Seed Germination and Early Seedling Growth of Barley at Negative Water Potentials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Seed Germination Assays and Treatments
2.3. Statistical Analysis
3. Results
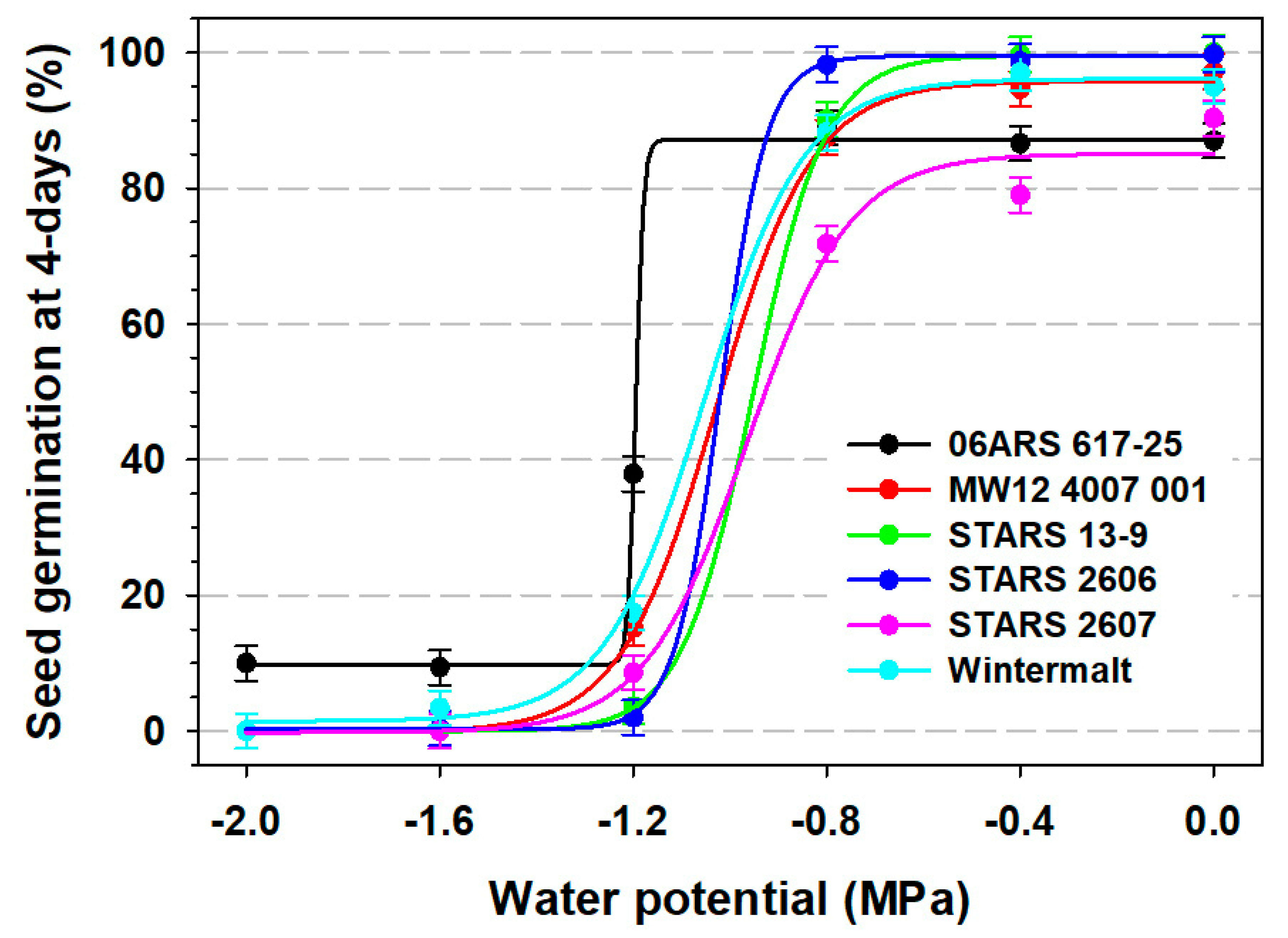
3.1. Seed Germination
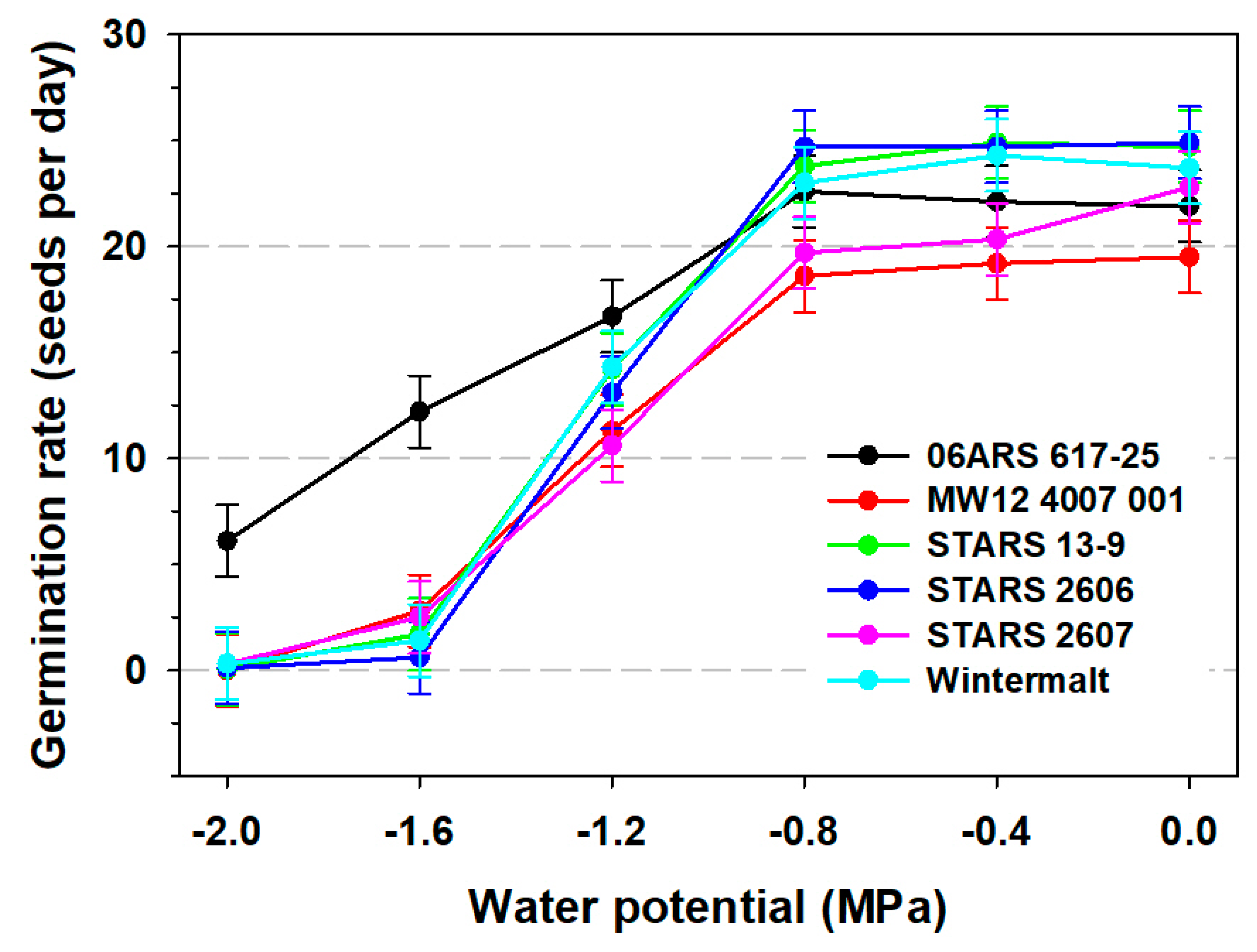
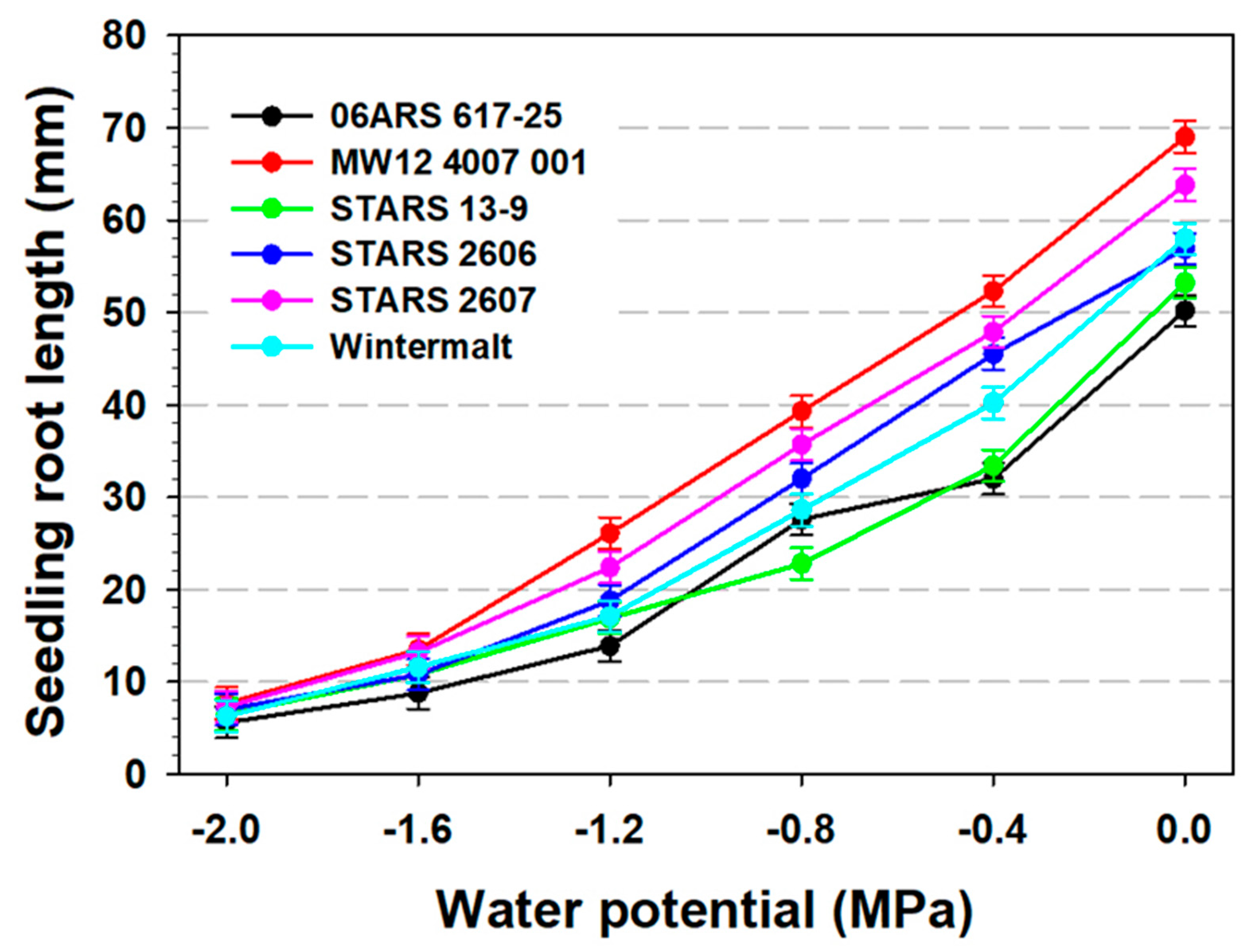
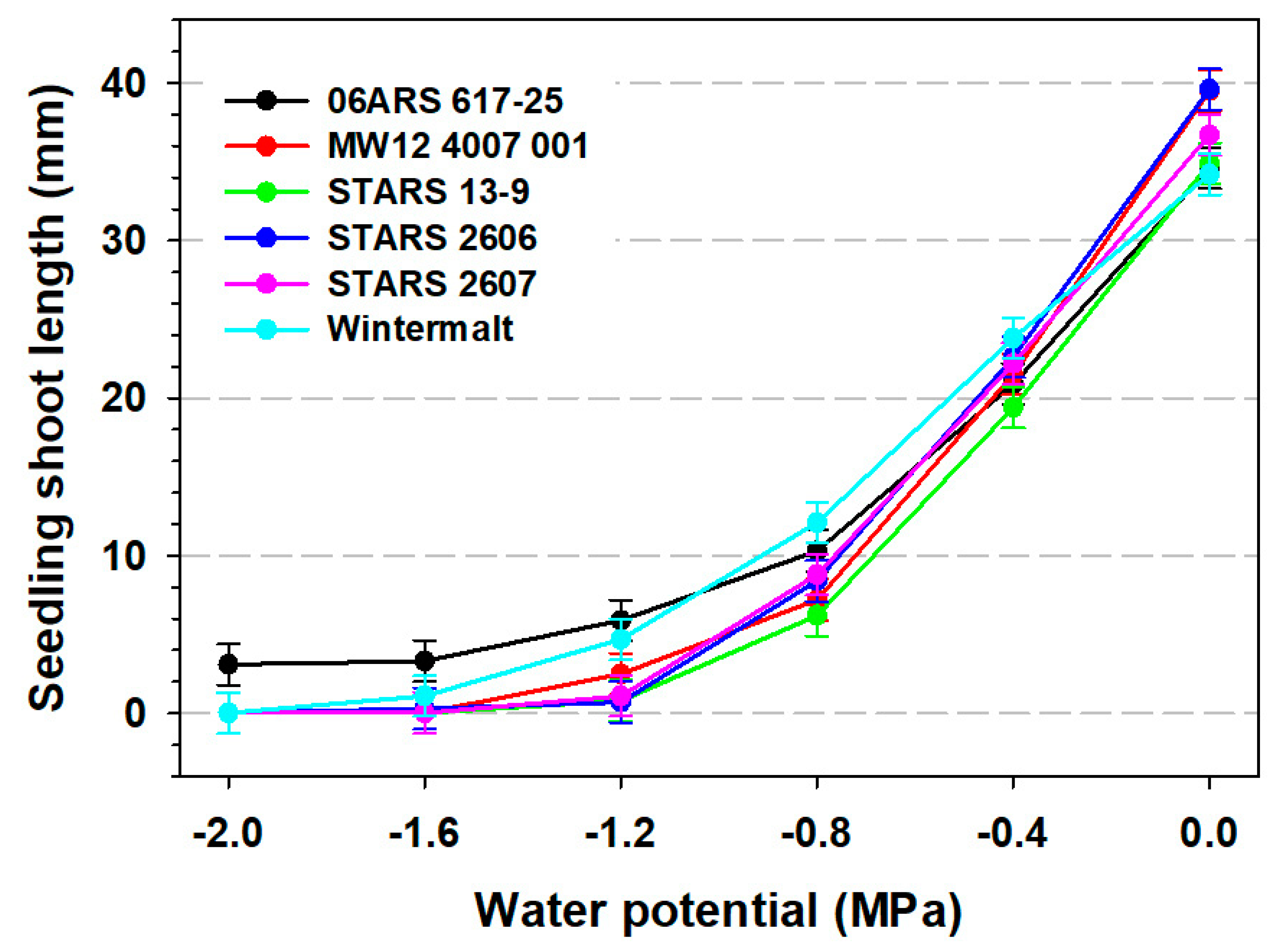
3.2. Seedling Root and Shoot Measurements and 100-Seed Weight
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Stambaugh, M.C.; Guyette, R.P.; McMurry, E.R.; Cook, E.R.; Meko, D.M.; Lupo, A.R. Drought duration and frequency in the U.S. corn belt during the last Millennium (AD 992–2004). Agric. For. Meteorol. 2011, 151, 154–162. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration [NOAA]. National Centers for Environmental Information. Available online: https://www.ncdc.noaa.gov/news/drought-monitoring-economic-environmental-and-social-impacts (accessed on 6 June 2019).
- Jeong, D.I.; Sushama, L.; Khaliq, M.N. The role of temperature in drought and projections over North America. Clim. Chang. 2014, 127, 289–303. [Google Scholar] [CrossRef]
- Federal Emergency Management Agency (FEMA). National Mitigation Strategy: Partnerships for Building Safer Communities; Federal Emergency Management Agency (FEMA): Washington, DC, USA, 1995. [Google Scholar]
- Blake, A.K. Viability and germination of seeds and early life history of prairie plants. Ecol. Monogr. 1935, 5, 405–460. [Google Scholar] [CrossRef]
- Springer, T.L. Recurrent selection for increased seed germination in sand bluestem (Andropogon hallii). Plant Breed. 2011, 131, 198–202. [Google Scholar] [CrossRef]
- Springer, T.L.; Wynia, R.L.; Rea, G.L. Field emergence and plant density of sand bluestem lines selected for increased seed germination. Crop Sci. 2012, 52, 2826–2829. [Google Scholar] [CrossRef]
- Springer, T.L.; Wynia, R.L.; Rea, G.L. Registration of ‘Centennial’ sand bluestem. J. Plant Regist. 2014, 8, 248–252. [Google Scholar] [CrossRef]
- Springer, T.L.; Dewald, C.L.; Sims, P.L.; Gillen, R.L.; Louthan, V.H.; Cooper, W.J.; Taliaferro, C.M.; Wynia, R.L.; Houck, M.J., Jr.; Esquivel, R.G.; et al. Registration of ‘Chet’ sand bluestem. Crop Sci. 2005, 45, 2125–2126. [Google Scholar] [CrossRef]
- Springer, T.L. Recurrent selection increases seed germination in little bluestem (Schizachyrium scoparium). Euphytica 2017, 213, 279. [Google Scholar] [CrossRef]
- Hodgson-Kratky, K.J.M.; Stoffyn, O.M.; Wolyn, D.J. Recurrent selection for improved germination under water stress in Russian dandelion. J. Am. Soc. Hortic. Sci. 2017, 142, 85–91. [Google Scholar] [CrossRef]
- Hodgson-Kratky, K. Breeding for Improved Germination under Water Stress, and Genetic Analyses of Flowering Habit and Male Sterility in Russian Dandelion (Taraxacum kok-saghyz). Ph.D. Thesis, MS-The University of Guelph, Guelph, ON, Canada, 2015. [Google Scholar]
- Czyczylo-Mysza, I.; Marciṅska, I.; Skrzypek, E.; Cyganek, K.; Juzoṅ, K.; Karbarz, M. QTL mapping for germination of seeds obtained from previous wheat generation under drought. Cent. Eur. J. Biol. 2014, 9, 374–382. [Google Scholar] [CrossRef]
- Palmer, T.P. Progressive improvement in self-fertilized crops. Heredity 1953, 7, 127–129. [Google Scholar] [CrossRef]
- Parlevliet, J.E.; van Ommeren, A. Recurrent selection for grain yield in early generations of two barley populations. Euphytica 1988, 38, 175–184. [Google Scholar] [CrossRef]
- Jensen, N.F.; Edwards, L.H.; Smith, E.L.; Sorrells, M.E. Registration of Wintermalt barley. Crop Sci. 1982, 22, 157. [Google Scholar] [CrossRef]
- Parmar, M.T.; Moore, R.P. Carbowax 6000, mannitol, and sodium chloride for simulating drought conditions in germination studies of corn (Zea mays L.) of strong and weak vigor. Agron. J. 1968, 60, 192–195. [Google Scholar] [CrossRef]
- Bell, D.T. The Influence of Osmotic Pressure in Tests for Allelopathy. Trans. Ill. State Acad. Sci. 1974, 67, 312–317. [Google Scholar]
- Maguire, J.D. Speed of Germination-aid in Selection and Evaluation for Seedling Emergence and Vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- SAS Institute, Inc. SAS/STAT®User’s Guide; Version 9.4; SAS Institute, Inc.: Cary, NC, USA, 2010. [Google Scholar]
- Introduction to SAS. UCLA: Statistical Consulting Group. Available online: https://stats.idre.ucla.edu/sas/faq/how-can-i-generate-a-dose-response-curve-sas/ (accessed on 14 August 2019).
- Helmerick, R.H.; Pfeifer, R.P. Differential varietal response of winter wheat germination and early growth to controlled limited moisture conditions. Agron. J. 1954, 46, 560–562. [Google Scholar] [CrossRef]
- Wiggins, S.C.; Gardner, F.P. Effectiveness of various solutions for simulating drought conditions as measured by germination and seedling growth. Agron. J. 1959, 51, 315–318. [Google Scholar] [CrossRef]
- Larsen, S.U.; Bailly, C.; Côme, D.; Corbineau, F. Use of the hydrothermal time model to analyze interacting effects of water and temperature on germination of three grass species. Seed Sci. Res. 2004, 14, 35–50. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Response of wheat and barley during germination to seed osmopriming at different water potential. J. Agron. Crop Sci. 1998, 181, 229–235. [Google Scholar] [CrossRef]
- Springer, T.L. Germination and early seedling growth of chaffy-seeded grasses at negative water potentials. Crop Sci. 2005, 45, 2075–2080. [Google Scholar] [CrossRef]
- Springer, T.L.; Goldman, J.J. Seed germination of five Poa species at negative water potentials. Am. J. Plant Sci. 2016, 7, 601–611. [Google Scholar] [CrossRef]
- Reid, D.A. Morphology and anatomy of the barley plant. In Barley; Rasmusson, R.C., Ed.; ASSA, CSSA, SSSA: Madison, WI, USA, 1985; pp. 73–101, Number 26. [Google Scholar]
- Shaidaee, G.; Dahl, B.E.; Hansen, R.M. Germination and emergence of different age seeds of six grasses. J. Range Manag. 1969, 22, 240–243. [Google Scholar] [CrossRef]

| Main Effects | Seed Germination (%) | Root Length (mm) | Shoot Length (mm) | Germination Rate | 100- Seed Weight (g) | |
|---|---|---|---|---|---|---|
| 4-Day | 7-Day | |||||
| Barley lines | ||||||
| 06ARS 617-25 | 53.3 a1 | 78.5 a | 23.0 d | 13.0 a | 16.9 a | 5.2 a |
| MW12 4007 001 | 48.9 b | 67.2 bc | 34.7 a | 11.8 bc | 11.8 a | 3.6 c |
| STARS 13-9 | 48.6 b | 67.9 bc | 23.9 d | 10.2 d | 14.9 a | 3.6 c |
| STARS 2606 | 49.8 b | 65.5 c | 28.5 c | 11.9 bc | 14.7 a | 4.8 b |
| STARS 2607 | 41.5 c | 58.4 d | 31.7 b | 11.5 c | 12.7 a | 3.2 c |
| Wintermalt | 50.1 b | 70.6 b | 27.0 c | 12.7 ab | 15.5 a | 4.4 b |
| SE of mean | 1.5 | 1.5 | 1.0 | 0.8 | 1.5 | 0.1 |
| Water potential (MPa) | ||||||
| 0 | 94.7 a | 95.4 a | 58.5 a | 36.6 a | 22.9 a | 4.2 a |
| −0.4 | 92.6 a | 94.6 a | 41.9 b | 21.7 b | 22.5 a | 4.1 a |
| −0.8 | 87.5 b | 96.4 a | 31.0 c | 8.8 c | 22.1 a | 4.2 a |
| −1.2 | 14.1 c | 85.5 b | 19.2 d | 2.6 d | 13.4 b | 4.2 a |
| −1.6 | 2.0 d | 30.1 c | 11.5 e | 0.8 d | 4.5 c | 4.2 a |
| −2.0 | 1.4 d | 6.1 d | 6.7 f | 0.5 d | 1.0 d | 4.1 a |
| SE of mean | 1.6 | 1.5 | 1.0 | 1.0 | 0.9 | 0.1 |
| Barley Line | y0 | a | x0 | Slope (b) | R2 |
|---|---|---|---|---|---|
| 4-day seed germination | |||||
| 06ARS 617-25 | 9.75 | 77.42 | −1.19 | 0.008 | 0.96 |
| MW12 4007 001 | −0.16 | 95.89 | −1.03 | 0.100 | 0.98 |
| STARS 13-9 | −0.04 | 99.53 | −0.95 | 0.075 | 0.98 |
| STARS 2606 | 0.25 | 99.25 | −1.02 | 0.048 | 0.99 |
| STARS 2607 | −0.21 | 85.29 | −0.97 | 0.028 | 0.96 |
| Wintermalt | 1.46 | 94.63 | −1.05 | 0.104 | 0.98 |
| 7-day seed germination | |||||
| 06ARS 617-25 | −14.28 | 105.10 | −1.96 | 0.180 | 0.89 |
| MW12 4007 001 | −0.57 | 97.14 | −1.49 | 0.110 | 0.97 |
| STARS 13-9 | −0.04 | 99.88 | −1.45 | 0.071 | 0.99 |
| STARS 2606 | 0.49 | 99.18 | −1.35 | 0.069 | 0.99 |
| STARS 2607 | −0.04 | 87.88 | −1.39 | 0.151 | 0.94 |
| Wintermalt | −21.63 | 118.00 | −1.69 | 0.217 | 0.92 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Springer, T.L.; Mornhinweg, D.W. Seed Germination and Early Seedling Growth of Barley at Negative Water Potentials. Agronomy 2019, 9, 671. https://doi.org/10.3390/agronomy9110671
Springer TL, Mornhinweg DW. Seed Germination and Early Seedling Growth of Barley at Negative Water Potentials. Agronomy. 2019; 9(11):671. https://doi.org/10.3390/agronomy9110671
Chicago/Turabian StyleSpringer, Tim L., and Dolores W. Mornhinweg. 2019. "Seed Germination and Early Seedling Growth of Barley at Negative Water Potentials" Agronomy 9, no. 11: 671. https://doi.org/10.3390/agronomy9110671
APA StyleSpringer, T. L., & Mornhinweg, D. W. (2019). Seed Germination and Early Seedling Growth of Barley at Negative Water Potentials. Agronomy, 9(11), 671. https://doi.org/10.3390/agronomy9110671





