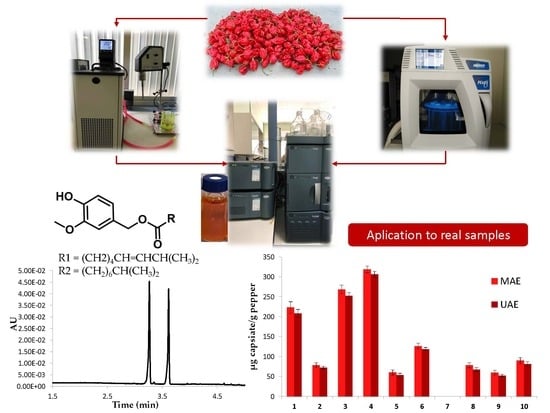
Optimizing and Comparing Ultrasound- and Microwave-Assisted Extraction Methods Applied to the Extraction of Antioxidant Capsinoids in Peppers
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Pepper Sample
2.3. Extraction Equipment and Procedure
2.3.1. Ultrasound-Assisted Extraction (UAE)
2.3.2. Microwave-Assisted Extraction (MAE)
2.4. UHPLC Analysis
2.5. Experimental Design and Data Treatment
3. Results and Discussion
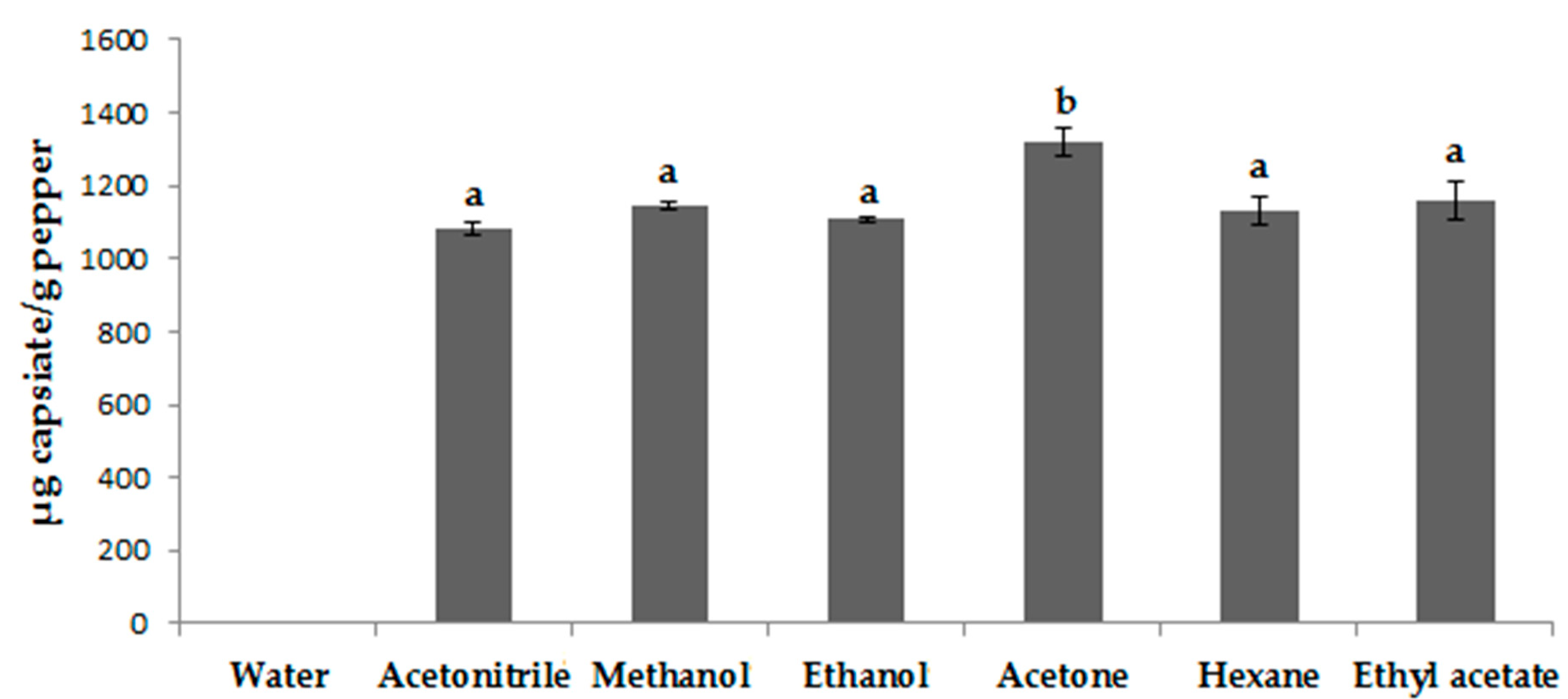
3.1. Selecting the Extraction Solvent
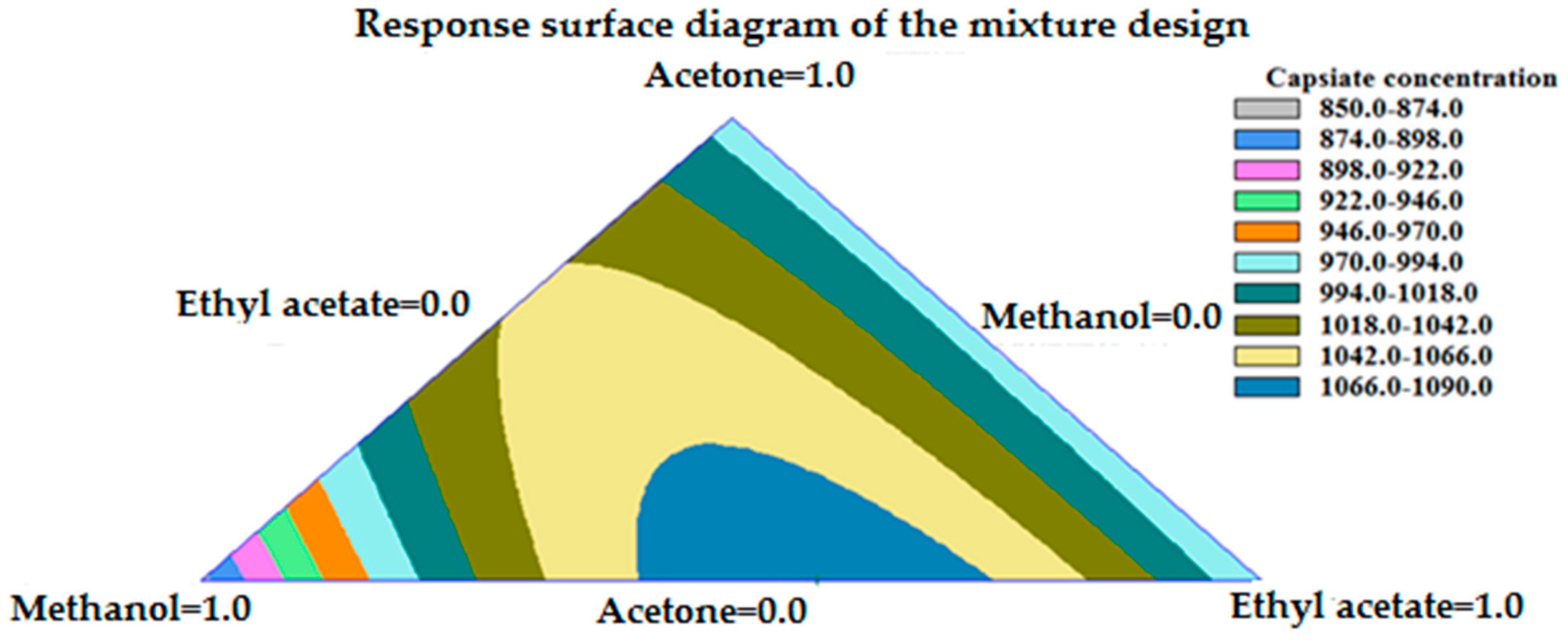
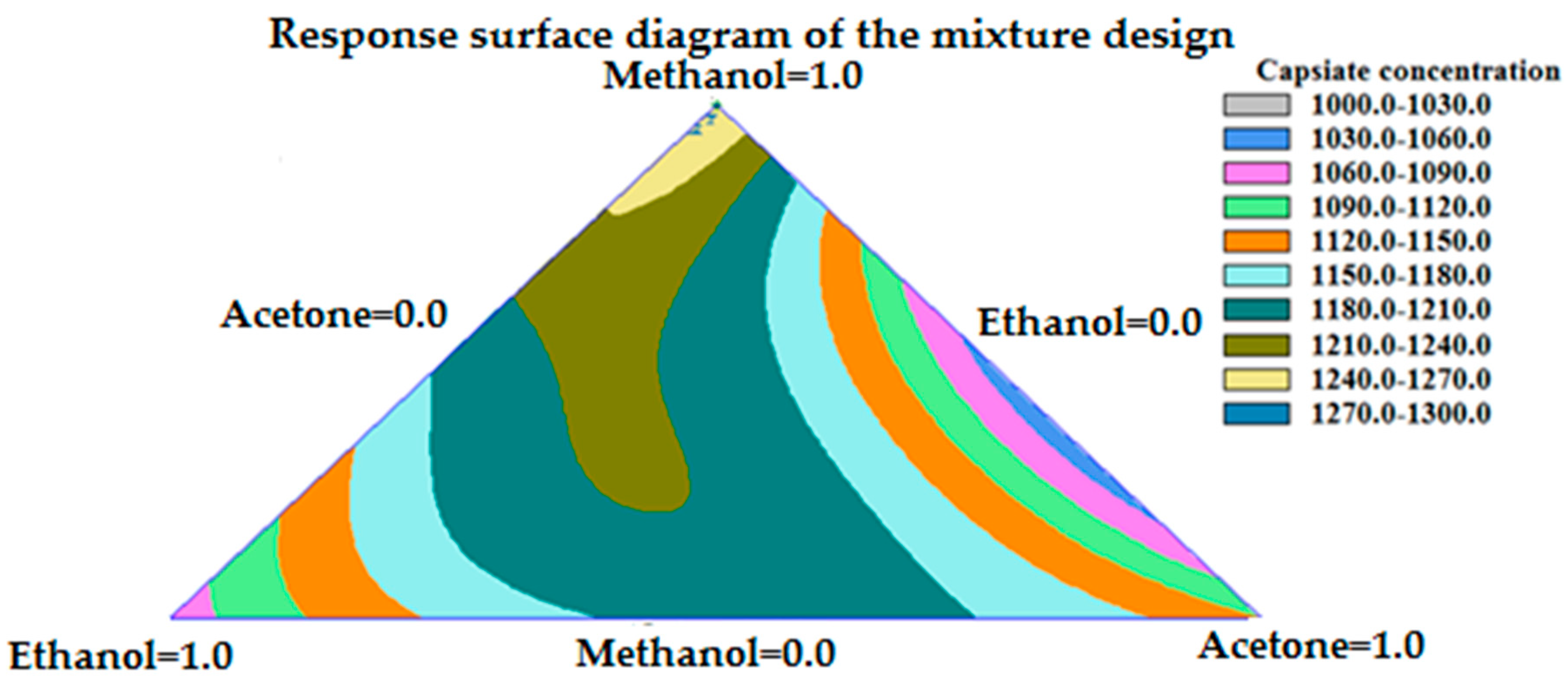
3.1.1. Solvent Selection Procedure for UAE
3.1.2. Solvent Selection Procedure for MAE
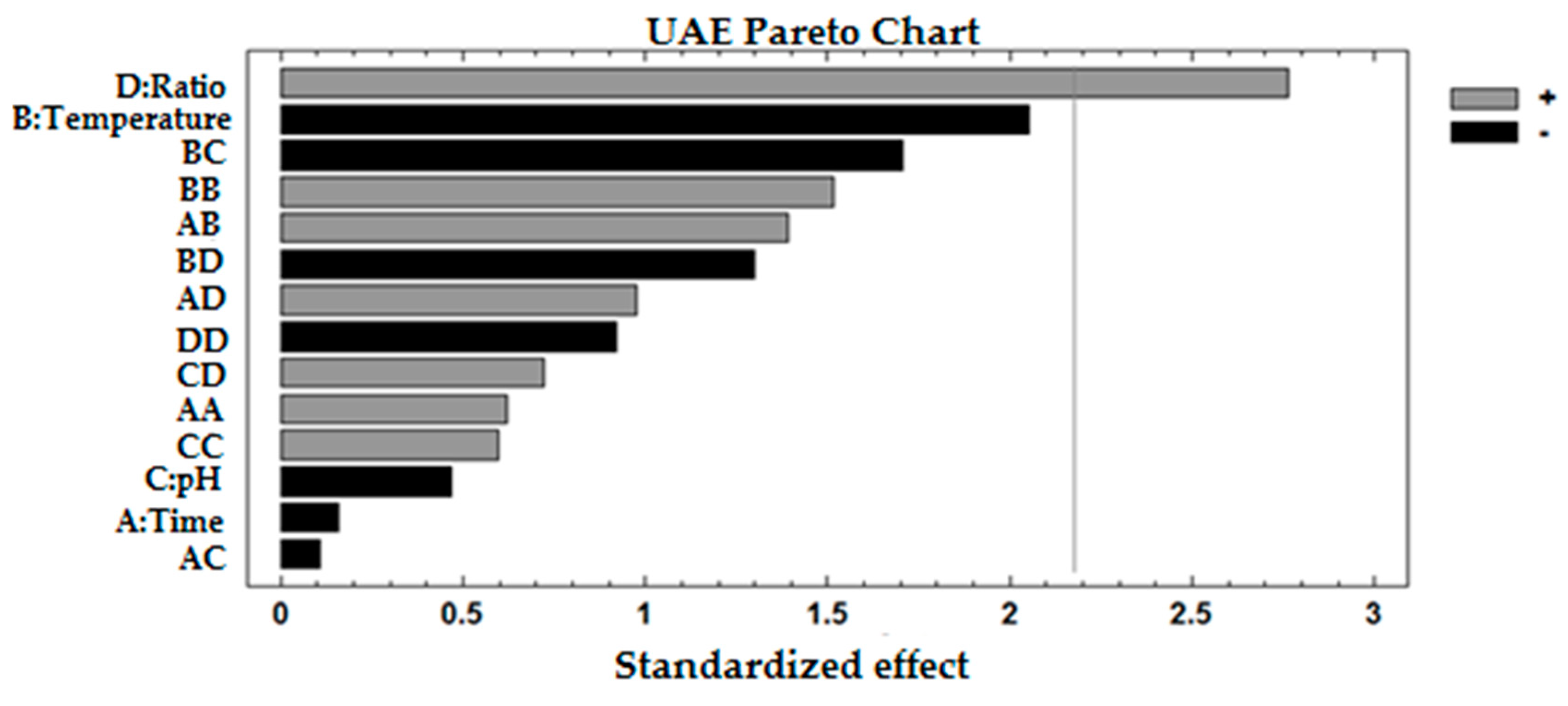
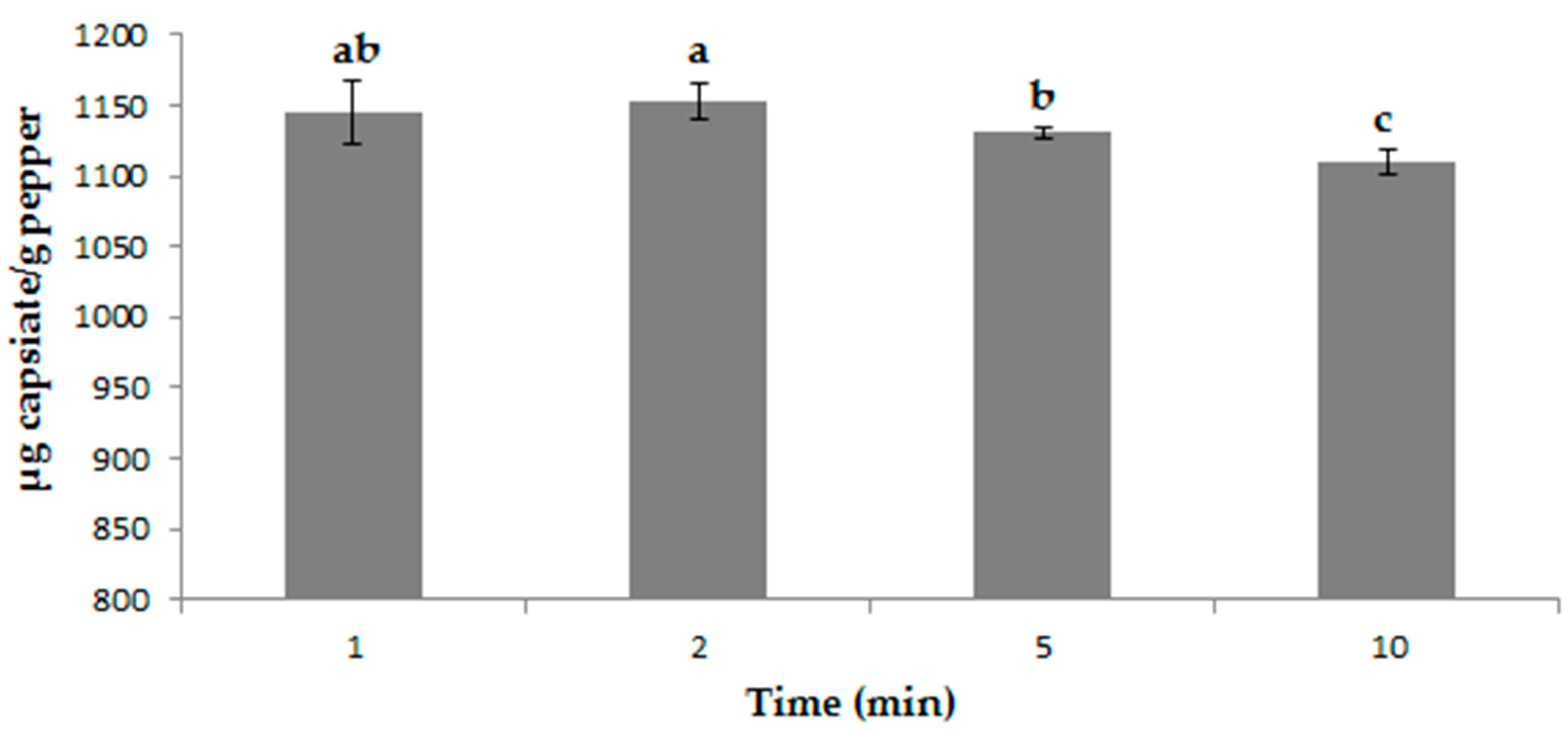
3.2. Determining Optimal Ultrasound Conditions
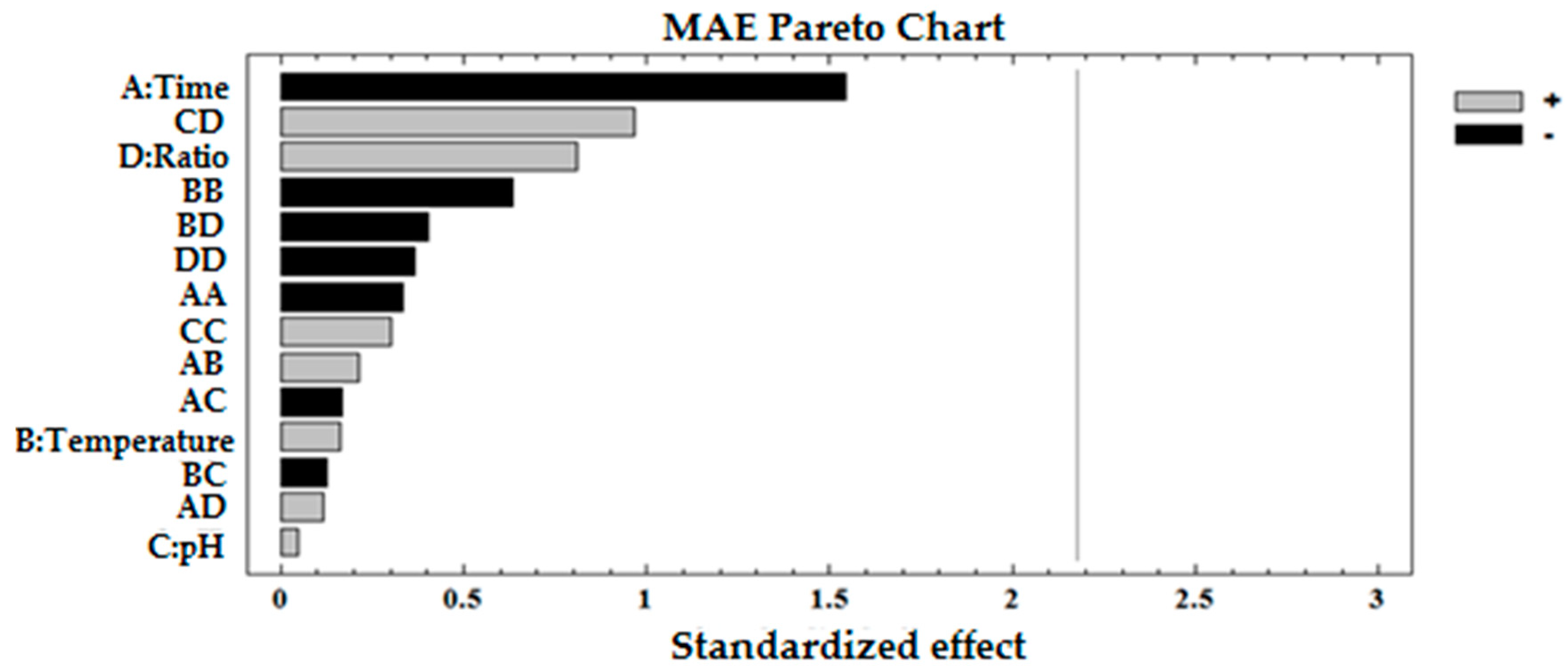
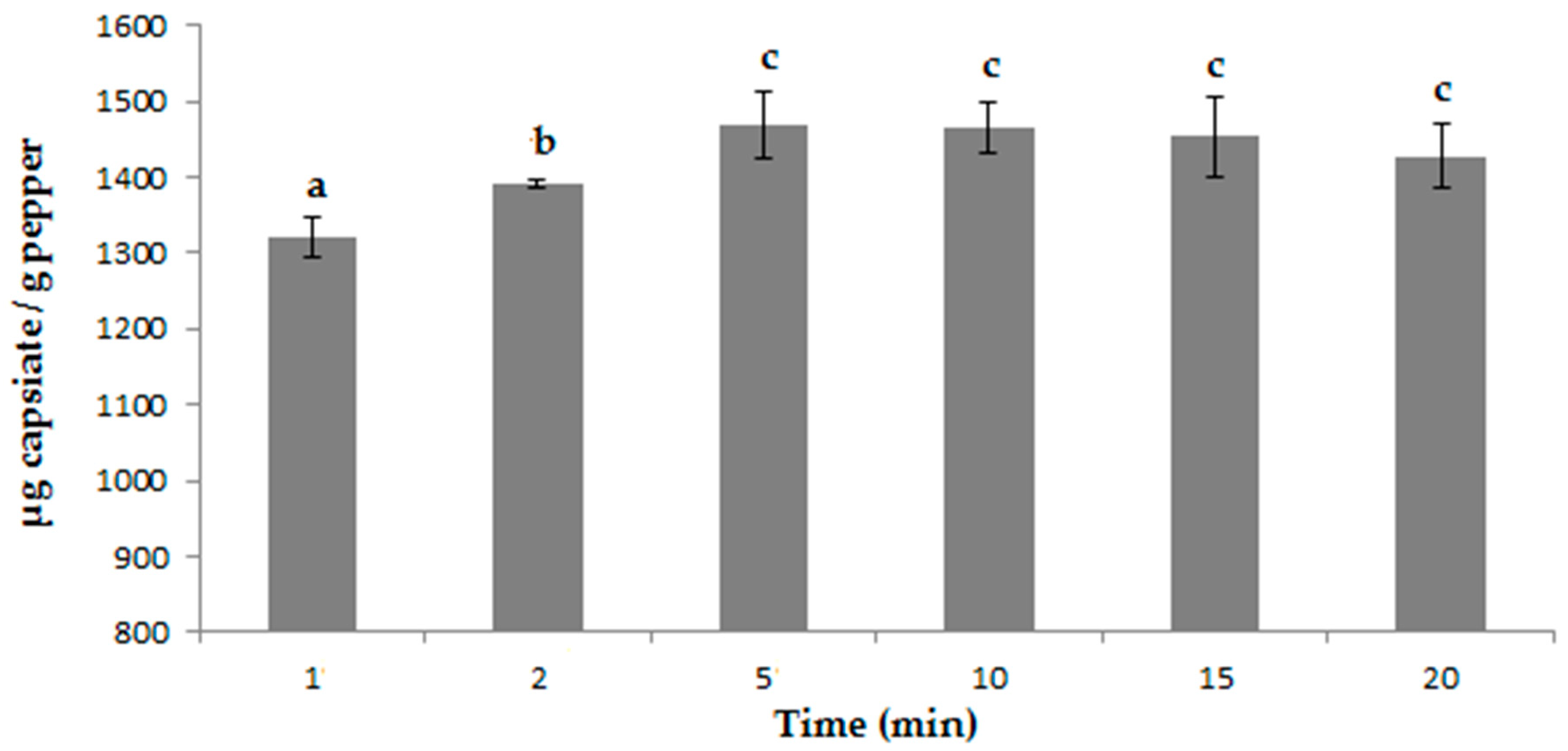
3.3. Determining Optimal Microwave Conditions
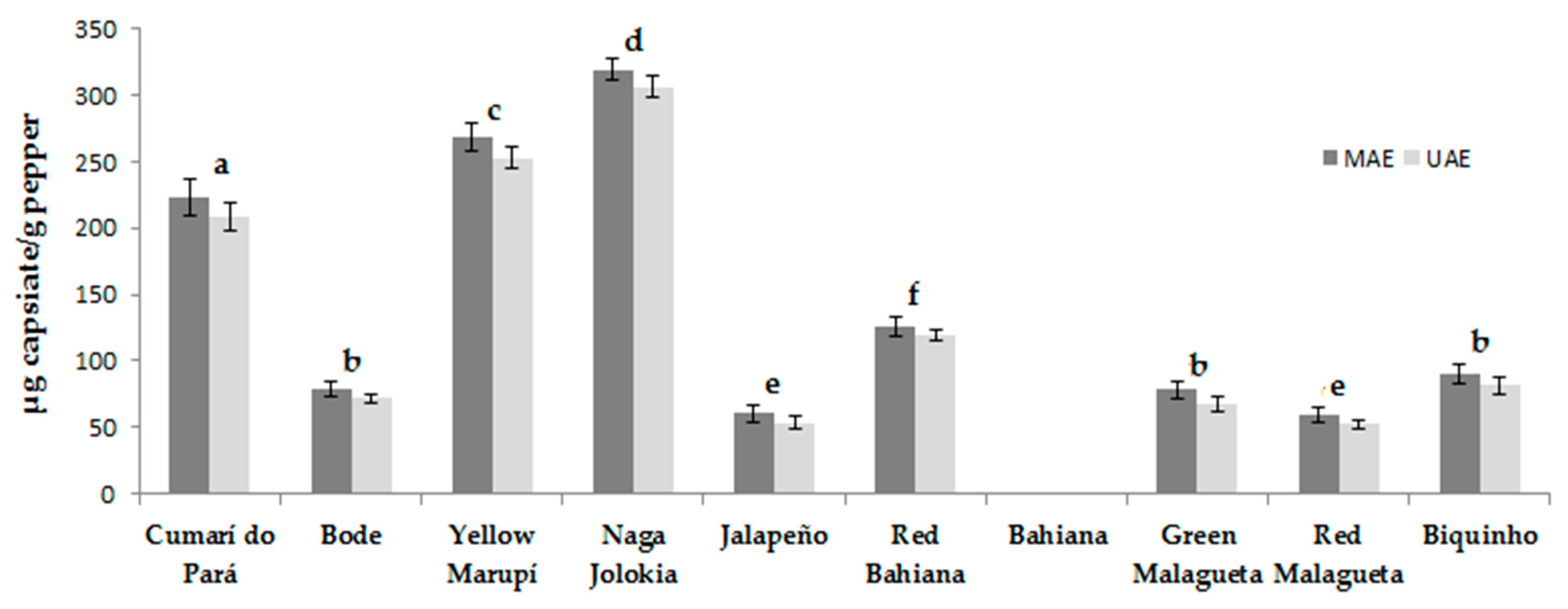
3.4. Real Samples Analysis
3.5. Comparison between the Two Extraction Methods
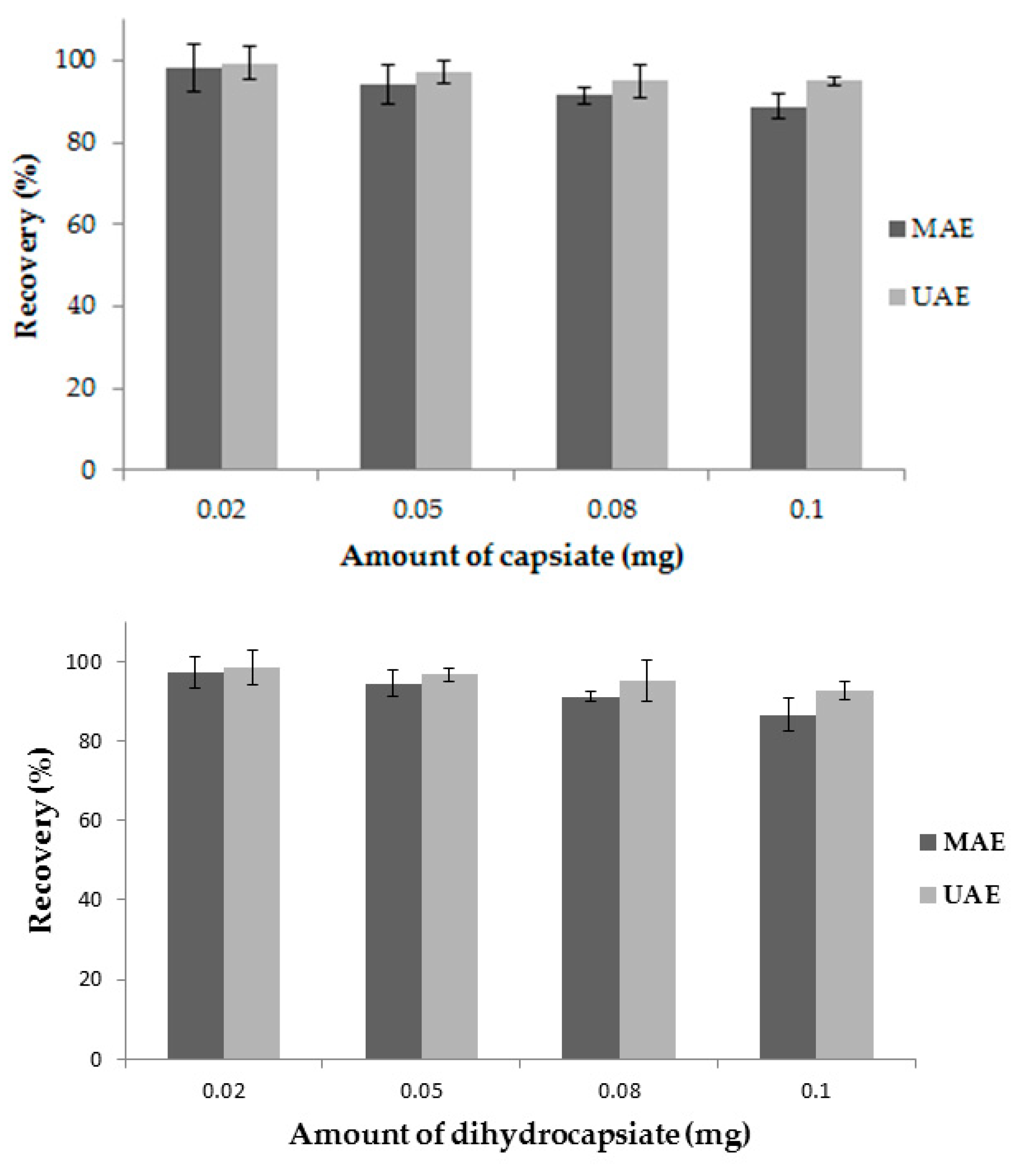
3.6. Recovery Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tripodi, P.; Cardi, T.; Bianchi, G.; Migliori, C.A.; Schiavi, M.; Rotino, G.L.; Lo Scalzo, R. Genetic and environmental factors underlying variation in yield performance and bioactive compound content of hot pepper varieties (Capsicum annuum) cultivated in two contrasting Italian locations. Eur. Food Res. Technol. 2018, 244, 1–13. [Google Scholar] [CrossRef]
- Olguin-Rojas, J.A.; Fayos, O.; Vázquez-León, L.A.; Ferreiro-González, M.; Rodríguez-Jimenes, G.C.; Palma, M.; Garcés-Claver, A.; Barbero, G.F. Progression of the total and individual capsaicinoids content in the fruits of three different cultivars of Capsicum chinense Jacq. Agronomy 2019, 9, 141. [Google Scholar] [CrossRef]
- Ananthan, R.; Subhash, K.; Longvah, T. Capsaicinoids, amino acid and fatty acid profiles in different fruit components of the world hottest Naga king chilli (Capsicum chinense Jacq). Food Chem. 2018, 238, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Matsufuji, H.; Ishikawa, K.; Nunomura, O.; Chino, M.; Takeda, M. Anti-oxidant content of different coloured sweet peppers, white, green, yellow, orange and red (Capsicum annuum L.). Int. J. Food Sci. Technol. 2007, 42, 1482–1488. [Google Scholar] [CrossRef]
- Santos, P.; Aguiar, A.C.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Supercritical carbon dioxide extraction of capsaicinoids from malagueta pepper (Capsicum frutescens L.) assisted by ultrasound. Ultrason. Sonochem. 2016, 22, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Chapa-Oliver, A.M.; Mejía-Teniente, L. Capsaicin: From plants to a cancer-suppressing agent. Molecules 2016, 21, 931. [Google Scholar] [CrossRef]
- Giuffrida, D.; Dugo, P.; Torre, G.; Bignardi, C.; Cavazza, A.; Corradini, C.; Dugo, G. Characterization of 12 Capsicum varieties by evaluation of their carotenoid profile and pungency determination. Food Chem. 2013, 140, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, S., Jr.; Libardi, S.H.; Dias, F.F.G.; Coutinho, J.P.; Bochi, V.C.; Rodrigues, D.; Melo, A.M.Y.; Godoy, H.T. Brazilian Capsicum peppers: Capsaicinoid content and antioxidant activity. J. Sci. Food Agric. 2018, 98, 217–224. [Google Scholar] [CrossRef]
- Ludy, M.J.; Moore, G.E.; Mattes, R.D. The effects of capsaicin and capsiate on energy balance: Critical review and meta-analyses of studies in humans. Chem. Senses 2012, 37, 103–121. [Google Scholar] [CrossRef]
- Trbojević Ivić, J.; Milosavic, N.; Dimitrijevic, A.; Gavrovic Jankulovic, M.; Bezbradica, D.; Kolaski, D.; Velickovic, D. Synthesis of medium-chain length capsinoids from coconut oil catalyzed by Candida rugosa lipases. Food Chem. 2017, 218, 505–508. [Google Scholar] [CrossRef]
- Fayos, O.; Savirón, M.; Orduna, J.; Barbero, G.F.; Mallor, C.; Garcés-Claver, A. Quantitation of capsiate and dihydrocapsiate and tentative identification of minor capsinoids in pepper fruits (Capsicum spp.) by HPLC-ESI-MS/MS(QTOF). Food Chem. 2019, 270, 264–272. [Google Scholar] [CrossRef] [PubMed]
- De Aguiar, A.C.; da Fonseca Machado, A.P.; Figueiredo Angolini, C.F.; Rodrigues de Morais, D.; Baseggio, A.M.; Eberlin, M.N.; Maróstica Junior, M.R.; Martínez, J. Sequential high-pressure extraction to obtain capsinoids and phenolic compounds from biquinho pepper (Capsicum chinense). J. Supercrit. Fluids 2019, 150, 112–121. [Google Scholar] [CrossRef]
- Rosa, A.; Deiana, M.; Casu, V.; Paccagnini, S.; Appendino, G.; Ballero, M.; Dessi, A. Antioxidant activity of capsinoids. J. Agric. Food Chem. 2002, 50, 7396–7401. [Google Scholar] [CrossRef] [PubMed]
- Sutoh, K.; Kobata, K.; Yazawa, S.; Watanabe, T. Capsinoid is biosynthesized from phenylalanine and valine in a non-pungent pepper, Capsicum annuum L. cv. CH-19 Sweet. Biosci. Biotechnol. Biochem. 2006, 70, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Swint, J.M.; Beining, K.M.; Bryant, J.A.; Tucker, R.M.; Ludy, M.J. Comparison of capsaicin and capsiate’s effects at a meal. Chemosens. Percept. 2015, 8, 174–182. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.S.; Abert-Vian, M. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Castejón, N.; Luna, P.; Señoráns, F.J. Alternative oil extraction methods from Echium plantagineum L. seeds using advanced techniques and green solvents. Food Chem. 2018, 244, 75–82. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops–A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-assisted extraction of natural products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef]
- Riciputi, Y.; Diaz-de-Cerio, E.; Akyol, H.; Capanoglu, E.; Cerretani, L.; Caboni, M.F.; Verardo, V. Establishment of ultrasound-assisted extraction of phenolic compounds from industrial potato by-products using response surface methodology. Food Chem. 2018, 269, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Böger, B.R.; Salviato, A.; Valezi, D.F.; Di Mauro, E.; Georgetti, S.R.; Kurozawa, L.E. Optimization of ultrasound-assisted extraction of grape-seed oil to enhance process yield and minimize free radical formation. J. Sci. Food Agric. 2018, 98, 5019–5026. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Guo, N.; Wang, S.X.; Kou, P.; Zhao, C.J.; Fu, Y.J. Ultrasound-negative pressure cavitation extraction of phenolic compounds from blueberry leaves and evaluation of its DPPH radical scavenging activity. Food Bioprod. Process. 2018, 108, 69–80. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W.L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Labbafi, M.; Saeid Hosseini, S.; Hojjati, M. Pistachio green hull pectin: Optimization of microwave-assisted extraction and evaluation of its physicochemical, structural and functional properties. Food Chem. 2019, 271, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Prieto, M.A.; Carvalho, A.M.; Barreiro, M.F.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: A nutraceutical-oriented optimization study. Sep. Purif. Technol. 2016, 164, 114–124. [Google Scholar] [CrossRef]
- Akhbari, M.; Masoum, S.; Aghababaei, F.; Hamedi, S. Optimization of microwave assisted extraction of essential oils from Iranian Rosmarinus officinalis L. using RSM. J. Food Sci. Technol. 2018, 55, 2197–2207. [Google Scholar] [CrossRef]
- Koyu, H.; Kazan, A.; Demir, S.; Haznedaroglu, M.Z.; Yesil-Celiktas, O. Optimization of microwave assisted extraction of Morus nigra L. fruits maximizing tyrosinase inhibitory activity with isolation of bioactive constituents. Food Chem. 2018, 248, 183–191. [Google Scholar] [CrossRef]
- Covarrubias-Cárdenas, A.G.; Martínez-Castillo, J.I.; Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; García-Cruz, N.U.; Pacheco, N. Antioxidant capacity and UPLC-PDA ESI-MS phenolic profile of Stevia rebaudiana dry powder extracts obtained by ultrasound assisted extraction. Agronomy 2018, 8, 170. [Google Scholar] [CrossRef]
- Barbero, G.F.; Palma, M.; Barroso, C.G. Determination of capsaicinoids in peppers by microwave-assisted extraction-high-performance liquid chromatography with fluorescence detection. Anal. Chim. Acta 2006, 578, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of capsaicinoids from peppers. Talanta 2008, 75, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Boonkird, S.; Phisalaphong, C.; Phisalaphong, M. Ultrasound-assisted extraction of capsaicinoids from Capsicum frutescens on a lab and pilot-plant scale. Ultrason. Sonochem. 2008, 15, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Jarret, R.; Russo, V.; Majetich, G.; Shimkus, J.; Bushway, R.; Perkins, B. Determination of capsinoids by HPLC-DAD in Capsicum species. J. Agric. Food Chem. 2009, 57, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; Junior, M.M.S.; Felix, C.S.A.; da Silva, D.L.F.; Santos, A.S.; Santos Neto, J.H.; de Souza, C.T.; Cruz Junior, R.A.; Souza, A.S. Multivariate optimization techniques in food analysis–A review. Food Chem. 2019, 273, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.M.Z.; de Oliveira, T.F.; Soares, P.K.; Bruns, R.E.; Scarminio, I.S. Statistical mixture design-Principal component determination of synergic solvent interactions for natural product extractions. Chemom. Intell. Lab. Syst. 2010, 103, 1–7. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Palma, M.; Barbero, G.F. A screening method based on headspace-ion mobility spectrometry to identify adulterated honey. Sensors 2019, 19, 1621. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, S.S.M.; Dessouky, S.S.; Elfaraskoury, A.A.; Abou Sharaf, A.B. Modelling and experimental verification of barrier effect on breakdown voltage of transformer oil using Box-Behnken Design. Measurement 2019, 147, 106829. [Google Scholar] [CrossRef]
- Iida, T.; Moriyama, T.; Kobata, K.; Morita, A.; Murayama, N.; Hashizune, S.; Fushiki, T.; Yazawa, S.; Watanabe, T.; Tominaga, M. TRPV1 activation and induction of nociceptive response by a non-pungent capsaicin-like compound, capsiate. Neuropharmacology 2003, 44, 958–967. [Google Scholar] [CrossRef]
- Barbero, G.F.; Molinillo, J.M.G.; Varela, R.M.; Palma, M.; MacÍas, F.A.; Barroso, C.G. Application of Hansch’s Model to capsaicinoids and capsinoids: A study using the quantitative structure-activity relationship. A novel method for the synthesis of capsinoids. J. Agric. Food Chem. 2010, 58, 3342–3349. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; Espada-Bellido, E.; González-de-Peredo, A.V.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of microwave-assisted extraction for the recovery of bioactive compounds from the Chilean superfruit (Aristotelia chilensis (Mol.) Stuntz). Agronomy 2018, 8, 240. [Google Scholar] [CrossRef]
- Samuel, S.M.; Abigail, M.E.A.; Chidambaram, R. Isotherm modelling, kinetic study and optimization of batch parameters using response surface methodology for effective removal of Cr (VI) using fungal biomass. PLoS ONE 2015, 10, e0116884. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.; Bin Hamid, M.S.; Webster, R.D. Quantification of capsaicinoids in chillies by solid-phase extraction coupled with voltammetry. Food Chem. 2018, 265, 152–158. [Google Scholar] [CrossRef] [PubMed]
- De Aguiar, A.C.; dos Santos, P.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Supercritical fluid extraction and low pressure extraction of Biquinho pepper (Capsicum chinense). J. Food Sci. Technol. 2014, 59, 1239–1246. [Google Scholar] [CrossRef]
- Lang, Y.; Kisaka, H.; Sugiyama, R.; Nomura, K.; Morita, A.; Watanabe, T.; Tanaka, Y.; Yazawa, S.; Miwa, T. Functional loss of pAMT results in biosynthesis of capsinoids, capsaicinoid analogs, in Capsicum annuum cv. CH-19 Sweet. Plant J. 2009, 59, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barbero, G.F.; Espada-Bellido, E. Assessment of ultrasound assisted extraction as an alternative method for the extraction of anthocyanins and total phenolic compounds from Maqui berries (Aristotelia chilensis (Mol.) Stuntz). Agronomy 2019, 9, 148. [Google Scholar] [CrossRef]
- Ohyama, K.; Nogusa, Y.; Shinoda, K.; Suzuki, K.; Bannai, M.; Kajimura, S. A synergistic antiobesity effect by a combination of capsinoids and cold temperature through promoting beige adipocyte biogenesis. Diabetes 2016, 65, 1410–1423. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Sachdev, A.; Kaur, C. Valorisation of black carrot pomace: Microwave assisted extraction of bioactive phytoceuticals and antioxidant activity using Box–Behnken design. J. Food Sci. Technol. 2019, 56, 995–1007. [Google Scholar] [CrossRef]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Amores-Arrocha, A.; Palma, M.; Barbero, G.F.; Jiménez-Cantizano, A. Alternative ultrasound-assisted method for the extraction of the bioactive compounds present in myrtle (Myrtus communis L.). Molecules 2019, 24, 882. [Google Scholar] [CrossRef]
- Zhang, H.; Birch, J.; Xie, C.; Yang, H.; Bekhit, A.E. Optimization of ultrasound assisted extraction method for phytochemical compounds and in-vitro antioxidant activity of New Zealand and China Asparagus cultivars (officinalis L.) roots extracts. Food Chem. 2019, 294, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Civan, M.; Kumcuoglu, S. Green ultrasound-assisted extraction of carotenoid and capsaicinoid from the pulp of hot pepper paste based on the bio-refinery concept. J. Food Sci. Technol. 2019, 113, 108320. [Google Scholar] [CrossRef]
- Baiao Dias, A.L.; Arroio Sergio, C.S.; Santos, P.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Effect of ultrasound on the supercritical CO2 extraction of bioactive compounds from dedo moça pepper (Capsicum baccatum L. var. pendulum). Ultrason. Sonochem. 2016, 31, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Gorgani, L.; Mohammadi, M.; Najafpou, G.; Nikzad, M. Sequential microwave-ultrasound-assisted extraction for isolation of Piperine from Black Pepper (Piper nigrum L.). Food Bioprocess Technol. 2017, 10, 2199–2207. [Google Scholar] [CrossRef]
- Zunun-Pérez, A.Y.; Guevara-Figueroa, T.; Jimenez-Garcia, S.N.; Feregrino-Pérez, A.A.; Gautier, F.; Guevara-González, R.G. Effect of foliar application of salicylic acid, hydrogen peroxide and a xyloglucan oligosaccharide on capsiate content and gene expression associated with capsinoids synthesis in Capsicum annuum L. J. Biosci. 2017, 42, 245–250. [Google Scholar] [CrossRef]


| Mathematical Model | p-Value | R2 Coefficient (%) | F-Value | |
|---|---|---|---|---|
| UAE | Linear | 0.7579 | 7.6159 | 0.29 |
| Quadratic | 0.0051 | 96.5392 | 22.32 | |
| Cubic | 0.0250 | 96.7146 | 14.72 |
| Mathematical Model | p-Value | R2 Coefficient (%) | F-Value | |
|---|---|---|---|---|
| MAE | Linear | 0.3851 | 23.8650 | 1.10 |
| Quadratic | 0.2538 | 71.8957 | 2.05 | |
| Cubic | 0.3299 | 78.6285 | 1.84 |
| Source | Degrees of Freedom | Sum of Squares | Mean Square | F-Value | p-Value | Coefficient |
|---|---|---|---|---|---|---|
| A: Time | 1 | 9.90 | 9.90 | 0.02 | 0.8777 | −0.9086 |
| B: Temperature | 1 | 1689.52 | 1689.52 | 4.22 | 0.0625 | −11.8656 |
| C: pH | 1 | 87.99 | 87.99 | 0.22 | 0.6478 | −2.7079 |
| D: Ratio | 1 | 3059.83 | 3059.83 | 7.63 | 0.0172 | 15.9683 |
| AA | 1 | 155.11 | 155.11 | 0.39 | 0.5455 | 5.3928 |
| AB | 1 | 775.61 | 775.61 | 1.94 | 0.1894 | 13.9249 |
| AC | 1 | 4.71 | 4.71 | 0.01 | 0.9155 | −1.0849 |
| AD | 1 | 380.88 | 380.88 | 0.95 | 0.3489 | 9.7581 |
| BB | 1 | 922.46 | 922.46 | 2.30 | 0.1551 | 13.1515 |
| BC | 1 | 1166.59 | 1166.59 | 2.91 | 0.1137 | −17.0777 |
| BD | 1 | 677.18 | 677.18 | 1.69 | 0.2181 | −13.0114 |
| CC | 1 | 142.12 | 142.12 | 0.35 | 0.5626 | 5.1621 |
| CD | 1 | 207.96 | 207.96 | 0.52 | 0.4851 | 7.2104 |
| DD | 1 | 341.03 | 341.03 | 0.85 | 0.3745 | −7.9964 |
| Residual | 12 | 4809.17 | 400.76 | |||
| Total | 26 | 14881.40 |
| Source | Degrees of Freedom | Sum of Squares | Mean Square | F-Value | p-Value | Coefficient |
|---|---|---|---|---|---|---|
| A: Time | 1 | 6647.48 | 6647.48 | 2.38 | 0.1489 | −23.5363 |
| B: Temperature | 1 | 74.42 | 74.42 | 0.03 | 0.8731 | 2.4904 |
| C: pH | 1 | 5.66 | 5.66 | 0.00 | 0.9648 | 0.6871 |
| D: Ratio | 1 | 1838.72 | 1838.72 | 0.66 | 0.4330 | 12.3785 |
| AA | 1 | 308.46 | 308.46 | 0.11 | 0.7454 | −7.6050 |
| AB | 1 | 129.49 | 129.49 | 0.05 | 0.8331 | 5.6896 |
| AC | 1 | 76.57 | 76.57 | 0.03 | 0.8713 | −4.3752 |
| AD | 1 | 38.29 | 38.29 | 0.01 | 0.9087 | 3.0938 |
| BB | 1 | 1122.19 | 1122.19 | 0.40 | 0.5381 | −14.5056 |
| BC | 1 | 43.93 | 43.93 | 0.02 | 0.9023 | −3.3138 |
| BD | 1 | 454.39 | 454.39 | 0.16 | 0.6938 | −10.6582 |
| CC | 1 | 251.40 | 251.40 | 0.09 | 0.7693 | 6.8656 |
| CD | 1 | 2621.09 | 2621.09 | 0.94 | 0.3518 | 25.5983 |
| DD | 1 | 377.87 | 377.87 | 0.14 | 0.7194 | −8.4173 |
| Residual | 12 | 33,520.90 | 2793.41 | |||
| Total | 26 | 47,794.30 |
| Optimal solvent | Most Influential Factor | Optimal Time | Average Amount of Capsiate Extracted (n = 3) | |
|---|---|---|---|---|
| UAE | 42% methanol + 58% ethyl acetate | Ratio | 2 min | 1323.68 ± 32.30 μg g−1 |
| MAE | 100% methanol | - | 5 min | 1403.98 ± 39.94 μg g−1 |
| Extraction Method | Capsiate Concentration (μg/g) | Solvent Used | References |
|---|---|---|---|
| Maceration | 11–369 | Acetonitrile | [34] |
| Sonication | 540–1310 | Acetonitrile | [55] |
| Shaking | 1.21–544.59 | Ethyl acetate | [11] |
| UAE | 150–250 | Ethanol, acetone, hexane, methanol | [44] |
| UAE | 1323.68 | Methanol + ethyl acetate | This work |
| MAE | 1403.98 | Methanol |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Espinosa, M.; González de Peredo, A.V.; Ferreiro-González, M.; Barroso, C.G.; Palma, M.; Barbero, G.F.; Espada-Bellido, E. Optimizing and Comparing Ultrasound- and Microwave-Assisted Extraction Methods Applied to the Extraction of Antioxidant Capsinoids in Peppers. Agronomy 2019, 9, 633. https://doi.org/10.3390/agronomy9100633
Vázquez-Espinosa M, González de Peredo AV, Ferreiro-González M, Barroso CG, Palma M, Barbero GF, Espada-Bellido E. Optimizing and Comparing Ultrasound- and Microwave-Assisted Extraction Methods Applied to the Extraction of Antioxidant Capsinoids in Peppers. Agronomy. 2019; 9(10):633. https://doi.org/10.3390/agronomy9100633
Chicago/Turabian StyleVázquez-Espinosa, Mercedes, Ana V. González de Peredo, Marta Ferreiro-González, Carmelo G. Barroso, Miguel Palma, Gerardo F. Barbero, and Estrella Espada-Bellido. 2019. "Optimizing and Comparing Ultrasound- and Microwave-Assisted Extraction Methods Applied to the Extraction of Antioxidant Capsinoids in Peppers" Agronomy 9, no. 10: 633. https://doi.org/10.3390/agronomy9100633
APA StyleVázquez-Espinosa, M., González de Peredo, A. V., Ferreiro-González, M., Barroso, C. G., Palma, M., Barbero, G. F., & Espada-Bellido, E. (2019). Optimizing and Comparing Ultrasound- and Microwave-Assisted Extraction Methods Applied to the Extraction of Antioxidant Capsinoids in Peppers. Agronomy, 9(10), 633. https://doi.org/10.3390/agronomy9100633