Domestication in Real Time: The Curious Case of a Trigenomic Sunflower Population
Abstract
1. Introduction
2. Materials and Methods
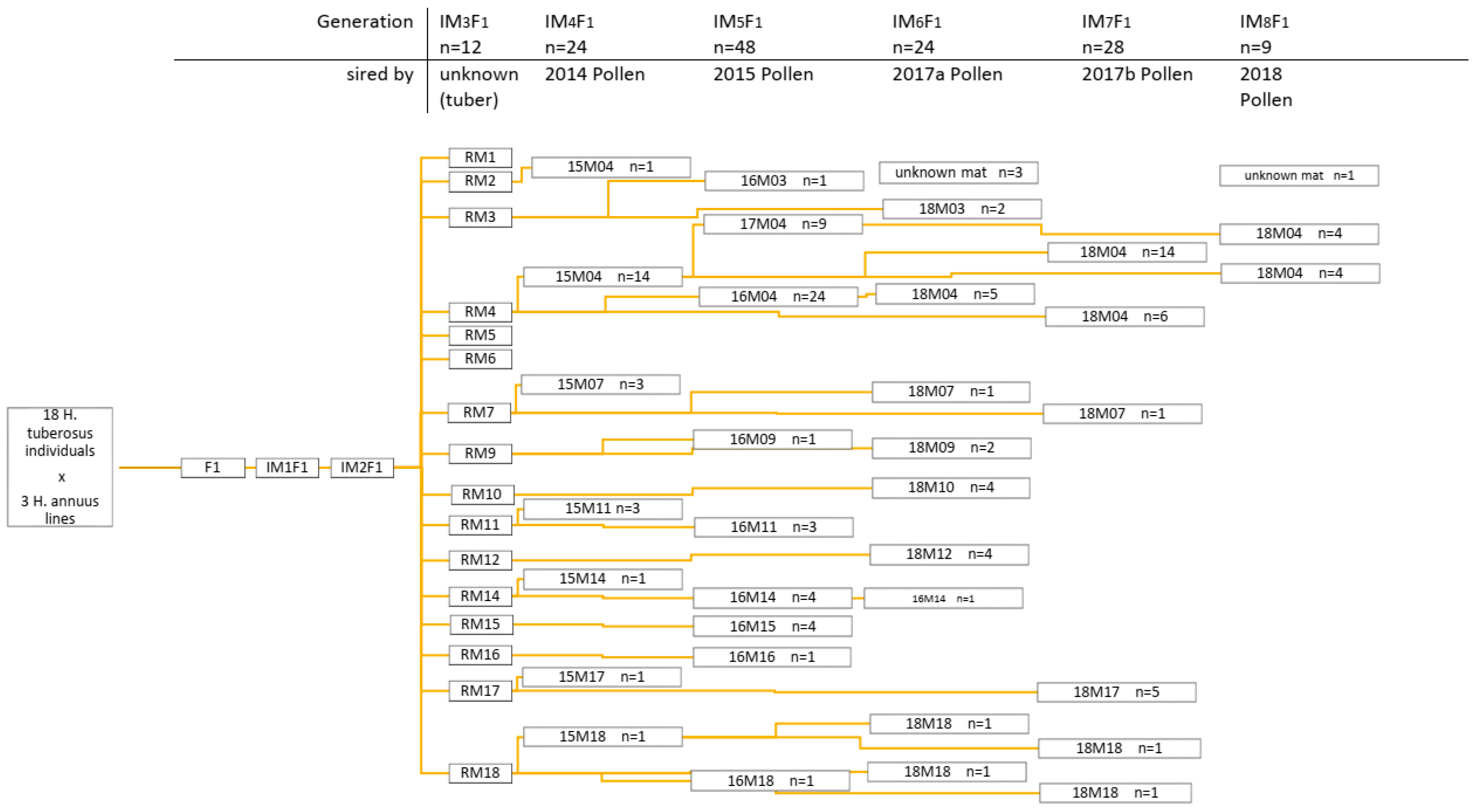
2.1. Population Development
2.2. Flow Cytometry
2.3. Phenotyping
2.4. Statistical Analyses
3. Results
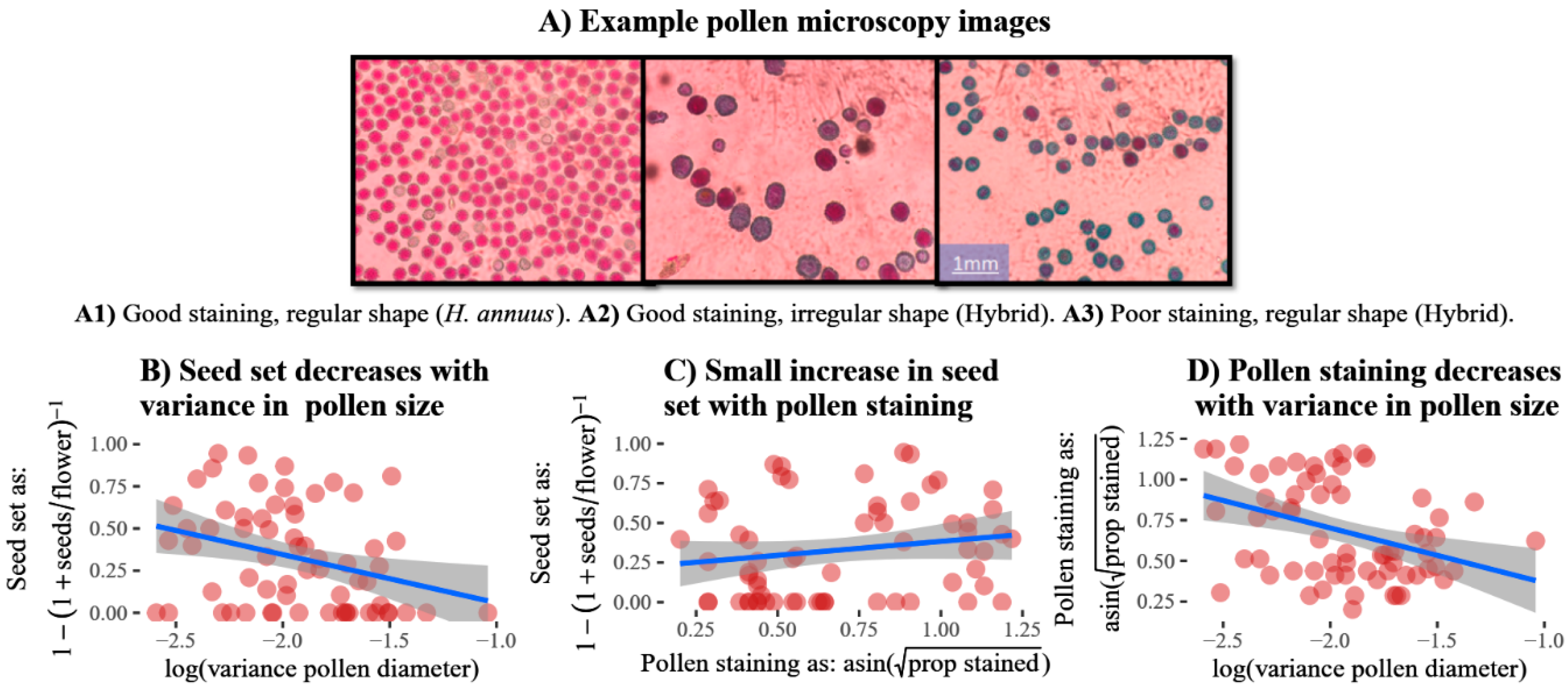
3.1. Trait Relationships with Fertility
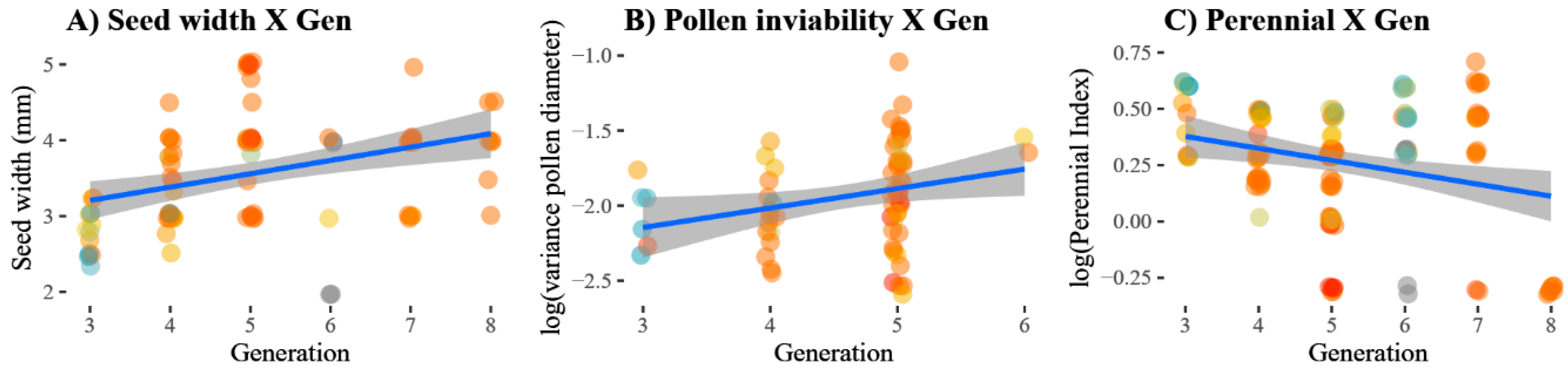
3.2. Changes in Traits over Time
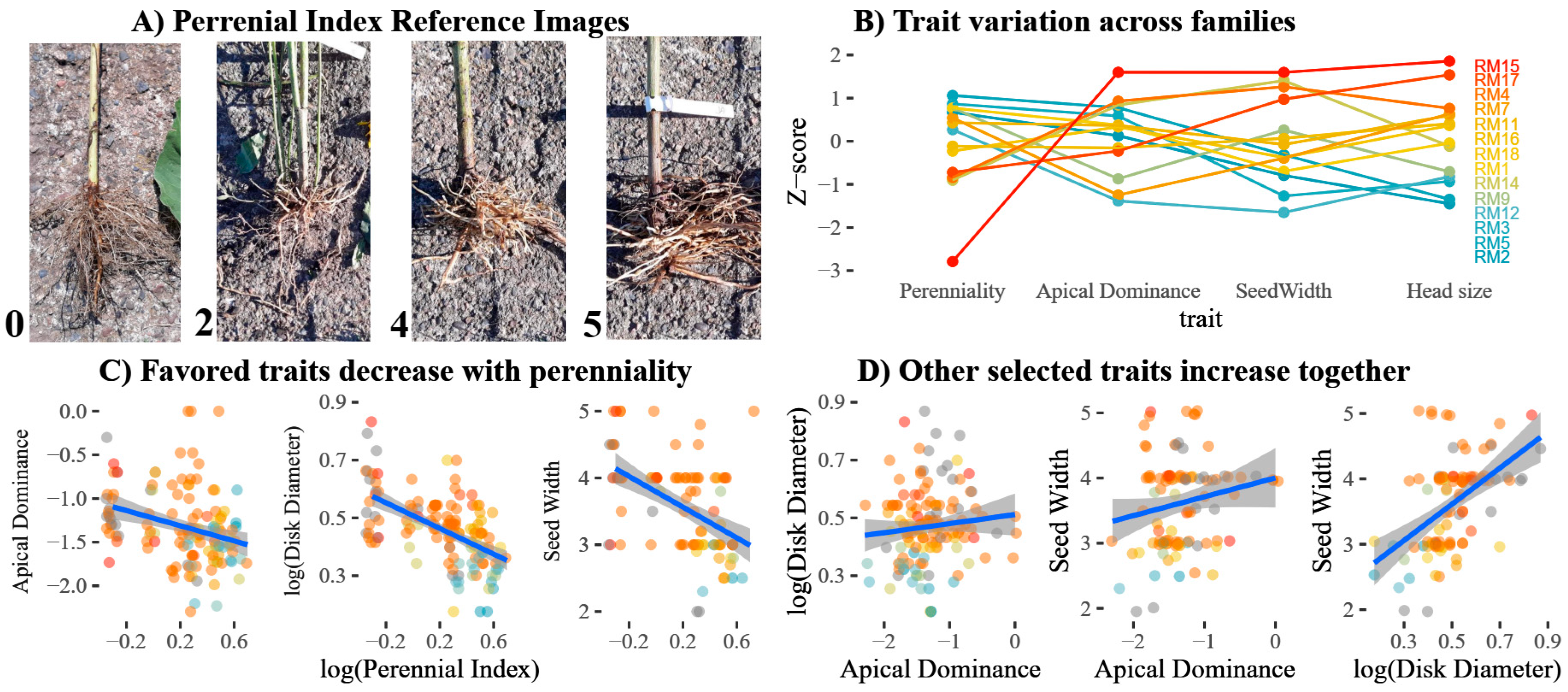
3.3. Negative Correlation Between Perenniality and Yield Traits
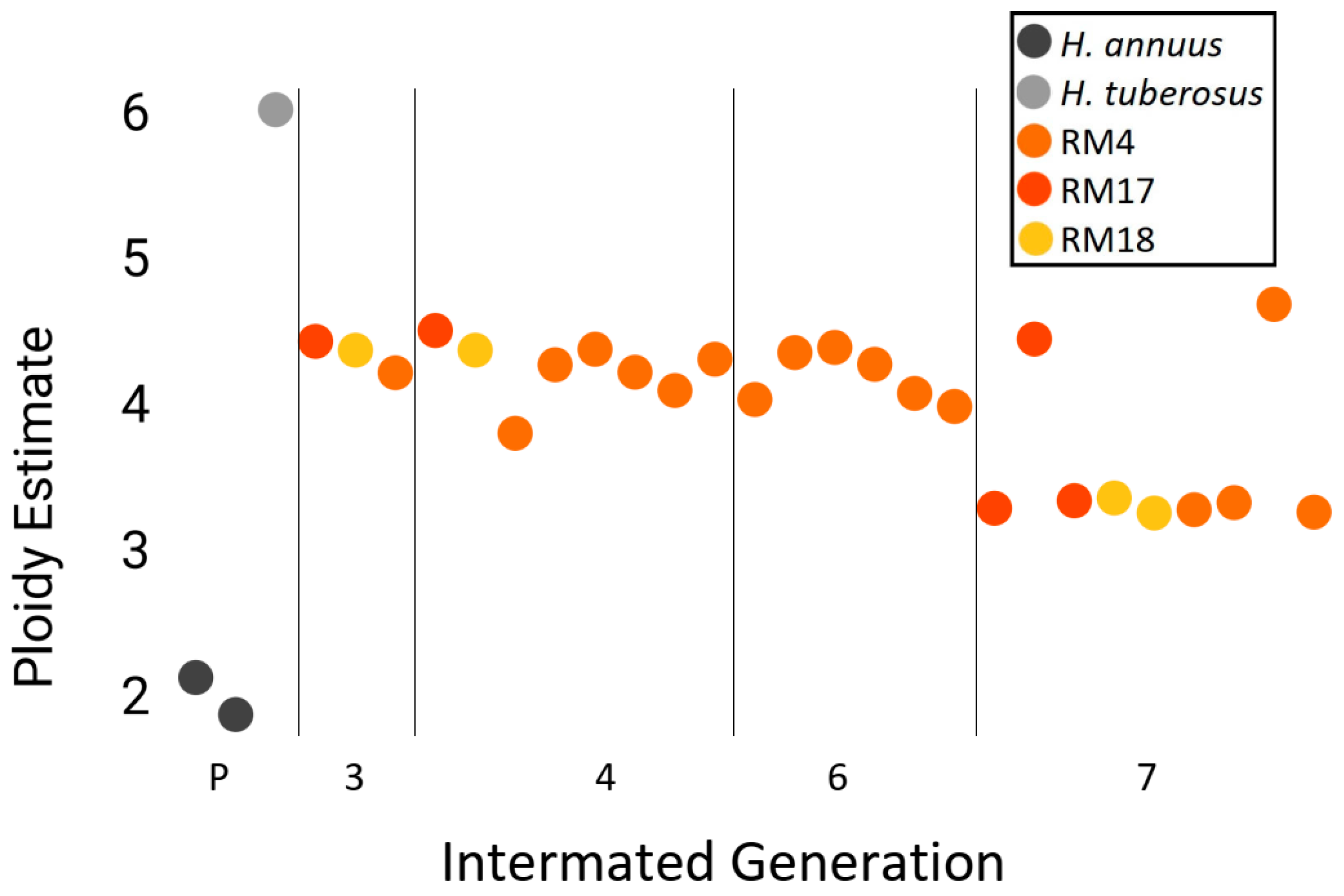
3.4. Genomic Stability
4. Discussion
4.1. Fertility
4.2. Pairing Structure of Trigenomic Tetraploids
4.3. Toward the Ideotype
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.M.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B.H. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Knapp, S.; Van Der Heijden, M.G.A. A global meta-analysis of yield stability in organic and conservation agriculture. Nat. Commun. 2018, 9, 3632. [Google Scholar] [CrossRef] [PubMed]
- Knowler, D.; Bradshaw, B. Farmers’ adoption of conservation agriculture: A review and synthesis of recent research. Food Policy 2007, 32, 25–48. [Google Scholar] [CrossRef]
- Kassam, A.; Friedrich, T.; Derpsch, R. Global spread of Conservation Agriculture. Int. J. Environ. Stud. 2019, 76, 29–51. [Google Scholar] [CrossRef]
- Rockström, J.; Williams, J.; Daily, G.; Noble, A.; Matthews, N.; Gordon, L.; Wetterstrand, H.; DeClerck, F.; Shah, M.; Steduto, P.; et al. Sustainable intensification of agriculture for human prosperity and global sustainability. Ambio 2017, 46, 4–17. [Google Scholar] [CrossRef]
- Kantar, M.B.; Tyl, C.E.; Dorn, K.M.; Zhang, X.; Jungers, J.M.; Kaser, J.M.; Schendel, R.R.; Eckberg, J.O.; Runck, B.C.; Bunzel, M.; et al. Perennial Grain and Oilseed Crops. Annu. Rev. Plant Boil. 2016, 67, 703–729. [Google Scholar] [CrossRef]
- Robertson, G.P. Greenhouse Gases in Intensive Agriculture: Contributions of Individual Gases to the Radiative Forcing of the Atmosphere. Science 2000, 289, 1922–1925. [Google Scholar] [CrossRef]
- Sperow, M.; Eve, M.; Paustian, K. Potential Soil C Sequestration on U.S. Agricultural Soils. Clim. Chang. 2003, 57, 319–339. [Google Scholar] [CrossRef]
- Culman, S.W.; Snapp, S.S.; Ollenburger, M.; Basso, B.; DeHaan, L.R. Soil and Water Quality Rapidly Responds to the Perennial Grain Kernza Wheatgrass. Agron. J. 2013, 105, 735. [Google Scholar] [CrossRef]
- Cox, T.S.; Glover, J.D.; Van Tassel, D.L.; Cox, C.M.; DeHaan, L.R. Prospects for Developing Perennial Grain Crops. BioScience 2006, 56, 649. [Google Scholar] [CrossRef]
- Glover, J.D.; Reganold, J.P.; Bell, L.W.; Borevitz, J.; Brummer, E.C.; Buckler, E.S.; Cox, C.M.; Cox, T.S.; Crews, T.E.; Culman, S.W.; et al. Increased Food and Ecosystem Security via Perennial Grains. Science 2010, 328, 1638–1639. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B. Breeding crop plants with deep roots: Their role in sustainable carbon, nutrient and water sequestration. Ann. Bot. 2011, 108, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Kort, J.; Collins, M.; Ditsch, D. A review of soil erosion potential associated with biomass crops. Biomass Bioenergy 1998, 14, 351–359. [Google Scholar] [CrossRef]
- Gyssels, G.; Poesen, J.; Bochet, E.; Li, Y. Impact of plant roots on the resistance of soils to erosion by water: A review. Prog. Phys. Geogr. Earth Environ. 2005, 29, 189–217. [Google Scholar] [CrossRef]
- Randall, G.; Goss, M. Nitrate Losses to Surface Water through Subsurface, Tile Drainage. In Nitrogen in the Environment: Sources, Problems and Management; Follett, R.F., Hatfield, J.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 95–122. [Google Scholar]
- Meyer, R.S.; Duval, A.E.; Jensen, H.R. Patterns and processes in crop domestication: An historical review and quantitative analysis of 203 global food crops. New Phytol. 2012, 196, 29–48. [Google Scholar] [CrossRef]
- Gaut, B.S.; Díez, C.M.; Morrell, P.L. Genomics and the Contrasting Dynamics of Annual and Perennial Domestication. Trends Genet. 2015, 31, 709–719. [Google Scholar] [CrossRef]
- Kantar, M.B.; Nashoba, A.R.; Anderson, J.E.; Blackman, B.K.; Rieseberg, L.H. The Genetics and Genomics of Plant Domestication. BioScience 2017, 67, 971–982. [Google Scholar] [CrossRef]
- Lye, Z.N.; Purugganan, M.D. Copy Number Variation in Domestication. Trends Plant Sci. 2019, 24, 352–365. [Google Scholar] [CrossRef]
- Runck, B.C.; Kantar, M.B.; Jordan, N.R.; Anderson, J.A.; Wyse, D.L.; Eckberg, J.O.; Barnes, R.J.; Lehman, C.L.; DeHaan, L.R.; Stupar, R.M.; et al. The Reflective Plant Breeding Paradigm: A Robust System of Germplasm Development to Support Strategic Diversification of Agroecosystems. Crop Sci. 2014, 54, 1939–1948. [Google Scholar] [CrossRef]
- DeHaan, L.R.; Van Tassel, D.L.; Anderson, J.A.; Asselin, S.R.; Barnes, R.; Baute, G.J.; Cattani, D.J.; Culman, S.W.; Dorn, K.M.; Hulke, B.S.; et al. A Pipeline Strategy for Grain Crop Domestication. Crop Sci. 2016, 56, 917. [Google Scholar] [CrossRef]
- Fernie, A.R.; Yan, J. De Novo Domestication: An Alternative Route toward New Crops for the Future. Mol. Plant 2019, 12, 615–631. [Google Scholar] [CrossRef] [PubMed]
- DeHaan, L.; Christians, M.; Crain, J.; Poland, J. Development and Evolution of an Intermediate Wheatgrass Domestication Program. Sustainability 2018, 10, 1499. [Google Scholar] [CrossRef]
- Van Tassel, D.L.; Albrecht, K.A.; Bever, J.D.; Boe, A.A.; Brandvain, Y.; Crews, T.E.; Gansberger, M.; Gerstberger, P.; González-Paleo, L.; Hulke, B.S.; et al. Accelerating Domestication: An Opportunity to Develop New Crop Ideotypes and Breeding Strategies Informed by Multiple Disciplines. Crop Sci. 2017, 57, 1274. [Google Scholar] [CrossRef]
- Braun, L.; Gillman, J.; Hoover, E.; Russelle, M. Nitrogen fertilization for young established hybrid hazelnuts in the Upper Midwest of the United States of America. Can. J. Plant Sci. 2011, 91, 907–918. [Google Scholar] [CrossRef]
- Wilkins, P.W.; Humphreys, M.O. Progress in breeding perennial forage grasses for temperate agriculture. J. Agric. Sci. 2003, 140, 129–150. [Google Scholar] [CrossRef]
- Karp, A.; Hanley, S.J.; Trybush, S.O.; MacAlpine, W.; Pei, M.; Shield, I. Genetic Improvement of Willow for Bioenergy and BiofuelsFree Access. J. Integr. Plant Boil. 2011, 53, 151–165. [Google Scholar] [CrossRef]
- Allwright, M.R.; Taylor, G.; Information, P.E.K.F.C. Molecular Breeding for Improved Second Generation Bioenergy Crops. Trends Plant Sci. 2016, 21, 43–54. [Google Scholar] [CrossRef]
- Kantar, M.B.; Hüber, S.; Herman, A.; Bock, D.G.; Baute, G.; Betts, K.; Ott, M.; Brandvain, Y.; Wyse, D.; Stupar, R.M.; et al. Neo-Domestication of an Interspecific Tetraploid × Population That Segregates for Perennial Habit. Genes 2018, 9, 422. [Google Scholar] [CrossRef]
- Kantar, M.; Betts, K.; Michno, J.-M.; Luby, J.J.; Morrell, P.L.; Hulke, B.S.; Stupar, R.; Wyse, D.L. Evaluating an interspecific Helianthus annuus × Helianthus tuberosus population for use in a perennial sunflower breeding program. Field Crops Res. 2014, 155, 254–264. [Google Scholar] [CrossRef]
- Ščibria, N.A. Hybrids between the Jerusalem artichoke (Helianthus tuberosus L.) and the sunflower(Helianthus annuus L.). C.R. Doklady L’Académie Sci. L’URSS 1936, 11, 193–196. [Google Scholar]
- Kostoff, D. Autosyndesis and structural hybridity in F1-hybrid Helianthus tuberosus L. x Helianthus annuus L. and their sequences. Genetica 1939, 21, 285–300. [Google Scholar] [CrossRef]
- Heiser, C.B.; Smith, D.M. Species crosses in Helianthus: II. Polyploid species. Rhodora 1964, 66, 344–358. [Google Scholar]
- Carter, J.F.; Whelan, E.D.P. Cytology and Interspecific Hybridization; American Society of Agronomy: Madison, WI, USA, 1978. [Google Scholar]
- Cauderon, Y. Selection pour la resistance au mildiou du tournesol a partir d’hybrides topinambour x tournesol. Ann. Améliof. Plantes 1970, 20, 363–373. [Google Scholar]
- Atlagic, J.; Dozet, B.; Škorić, D. Meiosis and Pollen Viability in Helianthus tuberosus L. and its Hybrids with Cultivated Sunflower. Plant Breed. 1993, 111, 318–324. [Google Scholar] [CrossRef]
- Atlagic, J.; Terzic, S. Cytogenetic study of hexaploid species Helianthus tuberosus and its F1 and BC1F1 hybrids with cultivated sunflower, H. annuus. Genetika 2006, 38, 203–213. [Google Scholar] [CrossRef]
- Hulke, B.S.; Wyse, D.L. Using interspecific hybrids with Helianthus tuberosus L. to transfer genes for quantitative traits into cultivated sunflower, H. annuus L. In Proceedings of the 17th International Sunflower Conference, Cordoba, Spain, 8–12 June 2008; pp. 729–734. [Google Scholar]
- Bock, D.G.; Kane, N.C.; Ebert, D.P.; Rieseberg, L.H. Genome skimming reveals the origin of the Jerusalem Artichoke tuber crop species: Neither from Jerusalem nor an artichoke. New Phytol. 2014, 201, 1021–1030. [Google Scholar] [CrossRef]
- Donald, C.M. The breeding of crop ideotypes. Euphytica 1968, 17, 385–403. [Google Scholar] [CrossRef]
- Miller, J.; Gulya, T.; Vick, B. Registration of Two Maintainer (HA 434 and HA 435) and Three Restorer (RHA 436 to RHA 438) High Oleic Oilseed Sunflower Germplasms. Crop Sci. 2004, 44, 1034. [Google Scholar] [CrossRef]
- Peterson, R.; Slovin, J.P.; Chen, C. A simplified method for differential staining of aborted and non-aborted pollen grains. Int. J. Plant Boil. 2010, 1, 13. [Google Scholar] [CrossRef]
- Singhal, V.K.; Kumar, P. Impact of cytomixis on meiosis, pollen viability and pollen size in wild populations of Himalayan poppy (Meconopsis aculeata Royle). J. Biosci. 2008, 33, 371–380. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Realini, M.F.; Poggio, L.; Cámara-Hernández, J.; González, G.E. Intra-specific variation in genome size in maize: Cytological and phenotypic correlates. AoB Plants 2016, 8, lv138. [Google Scholar] [CrossRef] [PubMed]
- Bomblies, K.; Higgins, J.D.; Yant, L. Meiosis evolves: Adaptation to external and internal environments. New Phytol. 2015, 208, 306–323. [Google Scholar] [CrossRef] [PubMed]
- Cisneros, A.; Tel-Zur, N. Evaluation of Interspecific-Interploid Hybrids (F1) and Back Crosses (BC1) in Hylocereus Species (Cactaceae). Meiosis Mol. Mech. Cytogenet. Divers. 2012. [Google Scholar] [CrossRef]
- Tel-Zur, N. Chromosome Doubling in Vine Cacti Hybrids. J. Hered. 2003, 94, 329–333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tel-Zur, N.; Abbo, S.; Bar-Zvi, D.; Mizrahi, Y. Clone identification and genetic relationship among vine cacti from the genera Hylocereus and Selenicereus based on RAPD analysis. Sci. Hortic. 2004, 100, 279–289. [Google Scholar] [CrossRef]
- De Nettancourt, D.; Grant, W.F. La Cytogénétique de Lotus (Leguminosae). Cytologia 1964, 29, 191–195. [Google Scholar] [CrossRef]
- Cauderon, Y. Analyse cytogénétique d’hybrides entre Helianthus tuberosus et H. annuus: Conséquences en matière de sélection. Ann. L’amélioration Plantes 1965, 15, 243–261. [Google Scholar]
- Ustinova, E.I. Study of interspecies hybrids, Helianthus annuus L. × Helianthus tuberosus L. Byul. Moskov Obshchest. Ospytatelei Prirody Otd. Biol. 1966, 71, 100–106. [Google Scholar]
- Cedeno, J.R.; McMullen, M.S.; Miller, J.F. Cytogenetic relationship between Helainthus annuus L. and H. tuberosus L. In Proceedings of the 11th International Sunflower Conference, Mar del Plata, Argentina, 10–13 March 1985; pp. 541–546. [Google Scholar]
- Kostoff, D. A contribution to the meiosis of Helianthus tuberosus L. Z. Pflanzenzuchtg. 1934, 19, 423–438. [Google Scholar]
- Anisimova, I.N. On the nature of polyploid genomes of sunflower species. Bull. N. I. Vavilov Inst. Plant Ind. 1982, 118, 27–29. [Google Scholar]
- Mason, A.S.; Huteau, V.; Eber, F.; Coriton, O.; Yan, G.; Nelson, M.N.; Cowling, W.A.; Chèvre, A.-M. Genome structure affects the rate of autosyndesis and allosyndesis in AABC, BBAC and CCAB Brassica interspecific hybrids. Chromosom. Res. 2010, 18, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.; Schemske, D.W. Neopolyploidy in Flowering Plants. Annu. Rev. Ecol. Syst. 2002, 33, 589–639. [Google Scholar] [CrossRef]
- Gaeta, R.T.; Chris Pires, J. Homoeologous recombination in allopolyploids: The polyploid ratchet. New Phytol. 2010, 186, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Boumil, R.M.; Kemp, B.; Angelichio, M.; Nilsson-Tillgren, T.; Dawson, D.S. Meiotic segregation of a homeologous chromosome pair. Mol. Genet. Genom. 2003, 268, 750–760. [Google Scholar]
- Santos, J.L.; Alfaro, D.; Sanchez-Moran, E.; Armstrong, S.J.; Franklin, F.C.H.; Jones, G.H. Partial diploidization of meiosis in autotetraploid Arabidopsis thaliana. Genetics 2003, 165, 1533–1540. [Google Scholar]
- Srivastava, S.; Lavania, U.C. Meiotic Regularization, Restoration of Seed Fertility and Alkaloid Content in the Induced Autotetraploids of Hyoscyamus albus L. Plant Breed. 1990, 104, 160–166. [Google Scholar] [CrossRef]
- Hilpert, G. Effect of selection for meiotic behaviour in autotetraploid rye. Hereditas 1957, 43, 318–322. [Google Scholar] [CrossRef]
- Bomblies, K.; Jones, G.; Franklin, C.; Zickler, D.; Kleckner, N. The challenge of evolving stable polyploidy: Could an increase in “crossover interference distance” play a central role? Chromosoma 2016, 125, 287–300. [Google Scholar] [CrossRef]
- Badouin, H.; Gouzy, J.; Grassa, C.J.; Murat, F.; Staton, S.E.; Cottret, L.; Lelandais-Brière, C.; Owens, G.L.; Carrère, S.; Mayjonade, B.; et al. The sunflower genome provides insights into oil metabolism, flowering and Asterid evolution. Nature 2017, 546, 148–152. [Google Scholar] [CrossRef]
| Description | Sample Size (n) for a Specified IM Generation | ||||||
|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | 8 | ||
| Perenniality | A score from zero (annual: zero tuber presence) to five. A score of at least one implies that, minimally, a single shoot survived the first winter, with increasing scores indicating more abundant tubers with a stronger resemblance to those of H. tuberosus; Figure 4A. | 12 | 24 | 47 | 22 | 16 | 9 |
| Seed width | The average widths of three seeds per plant. | 12 | 22 | 25 | 8 | 16 | 9 |
| Disk diameter | The average diameter of composite heads of a plant. | 12 | 23 | 43 | 24 | 18 | 9 |
| Heads per plant | A measure of apical dominance, with more heads indicating less apical dominance. | 12 | 23 | 43 | 24 | 18 | 9 |
| Seeds per head | The average seeds per head of a plant. We use seeds per head as our metric for seed set and female fertility. | 12 | 23 | 43 | 24 | 18 | 9 |
| Pollen staining | Proportion (of 50) stained pollen grains of a plant. | 6 | 16 | 42 | 2 | 0 | 0 |
| Pollen size variance | Pollen diameter variance was determined for the same 50 pollen grains per individual using ImageJ software. | 6 | 16 | 42 | 2 | 0 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekar, J.M.; Betts, K.J.; Herman, A.C.; Stupar, R.M.; Wyse, D.L.; Brandvain, Y.; Kantar, M.B. Domestication in Real Time: The Curious Case of a Trigenomic Sunflower Population. Agronomy 2019, 9, 704. https://doi.org/10.3390/agronomy9110704
Ekar JM, Betts KJ, Herman AC, Stupar RM, Wyse DL, Brandvain Y, Kantar MB. Domestication in Real Time: The Curious Case of a Trigenomic Sunflower Population. Agronomy. 2019; 9(11):704. https://doi.org/10.3390/agronomy9110704
Chicago/Turabian StyleEkar, Jill M., Kevin J. Betts, Adam C. Herman, Robert M. Stupar, Donald L. Wyse, Yaniv Brandvain, and Michael B. Kantar. 2019. "Domestication in Real Time: The Curious Case of a Trigenomic Sunflower Population" Agronomy 9, no. 11: 704. https://doi.org/10.3390/agronomy9110704
APA StyleEkar, J. M., Betts, K. J., Herman, A. C., Stupar, R. M., Wyse, D. L., Brandvain, Y., & Kantar, M. B. (2019). Domestication in Real Time: The Curious Case of a Trigenomic Sunflower Population. Agronomy, 9(11), 704. https://doi.org/10.3390/agronomy9110704





