Changes in the Chemical Composition of Six Lettuce Cultivars (Lactuca sativa L.) in Response to Biofortification with Iodine and Selenium Combined with Salicylic Acid Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Plant Analysis
2.2.1. Analysis of Fresh Lettuce Heads (Leaves)
2.2.2. Analysis after Sample Drying
2.3. Data Analysis
3. Results
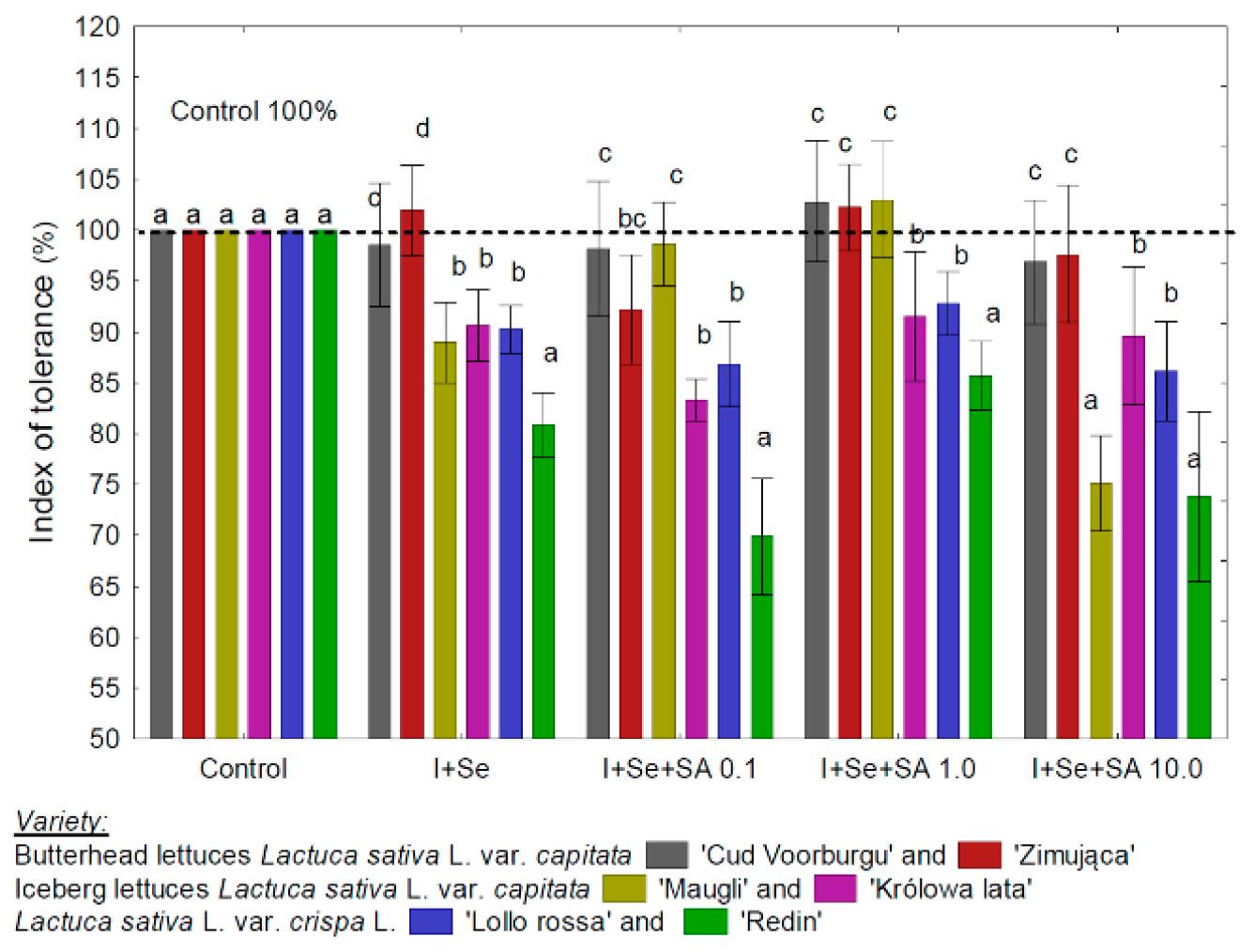
3.1. TI and Information on the Yield and Biofortification Effect
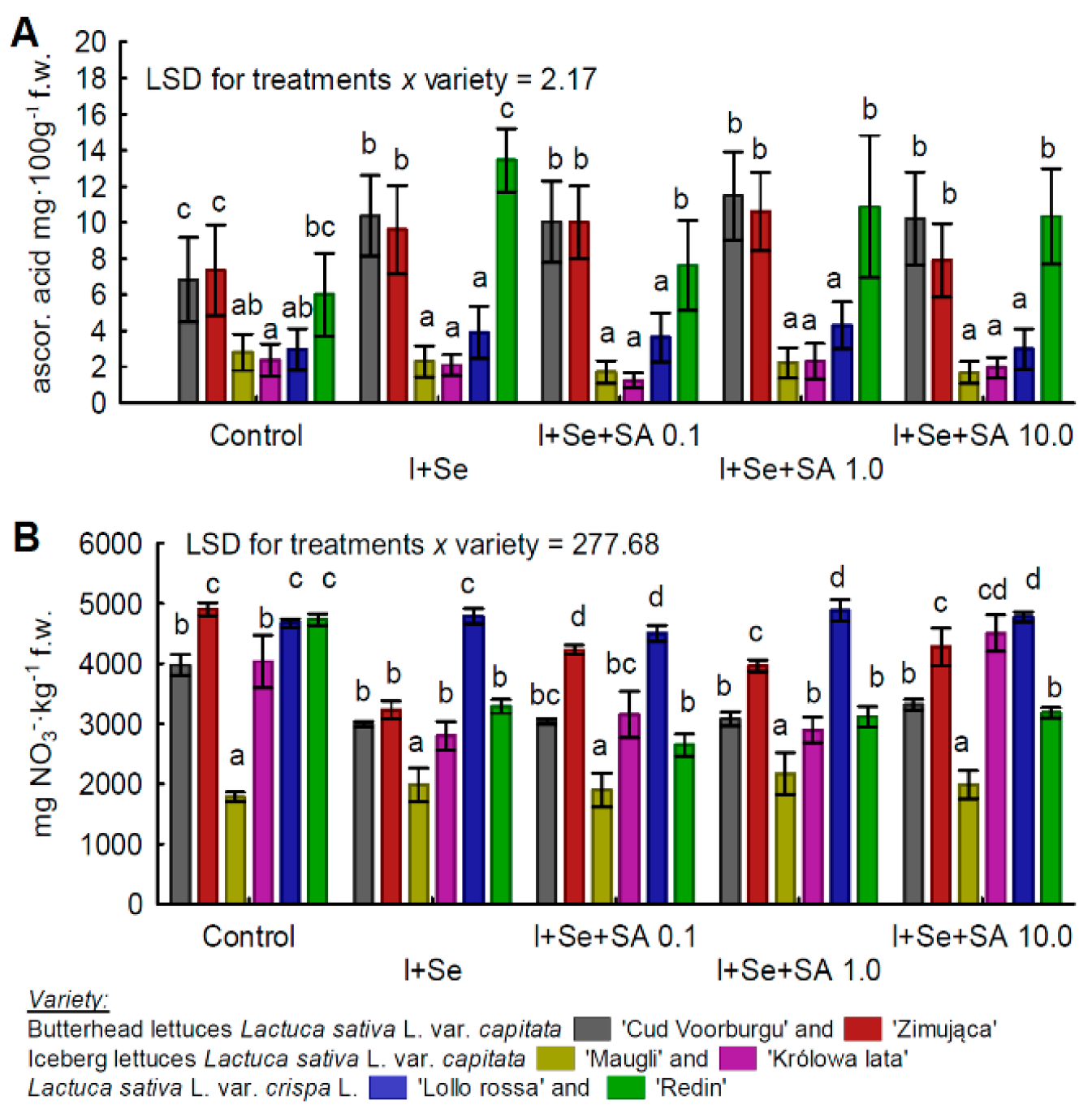
3.2. Ascorbic Acid and Nitrates(V)
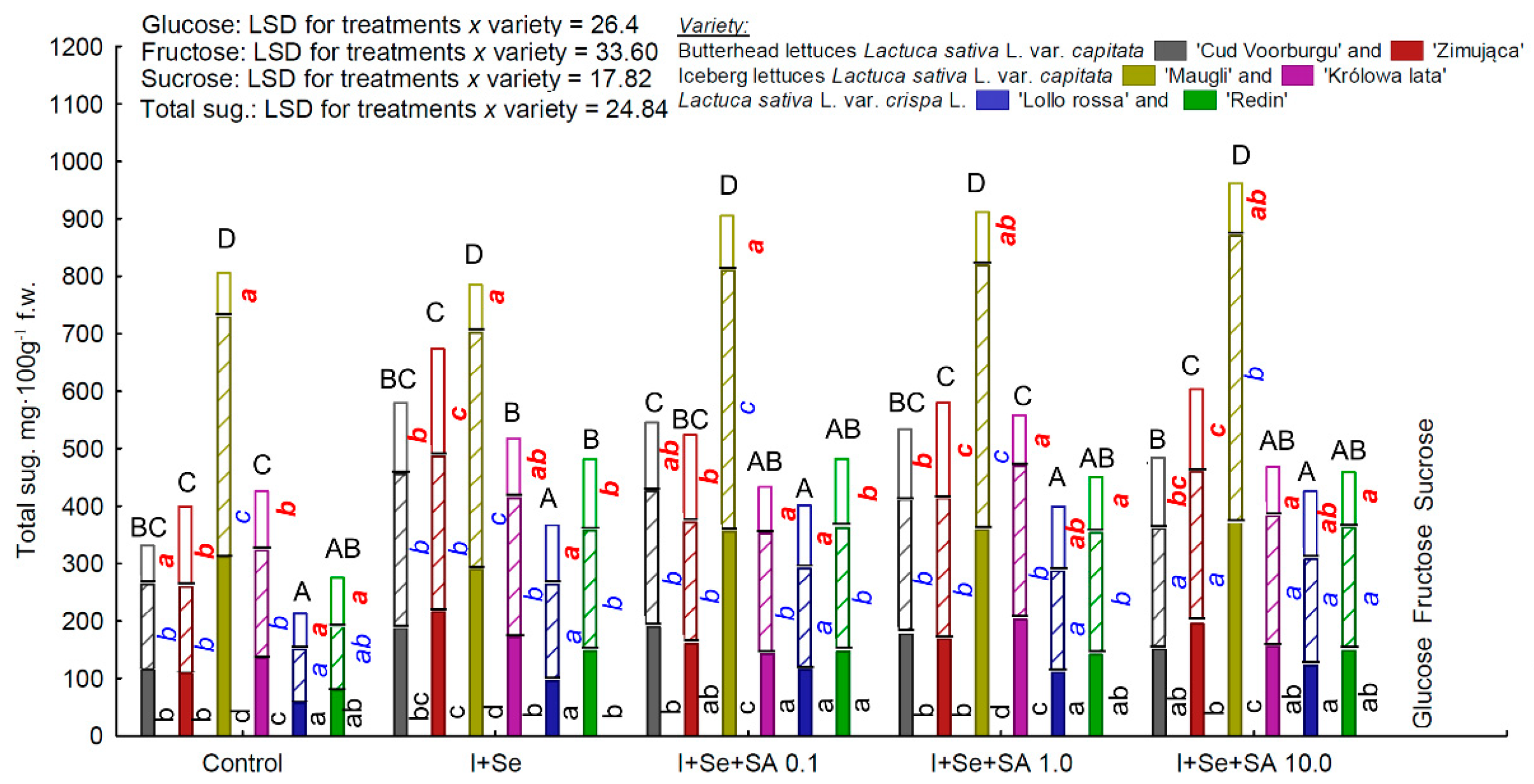
3.3. Sugars
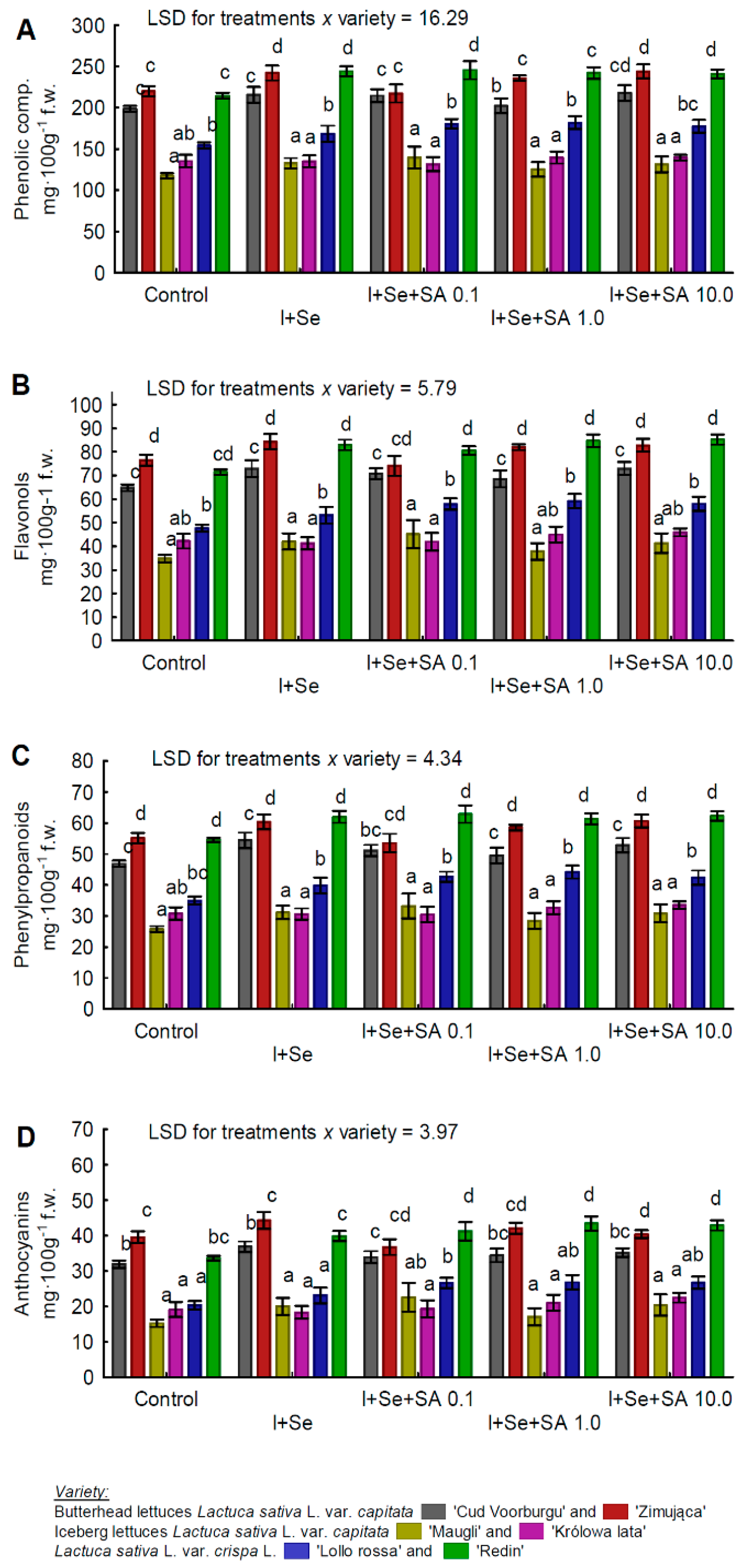
3.4. Phenols, Phenylpropanoids, Flavonols, and Anthocyanins
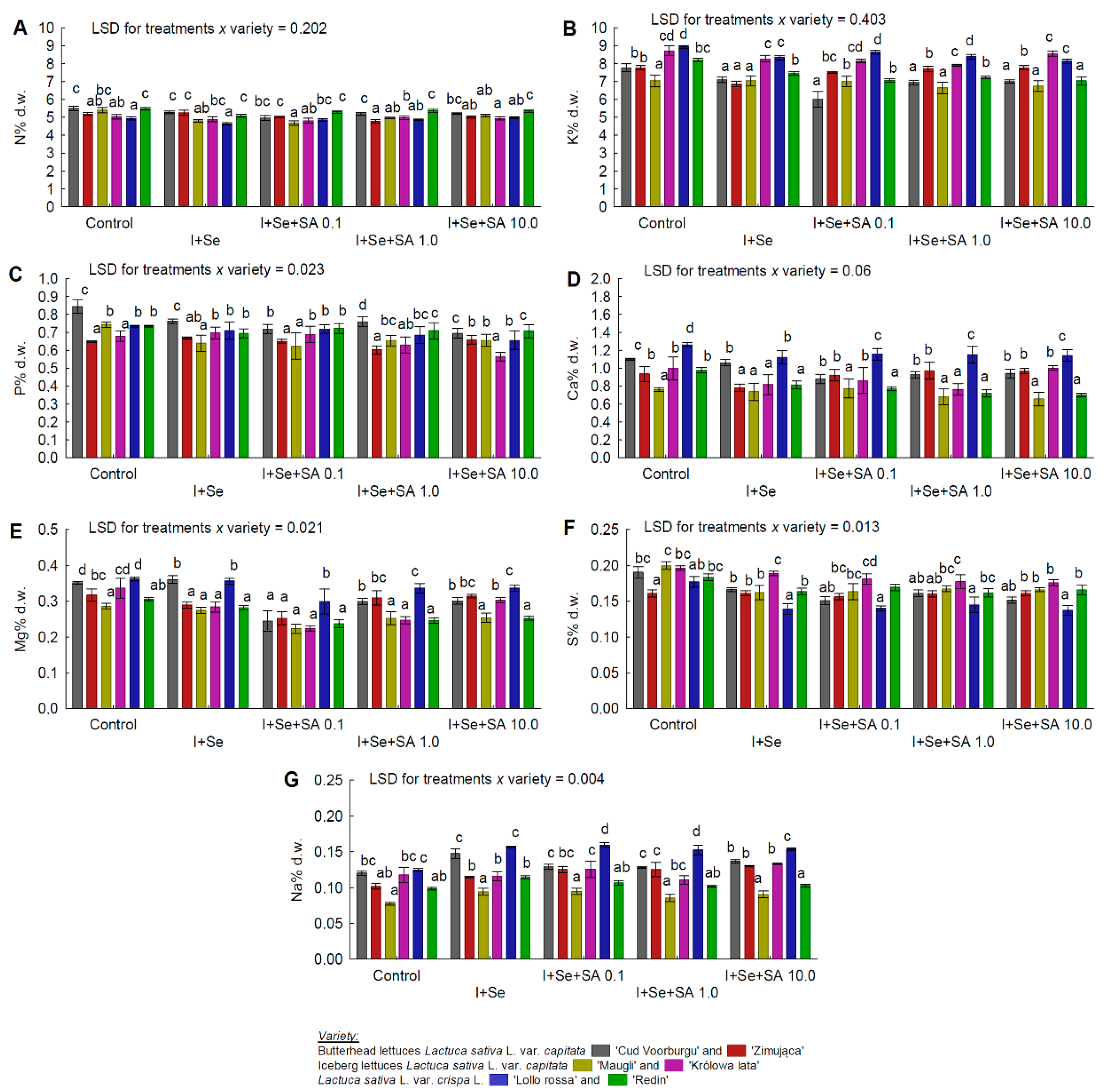
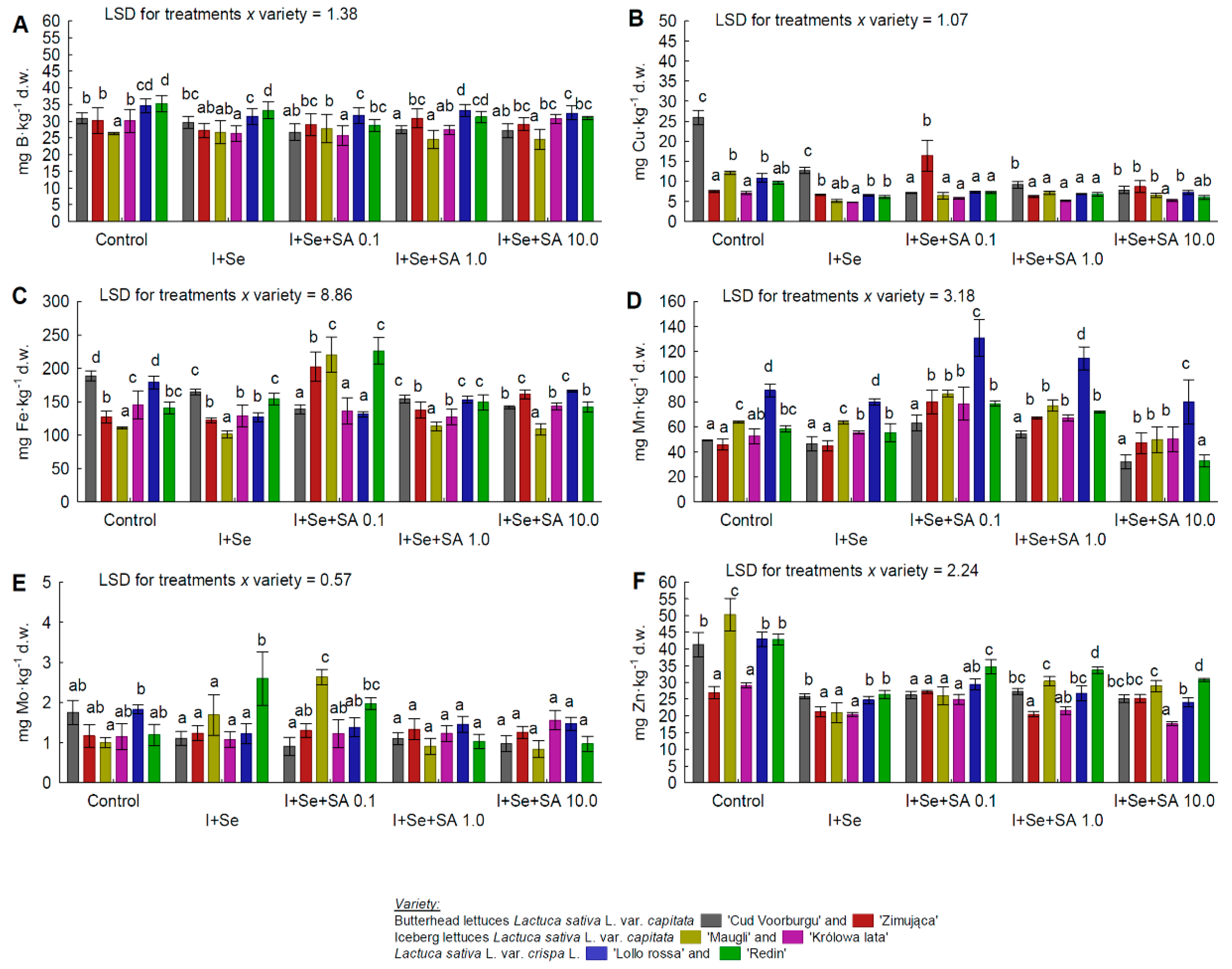
3.5. Macro- and Micronutrients
- No effect of SA application on the N content (in REDL “Lollorossa” and “Redin”);
- No increase in the Na content of the leaves of ICEL “Królowa lata”;
- No effect of the applied nutrient solutions on the Mo content of the leaves of BUTL “Cud Voorburgu”, ICEL “Królowa lata”, REDL “Lollorossa”, and BUTL “Zimująca”;
- In BUTL “Cud Voorburgu”, a reduction in the Mg content of the leaves but only in the combinations with SA application;
- In ICEL “Królowa lata”, a significant effect of SA at 10 mg∙dm−3 on increasing the Ca and B contents, as well as decreasing the P content;
- In the leaves of BUTL “Zimująca”, a significant increase in the Cu (after application of SA at 0.1 and 10 mg∙dm−3) and Fe (in all combinations with I, Se, and SA) contents. Also, for BUTL “Zimująca”, a significant reduction in the K content only after applying I+Se, a significant reduction in the P content only after applying I+Se+SA 1.0 mg∙dm−3, and no significant differences in the S content (all media tested) and N content (only I+Se).
4. Discussion
4.1. TI and Plant Chemical Composition—An Iindicator of Plant Physiological State
4.2. Accumulation of Nitrates(V) and Plant Nutritional Status with Respect to Macro- and Micronutrients
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Żuk-Gołaszewska, K.; Żerańska, A.; Krukowska, A.; Bojarczuk, J. Biofortification of the nutritional value of foods from the grain of Triticum durum Desf. by an agrotechnical method: A scientific review. J. Elem. 2016, 21, 963–975. [Google Scholar] [CrossRef]
- Hegedüsová, A.; Mezeyová, I.; Hegedüs, O.; Andrejiová, A.; Juríková, T.; Golian, M.; Lošák, T. Increasing of selenium content and qualitative parameters in garden pea (Pisum sativum L.) after its foliar application. Acta Scientiarum Polonorum. Hortorum Cultus 2017, 16, 3–17. [Google Scholar] [CrossRef]
- Zhao, F.J.; McGrath, S.P. Biofortification and phytoremediation. Curr. Opin. Plant Biol. 2009, 12, 373–380. [Google Scholar] [CrossRef]
- Dayod, M.; Tyerman, S.D.; Leigh, R.A.; Gilliham, M. Calcium storage in plants and the implications for calcium biofortification. Protoplasma 2010, 247, 215–231. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Lawson, P.G.; Daum, D.; Czauderna, R.; Meuser, H.; Härtling, J.W. Soil versus foliar iodine fertilization as a biofortification strategy for field-grown vegetables. Front. Plant Sci. 2015, 6, 450. [Google Scholar] [CrossRef]
- Cakmak, I.; Guilherme, L.R.G.; Rashid, A.; Hora, K.H.; Yazici, A.; Savasli, E.; Martins, F.A.D. Iodine biofortification of wheat, rice and maize through fertilizer strategy. Plant Soil 2017, 418, 319–335. [Google Scholar] [CrossRef]
- Li, R.; Li, D.W.; Liu, H.P.; Hong, C.L.; Song, M.Y.; Dai, Z.X.; Weng, H.X. Enhancing iodine content and fruit quality of pepper (Capsicum annuum L.) through biofortification. Sci. Hortic. 2017, 214, 165–173. [Google Scholar] [CrossRef]
- Ríos, J.J.; Rosales, M.A.; Blasco, B.; Cervilla, L.M.; Romero, L.; Ruiz, J.M. Biofortification of Se and induction of the antioxidant capacity in lettuce plants. Sci. Hortic. 2008, 116, 248–255. [Google Scholar] [CrossRef]
- Ríos, J.J.; Blasco, B.; Cervilla, L.M.; Rubio-Wilhelmi, M.M.; Rosales, M.A.; Sanchez-Rodriguez, E.; Ruiz, J.M. Nitrogen-use efficiency in relation to different forms and application rates of Se in lettuce plants. J. Plant Growth Regul. 2010, 29, 164–170. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiol. Plant. 2015, 37, 41. [Google Scholar] [CrossRef]
- Golubkina, N.A.; Kosheleva, O.V.; Krivenkov, L.V.; Dobrutskaya, H.G.; Nadezhkin, S.; Caruso, G. Intersexual differences in plant growth, yield, mineral composition and antioxidants of spinach (Spinacia oleracea L.) as affected by selenium form. Sci. Hortic. 2017, 225, 350–358. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E. Selenium. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2007; pp. 515–549. [Google Scholar]
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; Benavides-Mendoza, A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef] [PubMed]
- Gonzali, S.; Kiferle, C.; Perata, P. Iodine biofortification of crops: Agronomic biofortification, metabolic engineering and iodine bioavailability. Curr. Opin. Biotechnol. 2017, 44, 16–26. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Huang, Y.; Hu, Y.; Liu, Y.; Christie, P. Interactions between selenium and iodine uptake by spinach (Spinacia oleracea L.) in solution culture. Plant Soil 2004, 261, 99–105. [Google Scholar] [CrossRef]
- Mao, H.; Wang, J.; Wang, Z.; Zan, Y.; Lyons, G.; Zou, C. Using agronomic biofortification to boost zinc, selenium, and iodine concentrations of food crops grown on the loess plateau in China. J. Soil Sci. Plant Nutr. 2014, 14, 459–470. [Google Scholar] [CrossRef]
- Germ, M.; Maršić, N.K.; Turk, J.; Pirc, M.; Golob, A.; Jerše, A.; Stibilj, V. The effect of different compounds of selenium and iodine on selected biochemical and physiological characteristics in common buckwheat and pumpkin sprouts. Acta Biol. Slov. 2015, 58, 35–44. [Google Scholar]
- Jerše, A.; Maršić, N.K.; Kroflič, A.; Germ, M.; Šircelj, H.; Stibilj, V. Is foliar enrichment of pea plants with iodine and selenium appropriate for production of functional food? Food Chem. 2018, 267, 368–375. [Google Scholar] [CrossRef]
- Osmić, A.; Golob, A.; Germ, M. The effect of selenium and iodine on selected biochemical and morphological characteristics in kohlrabi sprouts (Brassica oleracea L. var. gongylodes L.). Acta Biol. Slov. 2017, 60, 41–51. [Google Scholar]
- Sady, W.; Rożek, S.; Gregorczyk, J. The effect of fertilization with different forms of nitrogen on yield and nitrate metabolism in leaves of greenhouse lettuce. I. Yield and content of selected components in lettuce leaves. Folia Hortic. 1990, 2, 65–76. [Google Scholar]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Czernicka, M.; Halka, M.; Kęska, K.; Sady, W. Iodine and selenium biofortification with additional application of salicylic acid affects yield, selected molecular parameters and chemical composition of lettuce plants (Lactuca sativa L. var. capitata). Front. Plant Sci. 2016, 7, 1553. [Google Scholar] [CrossRef]
- Fakumoto, L.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 13395. Water Quality—Determination of Nitrite Nitrogen and Nitrate and the Sum of Both by Flow Analysis (CFA and FIA) and Spectrometric Detection; Polish Committee of Standardization: Warsaw, Poland, 2001. (In Polish) [Google Scholar]
- Hawrylak-Nowak, B. Effect of selenium on selected macronutrients in maize plants. J. Elem. 2008, 13, 513–519. [Google Scholar]
- Smoleń, S.; Kowalska, I.; Kováčik, P.; Halka, H.; Sady, W. Biofortification of six varieties of lettuce (Lactuca sativa L.) with iodine and selenium in combination with the application of salicylic acid. Front. Plant Sci. 2019, 10, 143. [Google Scholar] [CrossRef]
- Siomos, A.S.; Beis, G.; Papadopoulou, P.P.; Nasi, P.; Kaberidou, I.; Barbayiannis, N. Aerial biomass, root biomass and quality of four lettuce cultivars grown hydroponically in perlite and pumice. Acta Hortic. 2001, 548, 437–444. [Google Scholar] [CrossRef]
- Ramos, S.J.; Rutzke, M.A.; Hayes, R.J.; Faquin, V.; Guilherme, L.R.G.; Li, L. Selenium accumulation in lettuce germplasm. Planta 2011, 233, 649–660. [Google Scholar] [CrossRef]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sanchez-Rodriguez, E.; Rubio-Wilhelmi, M.M.; Rosales, M.A.; Romero, L. Photorespiration process and nitrogen metabolism in lettuce plants (Lactuca sativa L.): Induced changes in response to iodine biofortification. J. Plant Growth Regul. 2010, 29, 477–486. [Google Scholar] [CrossRef]
- Blasco, B.; Rios, J.J.; Leyva, R.; Cervilla, L.M.; Sanchez-Rodriguez, E. Does iodine biofortification affect oxidative metabolism in lettuce plants? Biol. Trace Elem. Res. 2011, 142, 831–842. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef]
- Esringu, A.; Ekinci, M.; Usta, S.; Turan, M.; Dursun, A.; Ercisli, S.; Yildirim, E. Selenium supplementation affects the growth, yield and selenium accumulation in lettuce (Lactuca sativa L.). Comptes rendus de l’Académie bulgare des Sciences 2015, 68, 801–810. [Google Scholar]
- Korkina, L.G. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell. Mol. Biol. 2007, 53, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Wheeler, G.L. Ascorbic Acid in Plants: Biosynthesis and Function. Critical. Rev. Biochem. Mol. Biol. 2000, 35, 291–314. [Google Scholar] [CrossRef] [PubMed]
- Zechmann, B. Subcellular distribution of ascorbate in plants. Plant Signal. Behav. 2011, 6, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, J.; Park, S.W. Plastid genetic engineering in Solanaceae. Protoplasma 2012, 249, 981–999. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Dempsey, D.A.; Vlot, A.C.; Wildermuth, M.C.; Klessing, D.F. Salicylic acid biosynthesis and methabolism. Arab. Book 2011, 9, e0156. [Google Scholar] [CrossRef]
- Tieman, D.; Zeigler, M.; Schmelz, E.; Taylor, M.G.; Rushing, S.; Jones, J.B.; Klee, H.J. Functional analysis of a tomato salicylic acid methyl transferase and its role in synthesis of the flavor volatile methyl salicylate. Plant J. 2010, 62, 113–123. [Google Scholar] [CrossRef]
- Ross, J.R.; Nam, K.H.; D’Auria, J.C.; Pichersky, E. S-adenosyl-L-methionine: Salicylic acid carboxyl methyltransferase, an enzyme involved in floral scent production and plant defense, represents a new class of plant methyltransferases. Arch. Biochem. Biophys. 1999, 367, 9–16. [Google Scholar] [CrossRef]
- Kwon, S.; Hamada, K.; Matsuyama, A.; Yasuda, M.; Nakashita, H.; Yamakawa, T. Biotic and abiotic stresses induce AbSAMT1, encoding S-adenosyl-L-methionine: Salicylic acid carboxyl methyltransferase, in Atropa belladonna. Plant Biotechnol. 2009, 26, 207–215. [Google Scholar] [CrossRef]
- Bartsch, M.; Bednarek, P.; Vivancos, P.D.; Schneider, B.; von Roepenack-Lahaye, E.; Foyer, C.H.; Parker, J.E. Accumulation of isochorismate-derived 2, 3-dihydroxybenzoic 3-O-β-D-xyloside in Arabidopsis resistance to pathogens and ageing of leaves. J. Biol. Chem. 2010, 285, 25654–25665. [Google Scholar] [CrossRef] [PubMed]
- Blom-Zandstra, M. Nitrate accumulation in vegetables and its relationship to quality. Ann. Appl. Biol. 1989, 115, 553–561. [Google Scholar] [CrossRef]
- Konstantopoulou, E.; Kapotis, G.; Salachas, G.; Petropoulos, S.A.; Karapanos, I.C.; Passam, H.C. Nutritional quality of greenhouse lettuce at harvest and after storage in relation to N application and cultivation season. Sci. Hortic. 2010, 125, e1–e5. [Google Scholar] [CrossRef]
- Wojciechowska, R.; Kowalska, I. The effect of foliar application of urea, Mo and BA on nitrate metabolism in lettuce leaves in the spring and summer-autumn seasons. Folia Hortic. 2011, 23, 119–123. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) No 1258/2011 of 2 December 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for nitrates in foodstuffs. Off. J. Eur. Union 2011, 320, 15–17. Available online: https://www.fsai.ie/uploadedFiles/Reg1258_2011.pdf (accessed on 23 April 2019).
- Fariduddin, Q.; Hayat, S.; Ahmad, A. Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica 2003, 41, 281–284. [Google Scholar] [CrossRef]
- Aslam, M.; Harbit, K.B.; Huffaker, R.C. Comparative effects of selenite and selenate on nitrate assimilation in barley seedlings. Plant Cell Environ. 1990, 13, 773–782. [Google Scholar] [CrossRef]
- Nowak, J.; Kaklewski, K.; Ligocki, M. Influence of selenium on oxidoreductive enzymes activity in soil and in plants. Soil Biol. Biochem. 2004, 36, 1553–1558. [Google Scholar] [CrossRef]
- Wong, G.T.F.; Hung, C.C. Speciation of dissolved iodine: Integrating nitrate uptake over time in the oceans. Cont. Shelf Res. 2001, 21, 113–128. [Google Scholar] [CrossRef]
- Hung, C.C.; Wong, G.T.F.; Dunstan, W.M. Iodate reduction activity in initrate reductase extracts from marine phytoplankton. Bull. Mar. Sci. 2005, 76, 61–72. [Google Scholar]
- Mills, H.A.; Jones, J.B. Plant Analysis Handbook II: A Practical Sampling, Preparation, Analysis, and Interpretation Guide; MicroMacro Publishing: Athens, GA, USA, 1996. [Google Scholar]
- Hartz, T.K.; Johnstone, P.R.; Williams, E.; Smith, R.F. Establishing lettuce leaf nutrient optimum ranges through DRIS analysis. HortScience 2007, 42, 143–146. [Google Scholar] [CrossRef]
- Blasco, B.; Ríos, J.J.; Sánchez-Rodríguez, E.; Rubio-Wilhelm, M.M.; Leyva, R.; Romero, L.; Ruiz, J.M. Study of the interactions between iodine and mineral nutrients in lettuce plants. J. Plant Nutr. 2012, 35, 1958–1969. [Google Scholar] [CrossRef]
- Al-Redhaiman, K.N.; Helal, M.I.D.; Shahin, R.R. Effect of sulfur blended N-fertilizers on nitrogen use efficiency and quality of lettuce yield. Pak. J. Biol. Sci. 2003, 6, 1408–1412. [Google Scholar] [CrossRef]
- Koudela, M.; Petříková, K. Nutrients content and yield in selected cultivars of leaf lettuce (Lactuca sativa L. var. crispa). Hortic. Sci. 2008, 35, 99–106. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Sady, W. Assessment of biofortification with iodine and selenium of lettuce cultivated in the NFT hydroponic system. Sci. Hortic. 2014, 166, 9–16. [Google Scholar] [CrossRef]
- Winkel, L.H.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef] [PubMed]
| Compounds/Elements | Lettuce varieties ordered from the highest to the lowest content of individual compounds and elements in the leaves (heads) (n = 8). |
|---|---|
| Ascorbic acid | BUTL “Zimująca” = BUTL “Cud Voorburgu” = REDL “Redin” >REDL “Lollorossa” >ICEL “Królowa lata” = “ICEL “Maugli” |
| Nitrate(V) | REDL “Lollorossa” >BUTL “Zimująca” >ICEL “Królowa lata” = REDL “Redin” >BUTL “Cud Voorburgu” >ICEL “Maugli”. |
| Glucose, fructose, and total sugars | ICEL “Maugli” >BUTL “Zimująca” BUTL “Cud Voorburgu” = ICEL “Królowa lata” >REDL “Redin” > REDL “Lollorossa” |
| Sucrose only | BUTL “Zimująca” >BUTL “Cud Voorburgu” >REDL “Redin” = REDL “Lollorossa” >ICEL “Maugli” = ICEL “Królowa lata” |
| Phenols, phenylpropanoids, flavonols, and anthocyanins | BUTL “Zimująca” >REDL “Redin” >BUTL “Cud Voorburgu” >REDL “Lollorossa” >ICEL “Królowa lata” >ICEL “Maugli”. |
| N | REDL “Redin” >BUTL “Cud Voorburgu” >BUTL “Zimująca” = ICEL “Maugli” = ICEL “Królowa lata” >REDL “Lollorossa” |
| P | BUTL “Cud Voorburgu” >REDL “Redin” >REDL “Lollorossa” >ICEL “Maugli” >ICEL “Królowa lata” = BUTL “Zimująca” |
| K | REDL “Lollorossa” >ICEL “Królowa lata” >BUTL “Zimująca” = REDL “Redin” >BUTL “Cud Voorburgu” = ICEL “Maugli” |
| Mg | REDL “Lollorossa” >BUTL “Cud Voorburgu” >BUTL “Zimująca” >ICEL “Królowa lata” >REDL “Redin” = ICEL “Maugli” |
| Ca | REDL “Lollorossa” >BUTL “Cud Voorburgu” > BUTL “Zimująca” >ICEL “Królowa lata” >REDL “Redin” >ICEL “Maugli” |
| S | ICEL “Królowa lata” >REDL “Redin” = ICEL “Maugli” >BUTL “Cud Voorburgu” = BUTL “Zimująca” >REDL “Lollorossa” |
| Na | REDL “Lollorossa” >BUTL “Cud Voorburgu” >ICEL “Królowa lata” = BUTL “Zimująca” > REDL “Redin” >ICEL “Maugli” |
| B | REDL “Lollorossa” > REDL “Redin” > BUTL “Zimująca” >BUTL “Cud Voorburgu” = ICEL “Królowa lata” >ICEL “Maugli” |
| Cu | BUTL “Cud Voorburgu” >BUTL “Zimująca” >REDL “Lollorossa” >ICEL “Maugli” = REDL “Redin” >ICEL “Królowa lata” |
| Fe | REDL “Redin” > BUTL “Cud Voorburgu” >REDL “Lollorossa” = BUTL “Zimująca” > ICEL “Królowa lata” >ICEL “Maugli” |
| Mn | REDL “Lollorossa” >ICEL “Maugli” >ICEL “Królowa lata” > REDL “Redin” = BUTL “Zimująca” > BUTL “Cud Voorburgu” |
| Mo | REDL “Redin” = REDL “Lollorossa” >ICEL “Maugli” = BUTL “Zimująca”= ICEL “Królowa lata” >BUTL “Cud Voorburgu” |
| Zn | REDL “Redin” >REDL “Lollorossa”= ICEL “Maugli” = BUTL “Cud Voorburgu” >BUTL “Zimująca” >ICEL “Królowa lata” |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smoleń, S.; Kowalska, I.; Kováčik, P.; Sady, W.; Grzanka, M.; Kutman, U.B. Changes in the Chemical Composition of Six Lettuce Cultivars (Lactuca sativa L.) in Response to Biofortification with Iodine and Selenium Combined with Salicylic Acid Application. Agronomy 2019, 9, 660. https://doi.org/10.3390/agronomy9100660
Smoleń S, Kowalska I, Kováčik P, Sady W, Grzanka M, Kutman UB. Changes in the Chemical Composition of Six Lettuce Cultivars (Lactuca sativa L.) in Response to Biofortification with Iodine and Selenium Combined with Salicylic Acid Application. Agronomy. 2019; 9(10):660. https://doi.org/10.3390/agronomy9100660
Chicago/Turabian StyleSmoleń, Sylwester, Iwona Kowalska, Peter Kováčik, Włodzimierz Sady, Marlena Grzanka, and Umit Baris Kutman. 2019. "Changes in the Chemical Composition of Six Lettuce Cultivars (Lactuca sativa L.) in Response to Biofortification with Iodine and Selenium Combined with Salicylic Acid Application" Agronomy 9, no. 10: 660. https://doi.org/10.3390/agronomy9100660
APA StyleSmoleń, S., Kowalska, I., Kováčik, P., Sady, W., Grzanka, M., & Kutman, U. B. (2019). Changes in the Chemical Composition of Six Lettuce Cultivars (Lactuca sativa L.) in Response to Biofortification with Iodine and Selenium Combined with Salicylic Acid Application. Agronomy, 9(10), 660. https://doi.org/10.3390/agronomy9100660





