Nondestructive Estimation of the Chlorophyll b of Apple Fruit by Color and Spectral Features Using Different Methods of Hybrid Artificial Neural Network
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Used
2.2. Development of Visible and Near-Infrared Light Spectroscopy System
2.3. Extracting Color Features
2.4. Extraction of Chlorophyll b
2.5. Different Hybrids of Artificial Neural Networks Used for Selecting Effective Features and Predicting Chlorophyll b
2.6. Hybrid Neural Networks Used to Select the Most Effective Color Features
2.7. Neural Network Hybrid Used to Estimate the Amount of Chlorophyll b Using Color Features
2.8. Neural Network Hybrid Used to Select Effective Wavelengths
2.9. Neural Network Hybrid Used to Estimate Chlorophyll b Content Using Spectral Data
| Algorithm 1: Algorithm of biogeography-based optimization. |
| 1: Production of initial populations and sorting 2: Determining rates of immigrant receptivity and receiving 3: For (habitat like j) 4: For (variable such as k in the habitat j) 5: With the probability of immigrant receptivity in a settlement in the variable, changes are applied according to steps 6 to 8 6: Determining the origin of immigration using immigrant receptivity values randomly 7: Immigration from a settlement to another 8: With a certain probability, are applied to the variable component (random changes (mutation)) 9: end for 10: end for 11: The set of new answers is evaluated 12: Combining the main population (old) and the population from migration Create a new stage population 13: If the termination conditions are not fulfilled, the algorithm will be returned to step 3 14: End process |
2.10. Parameters Used to Evaluate the Performance of Proposed Methods for Estimating the Amount of Chlorophyll b
3. Results and Discussion
3.1. Response of Apple Samples to Visible/Near-Infrared Wavelengths
3.2. Estimation of chlorophyll b Using Color Features
3.3. Estimation of Chlorophyll b Content Using Non-Destructive Spectroscopy Method
3.4. Analyzing the Performance of Chlorophyll b Predictive Systems Based on Color and Spectroscopy Methods
3.5. Effective Wavelengths Selected by the Hybrid Artificial Neural Network—Differential Evolution Algorithm
3.6. The Performance of the Chlorophyll b Estimation System Based on the Effective Wavelengths Selected
4. Conclusions
- The cost of the configuration and set-up of the spectroscopy system is very important for real time aims. To reduce the cost of configuration, a small window of around 680 nm wavelength could be used instead of using spectroscopy over the entire visible/near-infrared range.
- The largest peak in spectral diagrams in the visible light region is related to the chlorophyll absorption because the chlorophyll b content was predicted to be high when the coefficient was predicted using the relevant spectral data of this region.
- There is a relationship between the color features of the apple and the amount of chlorophyll b so that the chlorophyll b values are estimated using these color features, with a coefficient of more than 0.996.
- Performance of the spectral method is higher than the color method in terms of the determination and regression coefficients as well as the error estimation parameters.
- When effective spectra selected by the hybrid artificial neural network-differential evolution algorithm are introduced as an input to a hybrid artificial neural network-biogeography-based algorithm, it has high regression and determination coefficients.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blasco, J.; Aleixos, N.; Moltó, E. Machine vision system for automatic quality grading of fruit. Biosyst. Eng. 2003, 85, 415–423. [Google Scholar] [CrossRef]
- Leemans, V.; Magein, H.; Destain, M.F. AE—Automation and emerging technologies: On-line fruit grading according to their external quality using machine vision. Biosyst. Eng. 2002, 83, 397–404. [Google Scholar] [CrossRef]
- Kondo, N.; Ahmad, U.; Monta, M.; Murase, H. Machine vision based quality evaluation of Iyokan orange fruit using neural networks. Comput. Electron. Agric. 2000, 29, 135–147. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.; Zhang, H.; Aiguo, O. Nondestructive measurement of internal quality of Nanfeng mandarin fruit by charge coupled device near infrared spectroscopy. Comput. Electron. Agric. 2010, 71, S10–S14. [Google Scholar] [CrossRef]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Opara, U.L. Non-destructive prediction of internal and external quality attributes of fruit with thick rind: A review. J. Food Eng. 2018, 217, 11–23. [Google Scholar] [CrossRef]
- Nicolai, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Gowen, A.; O’Donnell, C.; Cullen, P.; Downey, G.; Frias, J. Hyperspectral imaging—An emerging process analytical tool for food quality and safety control. Trends Food Sci. Technol. 2007, 18, 590–598. [Google Scholar] [CrossRef]
- Marcone, M.F.; Wang, S.; Albabish, W.; Nie, S.; Somnarain, D.; Hill, A. Diverse food-based applications of nuclear magnetic resonance (NMR) technology. Food Res. Int. 2013, 51, 729–747. [Google Scholar] [CrossRef]
- Zhang, L.; McCarthy, M.J. Assessment of pomegranate postharvest quality using nuclear magnetic resonance. Postharvest Biol. Technol. 2013, 77, 59–66. [Google Scholar] [CrossRef]
- Donis-González, I.R.; Guyer, D.E.; Fulbright, D.W.; Pease, A. Postharvest noninvasive assessment of fresh chestnut (Castanea spp.) internal decay using computer tomography images. Postharvest Biol. Technol. 2014, 94, 14–25. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L. Investigating non-destructive quantification and characterization of pomegranate fruit internal structure using X-ray computed tomography. Postharvest Biol. Technol. 2014, 95, 1–6. [Google Scholar] [CrossRef]
- Clément, A.; Dorais, M.; Vernon, M. Nondestructive measurement of fresh tomato lycopene content and other physicochemical characteristics using visible- NIR spectroscopy. J. Agric. Food Chem. 2008, 56, 9813–9818. [Google Scholar] [CrossRef] [PubMed]
- Baranska, M.; Schütze, W.; Schulz, H. Determination of lycopene and β-carotene content in tomato fruits and related products: Comparison of FT-Raman, ATR-IR, and NIR spectroscopy. Anal. Chem. 2006, 78, 8456–8461. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Lu, R.; Zhu, Q.; McGrath, J.M.; Tu, K. Measurement of moisture, soluble solids, sucrose content and mechanical properties in sugar beet using portable visible and near-infrared spectroscopy. Postharvest Biol. Technol. 2015, 102, 42–50. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Chen, K. Assessment of tomato soluble solids content and pH by spatially-resolved and conventional Vis/NIR spectroscopy. J. Food Eng. 2018, 236, 19–28. [Google Scholar] [CrossRef]
- Amoriello, T.; Ciccoritti, R.; Paliotta, M.; Carbone, K. Classification and prediction of early-to-late ripening apricot quality using spectroscopic techniques combined with chemometric tools. Sci. Hortic. 2018, 240, 310–317. [Google Scholar] [CrossRef]
- Moscetti, R.; Haff, R.P.; Monarca, D.; Cecchini, M.; Massantini, R. Near-infrared spectroscopy for detection of hailstorm damage on olive fruit. Postharvest Biol. Technol. 2016, 120, 204–212. [Google Scholar] [CrossRef]
- Bexiga, F.; Rodrigues, D.; Guerra, R.; Brázio, A.; Balegas, T.; Cavaco, A.M.; Antunes, M.D.; de Oliveira, J.V. A TSS classification study of ‘Rocha’pear (Pyrus communis L.) based on non-invasive visible/near infra-red reflectance spectra. Postharvest Biol. Technol. 2017, 132, 23–30. [Google Scholar] [CrossRef]
- Eisenstecken, D.; Stürz, B.; Robatscher, P.; Lozano, L.; Zanella, A.; Oberhuber, M. The potential of near infrared spectroscopy (NIRS) to trace apple origin: Study on different cultivars and orchard elevations. Postharvest Biol. Technol. 2019, 147, 123–131. [Google Scholar] [CrossRef]
- Ncama, K.; Opara, U.L.; Tesfay, S.Z.; Fawole, O.A.; Magwaza, L.S. Application of Vis/NIR spectroscopy for predicting sweetness and flavour parameters of ‘Valencia’orange (Citrus sinensis) and ‘Star Ruby’grapefruit (Citrus x paradisi Macfad). J. Food Eng. 2017, 193, 86–94. [Google Scholar] [CrossRef]
- Nordey, T.; Joas, J.; Davrieux, F.; Chillet, M.; Léchaudel, M. Robust NIRS models for non-destructive prediction of mango internal quality. Sci. Hortic. 2017, 216, 51–57. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Hu, D.; Chen, K. Quality assessment of tomato fruit by optical absorption and scattering properties. Postharvest Biol. Technol. 2018, 143, 78–85. [Google Scholar] [CrossRef]
- Uwadaira, Y.; Sekiyama, Y.; Ikehata, A. An examination of the principle of non-destructive flesh firmness measurement of peach fruit by using VIS-NIR spectroscopy. Heliyon 2018, 4, e00531. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Folador, G.; de Oliveira Bicudo, M.; de Andrade, E.F.; Renard, C.M.G.C.; Bureau, S.; de Castilhos, F. Quality traits prediction of the passion fruit pulp using NIR and MIR spectroscopy. LWT 2018, 95, 172–178. [Google Scholar] [CrossRef]
- Francis, F. Quality as influenced by color. Food Qual. Prefer. 1995, 6, 149–155. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer acceptance of ‘Brooks’ and ‘Bing’cherries is mainly dependent on fruit SSC and visual skin color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Nisha, P.; Singhal, R.S.; Pandit, A.B. Kinetic modelling of colour degradation in tomato puree (Lycopersicon esculentum L.). Food Bioprocess Technol. 2011, 4, 781–787. [Google Scholar] [CrossRef]
- Hertog, M.L.; Lammertyn, J.; Desmet, M.; Scheerlinck, N.; Nicolaı, B.M. The impact of biological variation on postharvest behaviour of tomato fruit. Postharvest Biol. Technol. 2004, 34, 271–284. [Google Scholar] [CrossRef]
- Pinheiro, J.; Alegria, C.; Abreu, M.; Gonçalves, E.M.; Silva, C.L. Kinetics of changes in the physical quality parameters of fresh tomato fruits (Solanum lycopersicum, cv.‘Zinac’) during storage. J. Food Eng. 2013, 114, 338–345. [Google Scholar] [CrossRef]
- Lana, M.M.; Tijskens, L.; De Theije, A.; Hogenkamp, M.; Van Kooten, O. Assessment of changes in optical properties of fresh-cut tomato using video image analysis. Postharvest Biol. Technol. 2006, 41, 296–306. [Google Scholar] [CrossRef]
- Schouten, R.E.; Huijben, T.P.; Tijskens, L.; Van Kooten, O. Modelling quality attributes of truss tomatoes: Linking colour and firmness maturity. Postharvest Biol. Technol. 2007, 45, 298–306. [Google Scholar] [CrossRef]
- Schouten, R.E.; Farneti, B.; Tijskens, L.; Alarcón, A.A.; Woltering, E.J. Quantifying lycopene synthesis and chlorophyll breakdown in tomato fruit using remittance VIS spectroscopy. Postharvest Biol. Technol. 2014, 96, 53–63. [Google Scholar] [CrossRef]
- Tilahun, S.; Park, D.S.; Seo, M.H.; Hwang, I.G.; Kim, S.H.; Choi, H.R.; Jeong, C.S. Prediction of lycopene and β-carotene in tomatoes by portable chroma-meter and VIS/NIR spectra. Postharvest Biol. Technol. 2018, 136, 50–56. [Google Scholar] [CrossRef]
- Clerici, M.; Kallmann, C.; Gaspi, F.; Morgano, M.; Martinez-Bustos, F.; Chang, Y. Physical, chemical and technological characteristics of Solanum lycocarpum A. St.-HILL (Solanaceae) fruit flour and starch. Food Res. Int. 2011, 44, 2143–2150. [Google Scholar] [CrossRef]
- Costa, G.; Noferini, M.; Fiori, G.; Torrigiani, P. Use of Vis/NIR spectroscopy to assess fruit ripening stage and improve management in post-harvest chain. Fresh Prod 2009, 1, 35–41. [Google Scholar]
- Eberhart, R.; Kennedy, J. Particle swarm optimization. In Proceedings of the IEEE International Conference on Neural Networks, Perth, WA, Australia, 27 November–1 December 1995; Volume 4, pp. 1942–1948. [Google Scholar]
- Ali, M.Z.; Awad, N.H.; Suganthan, P.N.; Duwairi, R.M.; Reynolds, R.G. A novel hybrid Cultural Algorithms framework with trajectory-based search for global numerical optimization. Inf. Sci. 2016, 334, 219–249. [Google Scholar] [CrossRef]
- Storn, R.; Price, K. Minimizing the real functions of the ICEC’96 contest by differential evolution. In Proceedings of the IEEE International Conference on Evolutionary Computation, Nagoya, Japan, 20–22 May 1996; pp. 842–844. [Google Scholar]
- Simon, D. Biogeography-based optimization. IEEE Trans. Evol. Comput. 2008, 12, 702–713. [Google Scholar] [CrossRef]
- Sabzi, S.; Javadikia, P.; Rabani, H.; Adelkhani, A. Mass modeling of Bam orange with ANFIS and SPSS methods for using in machine vision. Measurement 2013, 46, 3333–3341. [Google Scholar] [CrossRef]
- Sabzi, S.; Arribas, J.I. A visible-range computer-vision system for automated, non-intrusive assessment of the pH value in Thomson oranges. Comput. Ind. 2018, 99, 69–82. [Google Scholar] [CrossRef]
- Cayuela, J.A. Vis/NIR soluble solids prediction in intact oranges (Citrus sinensis L.) cv. Valencia Late by reflectance. Postharvest Biol. Technol. 2008, 47, 75–80. [Google Scholar] [CrossRef]
- Martínez-Valdivieso, D.; Font, R.; Blanco-Díaz, M.T.; Moreno-Rojas, J.M.; Gómez, P.; Alonso-Moraga, Á.; Del Río-Celestino, M. Application of near-infrared reflectance spectroscopy for predicting carotenoid content in summer squash fruit. Comput. Electron. Agric. 2014, 108, 71–79. [Google Scholar] [CrossRef]
- Adebayo, S.E.; Hashim, N.; Abdan, K.; Hanafi, M.; Mollazade, K. Prediction of quality attributes and ripeness classification of bananas using optical properties. Sci. Hortic. 2016, 212, 171–182. [Google Scholar] [CrossRef]
- Betemps, D.L.; Fachinello, J.C.; Galarça, S.P.; Portela, N.M.; Remorini, D.; Massai, R.; Agati, G. Non-destructive evaluation of ripening and quality traits in apples using a multiparametric fluorescence sensor. J. Sci. Food Agric. 2012, 92, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Kuckenberg, J.; Tartachnyk, I.; Noga, G. Evaluation of fluorescence and remission techniques for monitoring changes in peel chlorophyll and internal fruit characteristics in sunlit and shaded sides of apple fruit during shelf-life. Postharvest Biol. Technol. 2008, 48, 231–241. [Google Scholar] [CrossRef]










| Parameter | Values |
|---|---|
| Number of layers | 2 |
| Number of neurons | First layer: 11, Second layer: 15 |
| Transfer function | First layer: tribas, Second layer: logsig |
| Backpropagation network training function | trainscg |
| Backpropagation weight/bias learning function | learnpn |
| Parameter | Values |
|---|---|
| Number of layers | 2 |
| Number of neurons | First layer: 19, Second layer: 13 |
| Transfer function | First layer: radbas, Second layer: tansig |
| Backpropagation network training function | traingda |
| Backpropagation weight/bias learning function | learngd |
| Parameter | Values |
|---|---|
| Number of layers | 3 |
| Number of neurons | First layer: 9, Second layer: 19, Third layer: 7 |
| Transfer function | First layer: satlin, Second layer: purelin, Third layer: satlins |
| Backpropagation network training function | traingdm |
| Backpropagation weight/bias learning function | learncon |
| Statistics | MSE | RMSE | MAE | SSE | R |
|---|---|---|---|---|---|
| Mean ± Standard Deviation | 0.051 ± 0.054 | 0.209 ± 0.086 | 0.144 ± 0.055 | 0.665 ± 0.702 | 0.882 0.047 |
| The best result | 0.002 | 0.039 | 0.031 | 0.021 | 0.9931 |
| Parameter | Values |
|---|---|
| Number of layers | 3 |
| Number of neurons | First layer: 16, Second layer: 17, Third layer: 13 |
| Transfer function | First layer: softmax, Second layer: poslin, Third layer: tansig |
| Backpropagation network training function | traingbr |
| Backpropagation weight/bias learning function | learnlv2 |
| Statistics | MSE | RMSE | MAE | SSE | R |
|---|---|---|---|---|---|
| Mean ± Standard Deviation | 0.025 ± 0.032 | 0.141 ± 0.073 | 0.099 ± 0.047 | 0.329 ± 0.421 | 0.932 0.046 |
| The best result | 0.001 | 0.031 | 0.027 | 0.013 | 0.9965 |
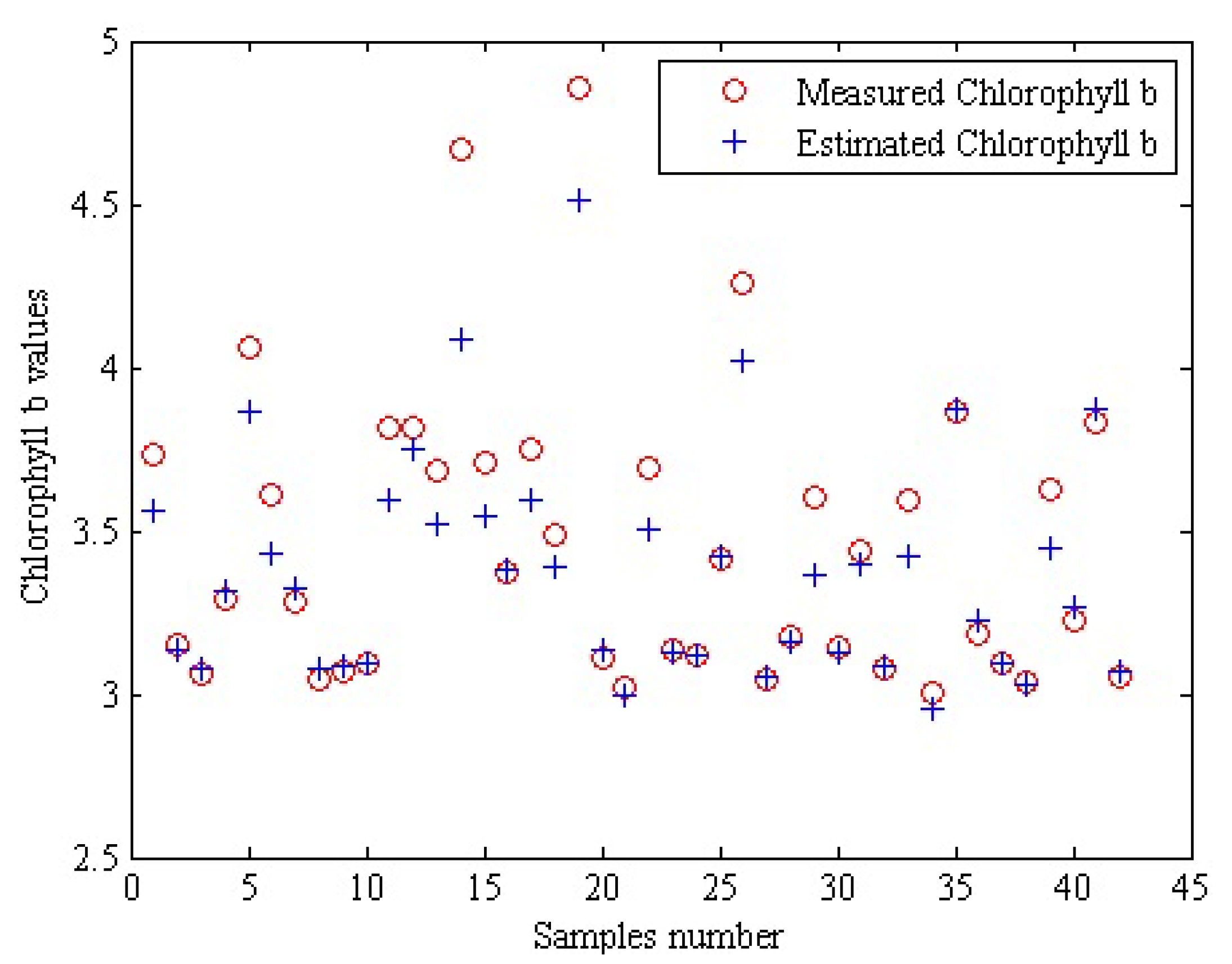
| Apple | Measured | Estimated | Apple | Measured | Estimated | Apple | Measured | Estimated |
|---|---|---|---|---|---|---|---|---|
| Number | Value | Value | Number | Value | Value | Number | Value | Value |
| 1 | 3.73 | 3.56 ± 0.169 | 15 | 3.71 | 3.54 ± 0.164 | 29 | 3.60 | 3.36 ± 0.140 |
| 2 | 3.15 | 3.13 ± 0.077 | 16 | 3.37 | 3.39 ± 0.128 | 30 | 3.14 | 3.12 ± 0.074 |
| 3 | 3.06 | 3.08 ± 0.062 | 17 | 3.75 | 3.59 ± 0.172 | 31 | 3.44 | 3.40 ± 0.125 |
| 4 | 3.29 | 3.31 ± 0.102 | 18 | 3.49 | 3.39 ± 0.117 | 32 | 3.08 | 3.09 ± 0.077 |
| 5 | 4.06 | 3.87 ± 0.218 | 19 | 4.86 | 3.51 ± 0.529 | 33 | 3.59 | 3.42 ± 0.134 |
| 6 | 3.61 | 3.43 ± 0.125 | 20 | 3.11 | 3.14 ± 0.074 | 34 | 3.00 | 2.96 ± 0.150 |
| 7 | 3.28 | 3.32 ± 0.108 | 21 | 3.02 | 2.99 ± 0.140 | 35 | 3.86 | 3.87 ± 0.263 |
| 8 | 3.04 | 3.07 ± 0.068 | 22 | 3.69 | 3.50 ± 0.157 | 36 | 3.19 | 3.23 ± 0.093 |
| 9 | 3.07 | 3.08 ± 0.057 | 23 | 3.13 | 3.12 ± 0.082 | 37 | 3.09 | 3.10 ± 0.159 |
| 10 | 3.09 | 3.09 ± 0.065 | 24 | 3.12 | 3.12 ± 0.092 | 38 | 3.04 | 3.02 ± 0.191 |
| 11 | 3.81 | 3.59 ± 0.173 | 25 | 3.42 | 3.43 ± 0.195 | 39 | 3.63 | 3.45 ± 0.136 |
| 12 | 3.82 | 3.75 ± 0.227 | 26 | 4.26 | 4.02 ± 0.241 | 40 | 3.23 | 3.27 ± 0.110 |
| 13 | 3.69 | 3.53 ± 0.159 | 27 | 3.04 | 3.05 ± 0.137 | 41 | 3.83 | 3.87 ± 0.292 |
| 14 | 4.67 | 3.08 ± 0.179 | 28 | 3.18 | 316 ± 0.213 | 42 | 3.06 | 3.07 ± 0.091 |
| Apple | Measured | Estimated | Apple | Measured | Estimated | Apple | Measured | Estimated |
|---|---|---|---|---|---|---|---|---|
| Number | Value | Value | Number | Value | Value | Number | Value | Value |
| 1 | 3.73 | 3.59 ± 0.167 | 15 | 3.71 | 3.56 ± 0.188 | 29 | 3.60 | 3.40 ± 0.119 |
| 2 | 3.15 | 3.21 ± 0.079 | 16 | 3.37 | 3.28 ± 0.090 | 30 | 3.14 | 3.16 ± 0.069 |
| 3 | 3.06 | 3.08 ± 0.049 | 17 | 3.75 | 3.62 ± 0.181 | 31 | 3.44 | 3.34 ± 0.107 |
| 4 | 3.29 | 3.28 ± 0.089 | 18 | 3.49 | 3.33 ± 0.105 | 32 | 3.08 | 3.09 ± 0.050 |
| 5 | 4.06 | 3.95 ± 0.249 | 19 | 3.84 | 4.55 ± 0.328 | 33 | 3.59 | 3.36 ± 0.121 |
| 6 | 3.61 | 3.55 ± 0.173 | 20 | 3.11 | 3.10 ± 0.056 | 34 | 3.00 | 2.96 ± 0.147 |
| 7 | 3.28 | 3.24 ± 0.083 | 21 | 3.02 | 3.04 ± 0.085 | 35 | 3.86 | 3.65 ± 0.241 |
| 8 | 3.04 | 3.07 ± 0.066 | 22 | 3.69 | 3.56 ± 0.164 | 36 | 3.19 | 3.22 ± 0.081 |
| 9 | 3.07 | 3.08 ± 0.069 | 23 | 3.13 | 3.18 ± 0.228 | 37 | 3.09 | 3.08 ± 0.058 |
| 10 | 3.09 | 3.09 ± 0.052 | 24 | 3.12 | 3.12 ± 0.064 | 38 | 3.04 | 3.05 ± 0.091 |
| 11 | 3.81 | 3.61 ± 0.176 | 25 | 3.42 | 3.33 ± 0.098 | 39 | 3.63 | 3.54 ± 0.159 |
| 12 | 3.82 | 3.66 ± 0.211 | 26 | 4.26 | 4.06 ± 0.228 | 40 | 3.23 | 3.28 ± 0.086 |
| 13 | 3.69 | 3.51 ± 0.154 | 27 | 3.04 | 3.04 ± 0.089 | 41 | 3.83 | 3.74 ± 0.239 |
| 14 | 4.67 | 4.03 ± 0.177 | 28 | 3.18 | 319 ± 0.069 | 42 | 3.06 | 3.07 ± 0.079 |
| Number of Effective Wavelengths | Effective Wavelengths |
|---|---|
| 2 | 687.152, 662.295 |
| 4 | 664.006, 687.724, 673.425, 697.180 |
| 6 | 669.428, 664.862, 680.571, 696.033, 683.431, 677.711 |
| 8 | 683.431, 997.753, 666.287, 685.148, 674.568, 671.141, 672.283, 684.862 |
| 10 | 662.866, 686.007, 671.997, 676.282, 696.033, 689.156, 690.015, 686.293, 693.453, 686.865 |
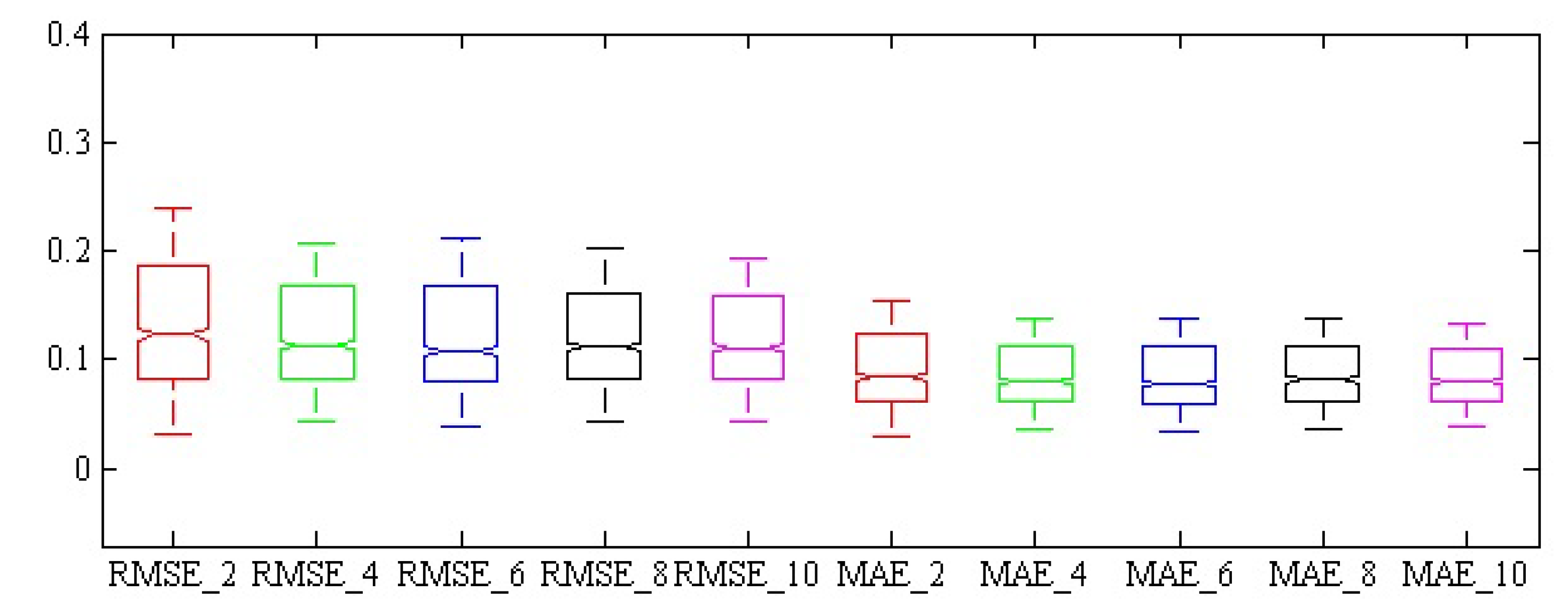
| The Number of Selected Effective Waveleghs | Statistics | MSE | RMSE | MAE | SSE | R2 |
|---|---|---|---|---|---|---|
| Mean ± | ||||||
| 2 | Standard Deviation | 0.032 ± 0.044 | 0.155 ± 0.095 | 0.099 ± 0.054 | 0.414 ± 0.5771 | 0.915 ± 0.106 |
| The best result | 0.006 | 0.025 | 0.019 | 0.008 | 0.997 | |
| Mean ± | ||||||
| 4 | Standard Deviation | 0.028 ± 0.112 | 0.138 ± 0.097 | 0.095 ± 0.073 | 0.368 ± 0.455 | 0.926 ± 0.087 |
| The best result | 0.0007 | 0.027 | 0.022 | 0.009 | 0.998 | |
| Mean ± | ||||||
| 6 | Standard Deviation | 0.026 ± 0.045 | 0.135 ± 0.087 | 0.093 ± 0.051 | 0.339 ± 0.587 | 0.924 ± 0.094 |
| The best result | 0.0007 | 0.026 | 0.020 | 0.008 | 0.996 | |
| Mean ± | ||||||
| 8 | Standard Deviation | 0.024 ± 0.034 | 0.134 ± 0.078 | 0.094 ± 0.048 | 0.315 ± 0.448 | 0.925 ± 0.090 |
| The best result | 0.0010 | 0.033 | 0.022 | 0.014 | 0.997 | |
| Mean ± | ||||||
| 10 | Standard Deviation | 0.024 ± 0.038 | 0.133 ± 0.079 | 0.093 ± 0.049 | 0.313 ± 0.496 | 0.930 ± 0.08 |
| The best result | 0.001 | 0.031 | 0.027 | 0.013 | 0.9965 |
| Method | Type of Fruit | Coefficient of Determination |
|---|---|---|
| Propose method using spectral features | Apple | 0.998 |
| Propose method using color features | Apple | 0.996 |
| Ncama et al. [20] | Grapefruit | 0.943 |
| Adebayo et al. [44] | Banana | 0.978 |
| Betemps et al. [45] | Apple | 0.934 |
| Kuckenberg et al. [46] | Apple | 0.927 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbaspour-Gilandeh, Y.; Sabzi, S.; Hernández-Hernández, M.; Hernández-Hernández, J.L.; Azadshahraki, F. Nondestructive Estimation of the Chlorophyll b of Apple Fruit by Color and Spectral Features Using Different Methods of Hybrid Artificial Neural Network. Agronomy 2019, 9, 735. https://doi.org/10.3390/agronomy9110735
Abbaspour-Gilandeh Y, Sabzi S, Hernández-Hernández M, Hernández-Hernández JL, Azadshahraki F. Nondestructive Estimation of the Chlorophyll b of Apple Fruit by Color and Spectral Features Using Different Methods of Hybrid Artificial Neural Network. Agronomy. 2019; 9(11):735. https://doi.org/10.3390/agronomy9110735
Chicago/Turabian StyleAbbaspour-Gilandeh, Yousef, Sajad Sabzi, Mario Hernández-Hernández, Jose Luis Hernández-Hernández, and Farzad Azadshahraki. 2019. "Nondestructive Estimation of the Chlorophyll b of Apple Fruit by Color and Spectral Features Using Different Methods of Hybrid Artificial Neural Network" Agronomy 9, no. 11: 735. https://doi.org/10.3390/agronomy9110735
APA StyleAbbaspour-Gilandeh, Y., Sabzi, S., Hernández-Hernández, M., Hernández-Hernández, J. L., & Azadshahraki, F. (2019). Nondestructive Estimation of the Chlorophyll b of Apple Fruit by Color and Spectral Features Using Different Methods of Hybrid Artificial Neural Network. Agronomy, 9(11), 735. https://doi.org/10.3390/agronomy9110735








