One-carbon metabolism in plants. Regulation of tetrahydrofolate synthesis during germination and seedling development. Plant Physiol. 2003, 131, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Goyer, A.; Navarre, D.A. Folate is higher in developmentally younger potato tubers. J. Sci. Food Agric. 2009, 89, 579–583. [Google Scholar] [CrossRef]
- Robinson, B.R.; Sathuvalli, V.R.; Bamberg, J.B.; Goyer, A. Exploring folate diversity in wild and primitive potatoes for modern crop improvement. Genes 2015, 6, 1300–1314. [Google Scholar] [CrossRef] [PubMed]
- Waller, J.C.; Akhtara, T.A.; Lara-Nunez, A.; Gregory, J.F., 3rd; McQuinn, R.P.; Giovannoni, J.J.; Hanson, A.D. Developmental and feedforward control of the expression of folate biosynthesis genes in tomato fruit. Mol. Plant 2010, 3, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, T.A.; Orsomando, G.; Mehrshahi, P.; Lara-Nunez, A.; Bennett, M.J.; Gregory, J.F., III; Hanson, A.D. A central role for gamma-glutamyl hydrolases in plant folate homeostasis. Plant J. 2010, 64, 256–266. [Google Scholar] [CrossRef]
- Mehrshahi, P.; Gonzalez-Jorge, S.; Akhtar, T.A.; Ward, J.L.; Santoyo-Castelazo, A.; Marcus, S.E.; Lara-Núñez, A.; Ravanel, S.; Hawkins, N.D.; Beale, M.H.; et al. Functional analysis of folate polyglutamylation and its essential role in plant metabolism and development. Plant J. 2010, 64, 267–279. [Google Scholar] [CrossRef]
- Storozhenko, S.; De Brouwer, V.; Volckaert, M.; Navarrete, O.; Blancquaert, D.; Zhang, G.F.; Lambert, W.; Van Der Straeten, D. Folate fortification of rice by metabolic engineering. Nat. Biotechnol. 2007, 25, 1277–1279. [Google Scholar] [CrossRef]
- Diaz de la Garza, R.I.; Gregory, J.F., 3rd; Hanson, A.D. Folate biofortification of tomato fruit. Proc. Natl. Acad. Sci. USA 2007, 104, 4218–4222. [Google Scholar] [CrossRef]
- Blancquaert, D.; Storozhenko, S.; Van Daele, J.; Stove, C.; Visser, R.G.F.; Lambert, W.; Van Der Straeten, D. Enhancing pterin and para-aminobenzoate content is not sufficient to successfully biofortify potato tubers and Arabidopsis thaliana plants with folate. J. Exp. Bot. 2013, 64, 3899–3909. [Google Scholar] [CrossRef]
- De Lepeleire, J.; Strobbe, S.; Verstraete, J.; Blancquaert, D.; Ambach, L.; Visser, R.G.F.; Stove, C.; Van der Straeten, D. Folate biofortification of potato by tuber-specific expression of four folate biosynthesis genes. Mol. Plant 2018, 11, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Parra, P.A.; Garcia-Salinas, C.; Hernandez-Brenes, C.; de la Garza, R.I.D. Folate levels and polyglutamylation profiles of papaya (Carica papaya cv. Maradol) during fruit development and ripening. J. Agric. Food Chem. 2013, 61, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Rivera, N.G.R.; Garcia-Salinas, C.; Aragao, F.J.L.; de la Garza, R.I.D. Metabolic engineering of folate and its precursors in mexican common bean (Phaseolus vulgaris L.). Plant Biotechnol. J. 2016, 14, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.M.; Hansey, C.N.; Gowda, M.; Childs, K.L.; Lin, H.; Vaillancourt, B.; Sekhon, R.S.; de Leon, N.; Kaeppler, S.M.; Jiang, N.; et al. Utility of RNA sequencing for analysis of maize reproductive transcriptomes. Plant Genome 2011, 4, 191–203. [Google Scholar] [CrossRef]
- Xu, X.; Pan, S.K.; Cheng, S.F.; Zhang, B.; Mu, D.S.; Ni, P.X.; Zhang, G.Y.; Yang, S.; Li, R.Q.; Wang, J.; et al. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar]
- Cumbie, J.S.; Kimbrel, J.A.; Di, Y.M.; Schafer, D.W.; Wilhelm, L.J.; Fox, S.E.; Sullivan, C.M.; Curzon, A.D.; Carrington, J.C.; Mockler, T.C.; et al. Gene-counter: A computational pipeline for the analysis of RNA-seq data for gene expression differences. PLoS ONE 2011, 6, e25279. [Google Scholar] [CrossRef]
- Di, Y.M.; Schafer, D.W.; Cumbie, J.S.; Chang, J.H. The NBP negative binomial model for assessing differential gene expression from RNA-seq. Stat. Appl. Genet. Mol. Biol. 2011, 10, 1–28. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparitive Ct method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Mouillon, J.M.; Ravanel, S.; Douce, R.; Rebeille, F. Folate synthesis in higher-plant mitochondria: Coupling between the dihydropterin pyrophosphokinase and the dihydropteroate synthase activities. Biochem. J. 2002, 363, 313–319. [Google Scholar] [CrossRef]
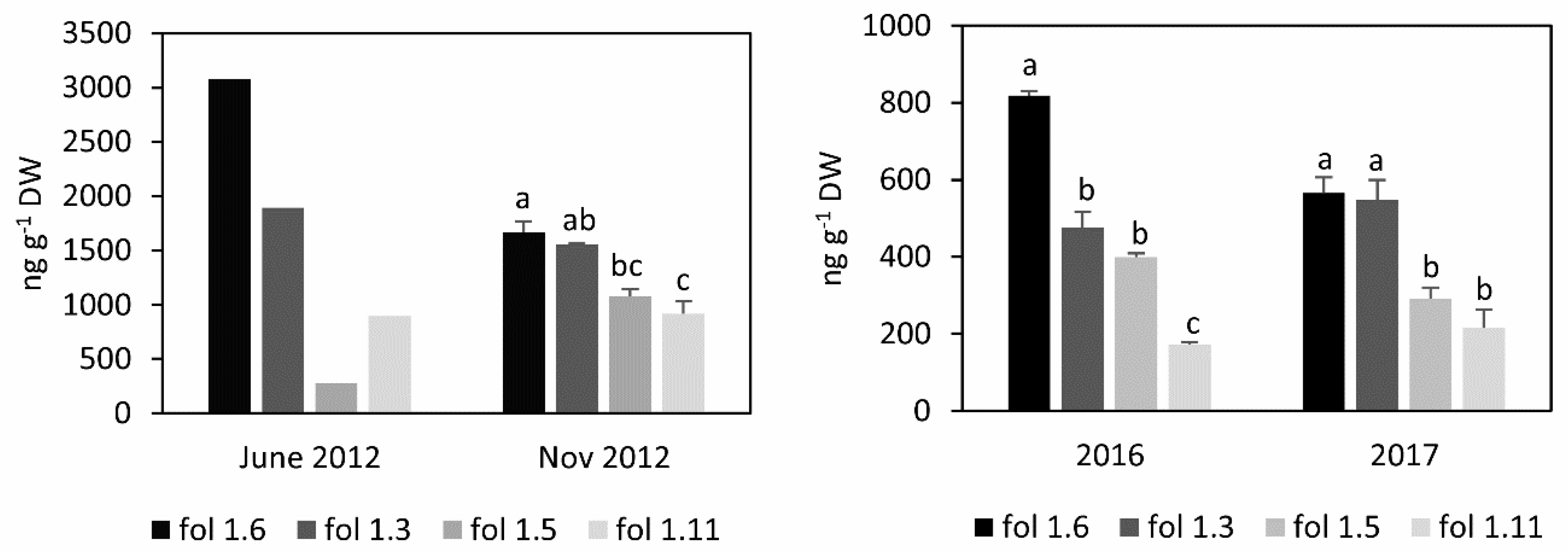

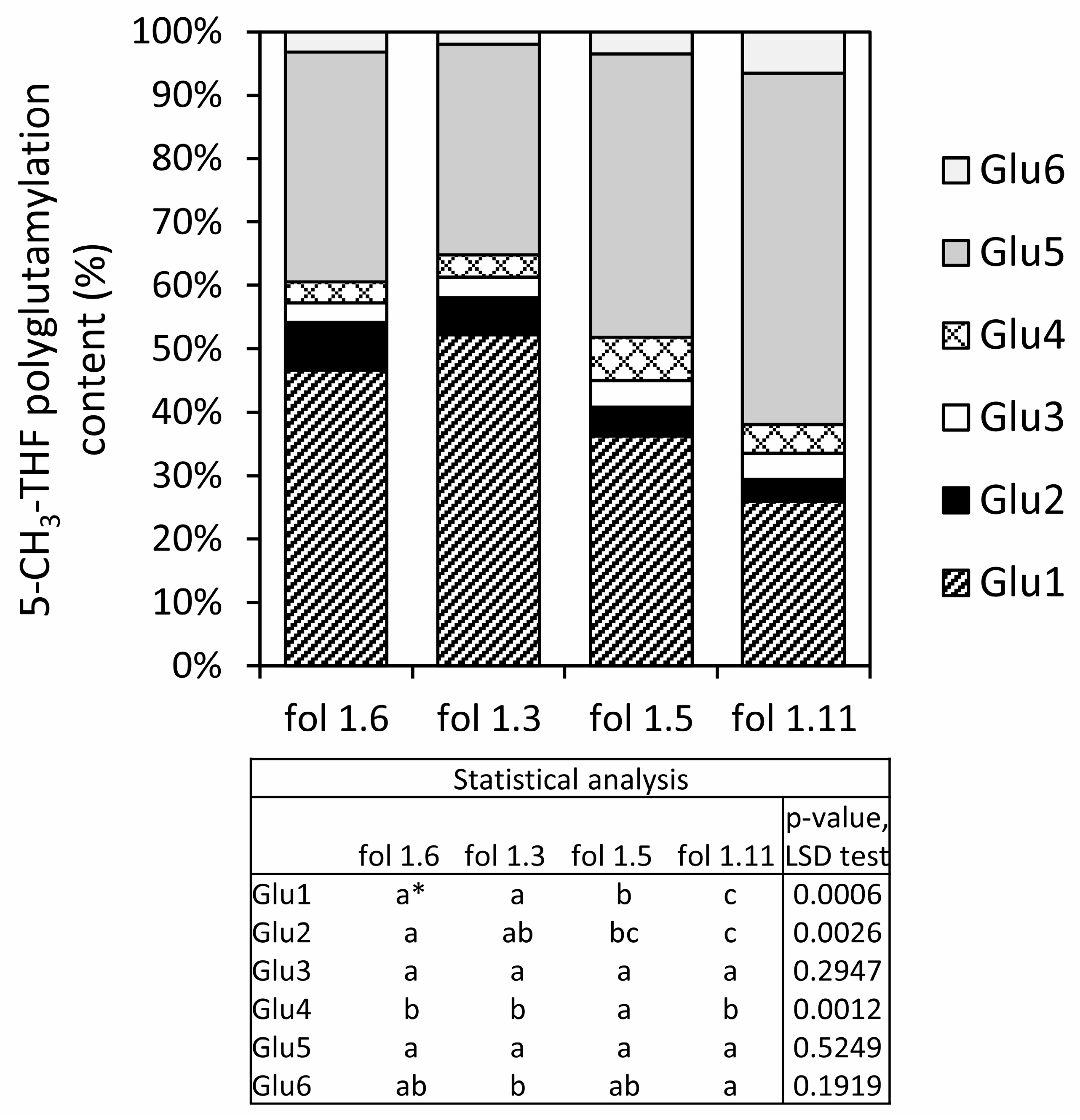
| Gene Name | PGSC Genecode | fol1.3_Rep1 | fol1.3_Rep2 | fol1.5_Rep1 | fol1.5_Rep2 | Log2 (Fold Change) | p-Value | q-Value |
|---|---|---|---|---|---|---|---|---|
| GCHI | PGSC0003DMG400020105 | 223 | 234 | 180 | 248 | 0.094 | 0.781 | 1 |
| DHN triphosphate diphosphatase | PGSC0003DMG400030259 | 51 | 38 | 26 | 49 | 0.246 | 0.590 | 0.930 |
| DHNA | PGSC0003DMG400029847 | 158 | 157 | 172 | 184 | −0.176 | 0.610 | 0.942 |
| ADCS | PGSC0003DMG400009777 | 194 | 220 | 217 | 211 | −0.047 | 0.892 | 1 |
| ADCL | PGSC0003DMG400018587 | 13 | 16 | 13 | 10 | 0.334 | 0.634 | 0.954 |
| HMDHP-PPK/DHPS | PGSC0003DMG400028362 | 64 | 68 | 70 | 81 | −0.194 | 0.630 | 0.952 |
| DHFS | PGSC0003DMG400002352 | 209 | 235 | 275 | 274 | −0.306 | 0.353 | 0.810 |
| DHFR | PGSC0003DMG400000736 | 614 | 629 | 642 | 733 | −0.145 | 0.643 | 0.957 |
| FPGS | PGSC0003DMG400027193 | 601 | 487 | 382 | 378 | 0.517 | 0.102 | 0.469 |
| UDP-glucose–pABA glucosyltransferase | PGSC0003DMG400004573 | 91 | 48 | 76 | 111 | −0.427 | 0.262 | 0.733 |
| PGSC0003DMG400004574 | - | - | - | - | - | - | - | |
| GGH1 | PGSC0003DMG400007066 | 399 | 390 | 67 | 57 | 2.669 | 7.526 × 10−14 | 1.874 × 10−11 |
| GGH2 | PGSC0003DMG400021256 | 746 | 744 | 670 | 652 | 0.172 | 0.581 | 0.924 |
| GGH3 | PGSC0003DMG400035974 | 3 | 3 | 0 | 0 | Inf | 0.057 | 0.336 |
| 5-FCL | PGSC0003DMG400024570 | 239 | 270 | 213 | 227 | 0.210 | 0.527 | 0.902 |
| Gene Name | PGSC Genecode | fol1.6_Rep1 | fol1.6_Rep2 | fol1.5_Rep1 | fol1.5_Rep2 | Log2 (Fold Change) | p-Value | q-Value |
|---|---|---|---|---|---|---|---|---|
| GCHI | PGSC0003DMG400020105 | 174 | 202 | 180 | 247 | −0.183 | 0.588 | 0.959 |
| DHN triphosphate diphosphatase | PGSC0003DMG400030259 | 32 | 59 | 26 | 53 | 0.204 | 0.658 | 0.980 |
| DHNA | PGSC0003DMG400029847 | 112 | 144 | 172 | 178 | −0.451 | 0.193 | 0.660 |
| ADCS | PGSC0003DMG400009777 | 207 | 215 | 217 | 213 | −0.027 | 0.942 | 1 |
| ADCL | PGSC0003DMG400018587 | 128 | 137 | 13 | 12 | 3.405 | 3.74 × 10−14 | 6.83 × 10−12 |
| HMDHP-PPK/DHPS | PGSC0003DMG400028362 | 52 | 59 | 70 | 85 | −0.481 | 0.224 | 0.714 |
| DHFS | PGSC0003DMG400002352 | 192 | 143 | 275 | 275 | −0.715 | 0.031 | 0.236 |
| DHFR | PGSC0003DMG400000736 | 282 | 430 | 642 | 739 | −0.955 | 0.002 | 0.037 |
| FPGS | PGSC0003DMG400027193 | 540 | 602 | 382 | 382 | 0.579 | 0.067 | 0.374 |
| UDP-glucose–pABA glucosyltransferase | PGSC0003DMG400004573 | 311 | 88 | 76 | 109 | 0.846 | 0.052 | 0.403 |
| PGSC0003DMG400004574 | - | - | - | - | - | - | - | |
| GGH1 | PGSC0003DMG400007066 | 201 | 205 | 67 | 59 | 1.688 | 3.19 × 10−6 | 1.352 × 10−4 |
| GGH2 | PGSC0003DMG400021256 | 445 | 499 | 670 | 637 | −0.469 | 0.135 | 0.562 |
| GGH3 | PGSC0003DMG400035974 | 3 | 8 | 0 | 0 | Inf | 0.004 | 0.058 |
| 5-FCL | PGSC0003DMG400024570 | 228 | 215 | 213 | 234 | −0.012 | 0.976 | 1 |
| Sample | Folate Concentration (ng/g DW) |
|---|---|
| BRR1 12 | 2373 1 |
| BRR1 27 | 471 1 |
| BRR3 90 | 2952 1 |
| BRR3 56 | 326 1 |
| Tbr 225710.3 | 2336 1,2 |
| Tbr 546023.4 | 626 1,2 |
| Vrn 558149.3 | 1688 1,2 |
| Vrn 500063.1 | 469 1,2 |
| High Folate Genotype | Ct Value 1 | Low Folate Genotype | Ct Value 1 | High/Low 2−ΔCt | Fold Change |
|---|---|---|---|---|---|
| BRR1 12 | 34.18 | BRR1 27 | 31.74 | 0.189/0.018 | 10 |
| BRR3 90 | 40.44 | BRR3 56 | 36.71 | 3.33 × 10−5/4.53 × 10−4 | 0.1 |
| Tbr PI 225710 | 29.66 | Tbr PI 546023 | 38.84 | 3.00 × 10−2/1.55 × 10−2 | 2 |
| Vrn PI 558149 | 35.33 | Vrn PI 500063 | 40.78 | 6.25 × 10−2/1.29 × 10−4 | 481 |
| fol 1-6 | 32.01 | fol 1-11 | 35.41 | 7.10 × 10−3/4.76 × 10−4 | 15 |
| fol 1-6 | 32.01 | fol 1-5 | 39.82 | 7.10 × 10−3/8.07 × 10−5 | 88 |
| fol 1-3 | 30.90 | fol 1-11 | 35.41 | 1.13 × 10−2/4.76 × 10−4 | 24 |
| fol 1-3 | 30.90 | fol 1-5 | 39.82 | 1.13 × 10−2/8.07 × 10−5 | 140 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, B.R.; Garcia Salinas, C.; Ramos Parra, P.; Bamberg, J.; Diaz de la Garza, R.I.; Goyer, A. Expression Levels of the γ-Glutamyl Hydrolase I Gene Predict Vitamin B9 Content in Potato Tubers. Agronomy 2019, 9, 734. https://doi.org/10.3390/agronomy9110734
Robinson BR, Garcia Salinas C, Ramos Parra P, Bamberg J, Diaz de la Garza RI, Goyer A. Expression Levels of the γ-Glutamyl Hydrolase I Gene Predict Vitamin B9 Content in Potato Tubers. Agronomy. 2019; 9(11):734. https://doi.org/10.3390/agronomy9110734
Chicago/Turabian StyleRobinson, Bruce R., Carolina Garcia Salinas, Perla Ramos Parra, John Bamberg, Rocio I. Diaz de la Garza, and Aymeric Goyer. 2019. "Expression Levels of the γ-Glutamyl Hydrolase I Gene Predict Vitamin B9 Content in Potato Tubers" Agronomy 9, no. 11: 734. https://doi.org/10.3390/agronomy9110734
APA StyleRobinson, B. R., Garcia Salinas, C., Ramos Parra, P., Bamberg, J., Diaz de la Garza, R. I., & Goyer, A. (2019). Expression Levels of the γ-Glutamyl Hydrolase I Gene Predict Vitamin B9 Content in Potato Tubers. Agronomy, 9(11), 734. https://doi.org/10.3390/agronomy9110734






