Growth, Physiological, Biochemical, and Transcriptional Responses to Drought Stress in Seedlings of Medicago sativa L., Medicago arborea L. and Their Hybrid (Alborea)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Seeds Pretreatment
2.3. Growth Conditions and Drought Treatment
2.4. Growth and Physiological Parameters
2.5. Sample Preparation for Antioxidant Assays
2.6. Total Antioxidant Capacity Assays
2.7. MDA
2.8. Antioxidant Enzyme Assays
2.9. Protein Determination
2.10. Primer Design
2.11. cDNA Synthesis and Gene Relative Expression Ratios
2.12. Statistical Analysis
3. Results
3.1. The Effect of Drought Stress on Seedling Growth
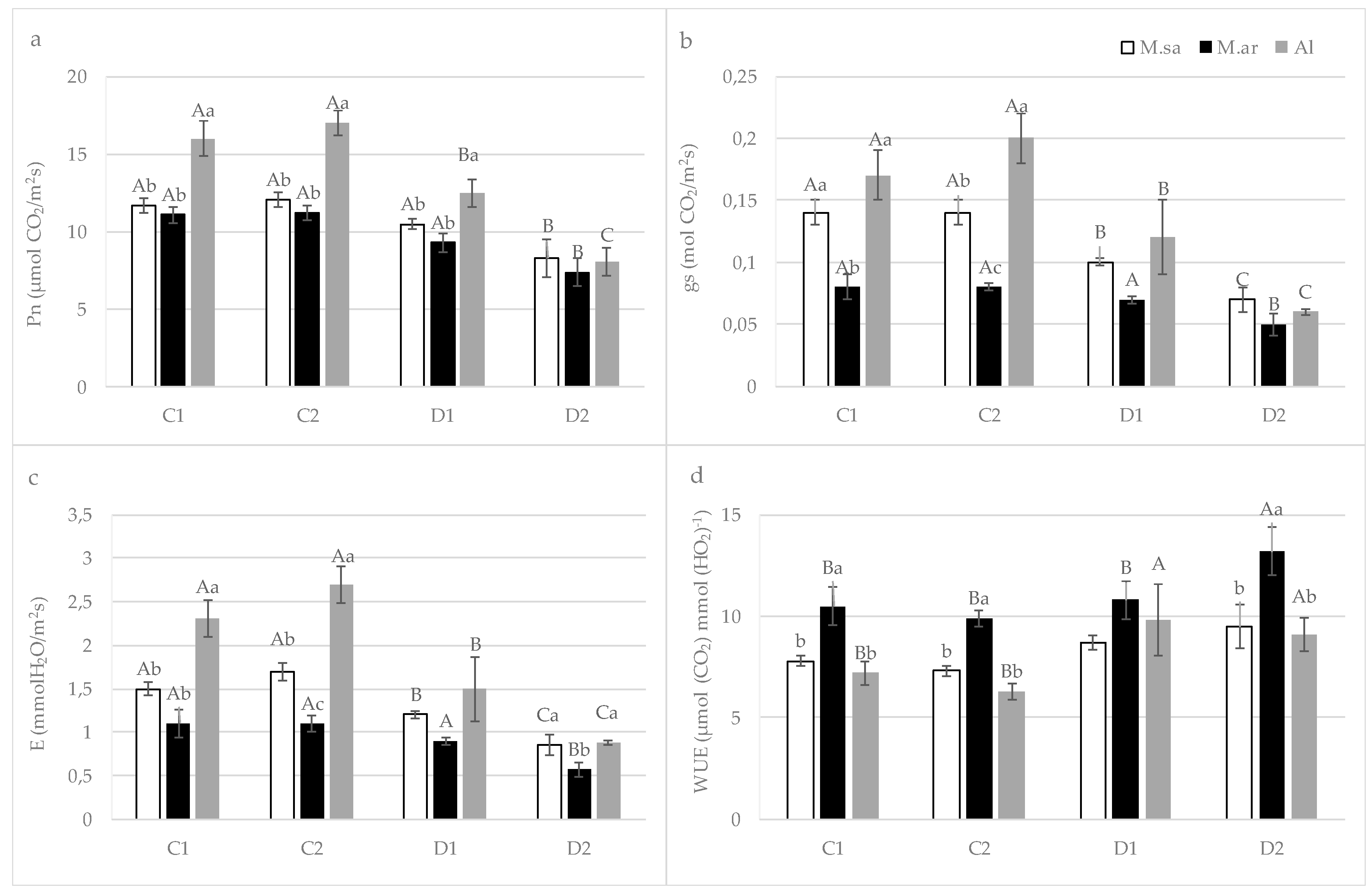
3.2. The Effect of Drought Stress on Physiological Parameters
3.3. The Effect of Drought Stress on the Antioxidant Capacity
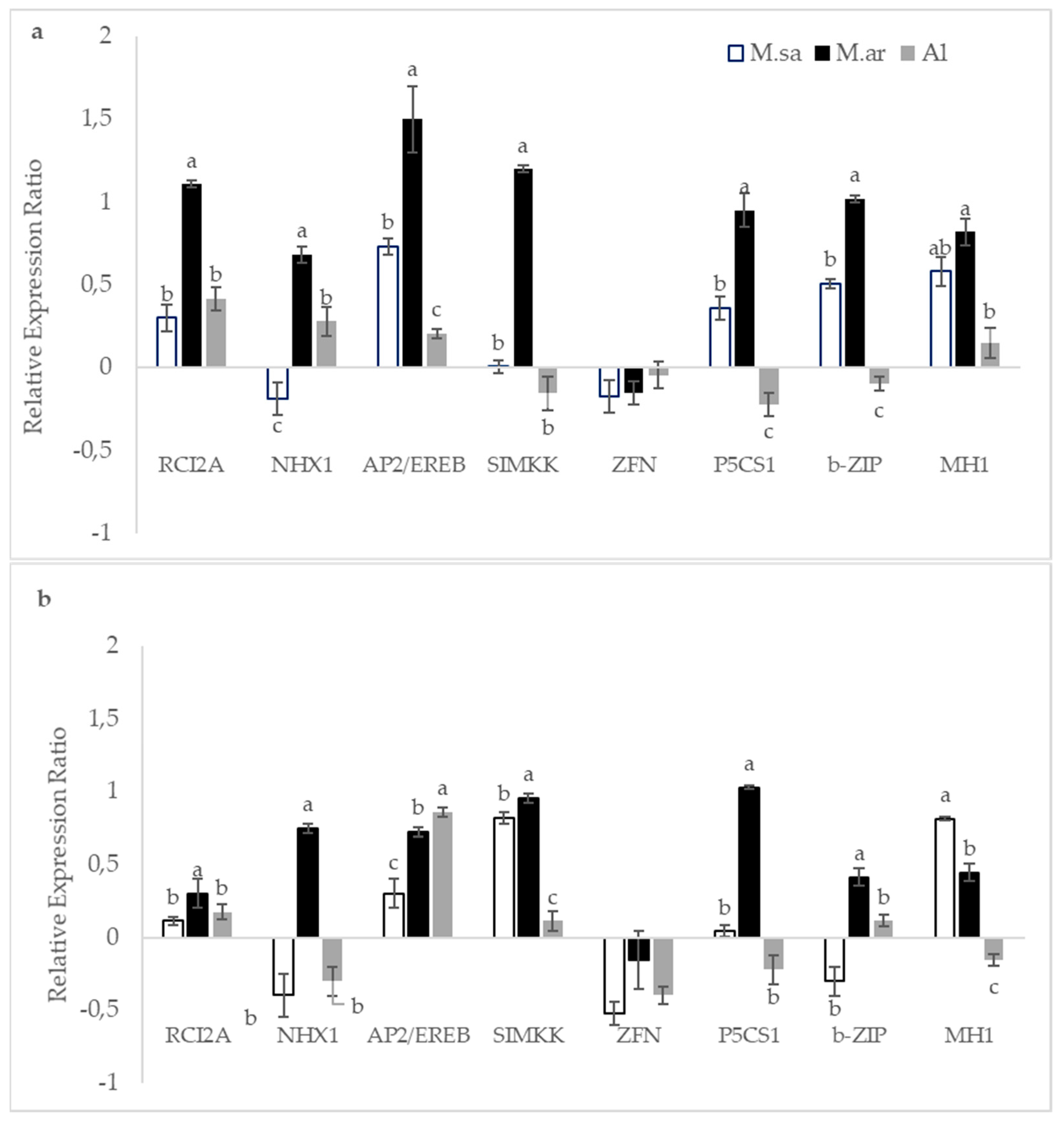
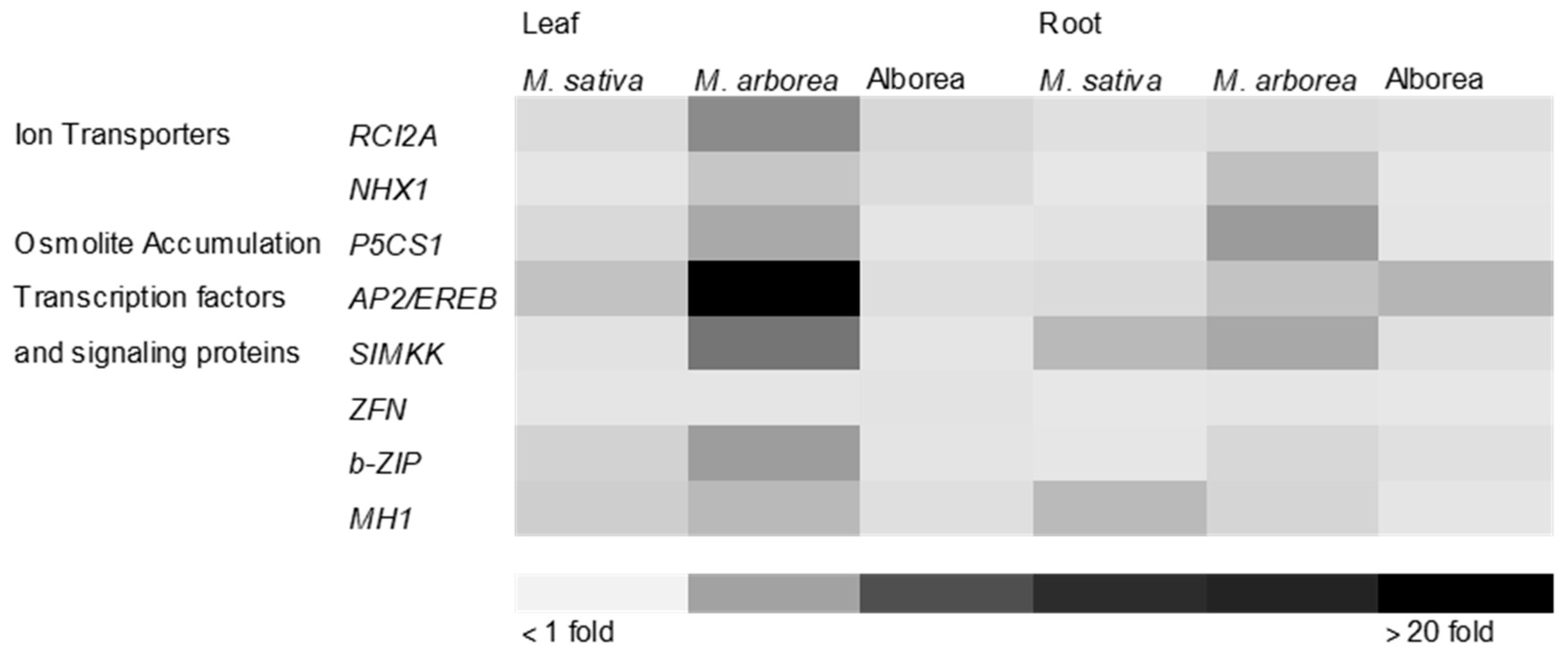
3.4. The Effect of Drought Stress on Transcriptional Responses
4. Discussion
4.1. Seedling Growth and Physiological Responses under Drought Stress
4.2. Drought Stress and Antioxidant Capacity
4.3. Drought Stress and Transcriptional Responses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Valliyodan, B.; Nguyen, H.T. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr. Opin. Plant Βiol. 2006, 9, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Reguera, M.; Peleg, Z.; Blumwald, E. Targeting metabolic pathways for genetic engineering abiotic stress-tolerance in crops. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2012, 1819, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Wang, L.; Farooq, M.; Xue, L.; Ali, S. Fulvic acid application improves the maize performance under well-watered and drought conditions. J. Agron. CropSci. 2011, 197, 409–417. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Xue, L.L.; Zou, C.M. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agron. CropSci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Ashraf, U.; Kanu, A.S.; Mo, Z.; Hussain, S.; Anjum, S.A.; Khan, I.; Abbas, R.N.; Tang, X. Lead toxicity in rice: Effects, mechanisms, and mitigation strategies—A mini review. Environ. Sci. Pollut. Res. Int. 2015, 22, 18318–18332. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Adebayo, M.A.; Menkir, A. Assessment of hybrids of drought tolerant maize (Zea mays L.) inbred lines for grain yield and other traits under stress managed conditions. Niger. J. Genet. 2014, 28, 19–23. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Ramanjulu, S.; Bartels, D. Drought- and desiccation-induced modulation of gene expression in plants. Plant Cell Environ. 2002, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, N.; Sopory, S.K.; KaviKishor, P.B. Deciphering the regulatory mechanisms of abiotic stress tolerance in plants by genomic approaches. Gene 2007, 388, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.S.; Clarke, T.R.; Inoue, Y.; Vidal, A. Estimating crop water deficit using the relation between surface-air temperature and spectral vegetation index. Remote. Sens. Environ. 1994, 49, 246–263. [Google Scholar] [CrossRef]
- Bhattarai, K.; Brummer, E.C.; Monteros, M.J. Alfalfa as a bioenergy crop. In Bioenergy Feedstocks: Breeding and Genetics; Saha, M.C., Bhandari, H.S., Bouton, J.H., Eds.; Wiley: New York, NY, USA, 2013; pp. 207–231. ISBN 9780470960332. [Google Scholar]
- Tang, L.L.; Cai, H.; Ji, W.; Luo, X.; Wang, Z.Y.; Wu, J.; Wang, X.D.; Cui, L.; Wang, Y.; Zhu, Y.M.; et al. Overexpression of GsZFP1 enhances salt and drought tolerance in transgenic alfalfa (Medicago sativa L.). Plant Physiol. Biochem. 2013, 71, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Wen, F.; Yao, D.; Wang, L.; Guo, J.; Ni, L.; Zhang, A.; Tan, M.; Jiang, M. A novel rice C2H2-type zinc finger protein, ZFP36, is a key player involved in abscisic acid-induced antioxidant defense and oxidative stress tolerance in rice. J. Exp. Bot. 2014, 65, 5795–5809. [Google Scholar] [CrossRef]
- Quan, W.; Liu, X.; Wang, H.; Chan, Z. Comparative physiological and transcriptional analyses of two contrasting drought tolerant alfalfa varieties. Front. Plant Sci. 2015, 6, 1256. [Google Scholar] [CrossRef]
- Wu, S.; Ning, F.; Zhang, Q.; Wu, X.; Wang, W. Enhancingomics research of crop responses to drought under field conditions. Front. Plant Sci. 2017, 8, 174. [Google Scholar]
- Noitsakis, B.; Radoglou, K.M.; Jarvis, P.G. Water relation and growth in two years old seedlings of Medicagoarborea under short-time water stress. PhytonAnn. Rei Bot. 1991, 31, 111–120. [Google Scholar]
- Bingham, E.T.; Armour, D.; Irwin, J.A.G. The hybridization barrier between herbaceous Medicago sativa and woody M. arborea is weakened by selection of seed parents. Plants 2013, 2, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Irwin, J.A.G.; Sewell, J.C.; Woodfield, D.R.; Bingham, E.T. Restructuring lucerne (Medicago sativa) through introgression of the Medicagoarborea genome. Agric. Sci. 2016, 28, 40–46. [Google Scholar]
- Tani, E.; Sarri, E.; Goufa, M.; Asimakopoulou, G.; Psychogiou, M.; Bingham, E.; Skaracis, G.; Abraham, E. Seedling growth and transcriptional responses to salt shock and stress in Medicago sativa L., Medicagoarborea L., and their hybrid (Alborea). Agronomy 2018, 8, 231. [Google Scholar] [CrossRef]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Y.B.; Dong, Y.X.; Gao, X.-Q.; Zhang, X.S. Expression of a putative alfalfa helicase increases tolerance to abiotic stress in arabidopsis by enhancing the capacities for ros scavenging and osmotic adjustment. J. Plant Physiol. 2009, 166, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Y.; Yang, Q.; Fang, F.; Kang, J.; Zhang, T. Isolation and characterization of a gene from Medicago sativa L., encoding a bZIP transcription factor. Mol. Biol. Rep. 2013, 40, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Suh, A. Evaluation of Bioactivity of Phytotoxins from Pathogenic Fungi of Orobanche sp. Ph.D. Thesis, Argicultural University of Athens, Athens Greece, 2011. [Google Scholar]
- Barrs, H.; Weatherley, P. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (frap) as a measure of “antioxidant power”: The frap assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Li, X.; Luo, Y.; Shen, H.; You, J. Antioxidant activities and functional properties of grass carp (Ctenopharyngodonidellus) protein hydrolysates. J. Sci. Food Agric. 2012, 92, 292–298. [Google Scholar] [CrossRef]
- Stewart, R.R.C.; Bewley, J.D. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase: An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [PubMed]
- Polle, A.; Otter, T.; Seifert, F. Apoplastic peroxidases and lignification in needles of Norway spruce (Piceaabies L.). Plant Physiol. 1994, 106, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M. Performance of wheat genotypes under osmotic stress at germination and early seedling growth stage. Sky J. Agric. Res. 2013, 2, 116–119. [Google Scholar]
- Ings, J.; Mur, L.A.J.; Robson, P.R.H.; Bosch, M. Physiological and growth responses to water deficit in the bioenergy crop Miscanthus x giganteus. Front. Plant Sci. 2013, 4, 468. [Google Scholar] [CrossRef]
- Mouradi, M.; Farissi, M.; Bouizgaren, A.; MAKOUDI, B.; Kabbadj, A.; Véry, A.-A.; Sentenac, H.; Qaddourya, A.; Ghoulam, C. Effects of water deficit on growth, nodulation and physiological and biochemical processes in Medicago sativa-rhizobia symbiotic association. Arid. Land Res. Manag. 2016, 30, 193–208. [Google Scholar] [CrossRef]
- Benjamin, J.G.; Nielsen, D.C.; Vigil, M.F.; Mikha, M.M.; Calderon, F. Water deficit stress effects on corn (Zea mays L.) root:shoot ratio. Open J. Soil Sci. 2014, 4, 10. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, S.; Wang, B.; Zhao, J. Physiological and biochemical changes in different drought-tolerant alfalfa (Medicago sativa L.) varieties under PEG-induced drought stress. Acta Physiol. Plant. 2018, 40, 25. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Periera, S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Clifton-Brown, J.C.; Lewandowski, I. Water use efficiency and biomass partitioning of three different miscanthus genotypes with limited and unlimited water supply. Ann. Bot. 2000, 86, 191–200. [Google Scholar] [CrossRef]
- Lugojan, C.; Ciulca, S. Evaluation of relative water content in winter wheat. J. Hortic. For. Biotechnol. 2011, 15, 173–177. [Google Scholar]
- Hassanzadeh, M.; Ebadi, A.; Panahyan-e-Kivi, M.G.; Eshghi, A.; Jamaati-e-Somarin, S.; Saeidi, M.; Zabihi-e-Mahmoodabad, R. Evaluation of drought stress on relative water content and chlorophyll content of sesame (Sesamumindicum L.) genotypes at early flowering stage. Res. J. Environ. Sci. 2009, 3, 345–350. [Google Scholar] [CrossRef]
- Jia, W.; Zhang, J. Stomatal movements and long-distance signaling in plants. Plant Signal. Behav. 2008, 3, 772–777. [Google Scholar] [CrossRef]
- Harb, A.; Krishnan, A.; Ambavaram, M.M.R.; Pereira, A. Molecular and physiological analysis of drought stress in arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 2010, 154, 1254–1271. [Google Scholar] [CrossRef]
- Galmés, J.; Flexas, J.; Savé, R.; Medrano, H. Water relations and stomatal characteristics of mediterranean plants with different growth forms and leaf habits: Responses to water stress and recovery. Plant Soil 2007, 290, 139–155. [Google Scholar] [CrossRef]
- Bousba, R.; Ykhlef, N.; Djekoun, A. Water use efficiency and flat leaf photosynthetic in response to water deficit of durum wheat (Triticum durum desf). WJAS 2009, 5, 609–616. [Google Scholar]
- Bramley, H.; Turner, N.C.; Turner, D.W.; Tyerman, S.D. Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots. Plant Physiol. 2009, 150, 348–364. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Ferreira, H.F.; Correia, C.M. Changes in growth, gas exchange, xylem hydraulic properties and water use efficiency of three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2007, 60, 183–192. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. Does enhanced photosynthesis enhance growth? Lessons learned from CO2 enrichment studies. Plant Physiol. 2011, 155, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, D.Y.; Mortier, F.; Gourlet-Fleury, S.; Freycon, V.; Picard, N.; Turnbull, M. Slow-growing species cope best with drought: Evidence from long-term measurements in a tropical semi-deciduous moist forest of central Africa. J. Ecol. 2013, 101, 1459–1470. [Google Scholar] [CrossRef]
- Zhou, Z.; Su, P.; González-Paleo, L.; Xie, T.; Li, S.; Zhang, H. Trade-off between leaf turnover and biochemical responses related to drought tolerance in desert woody plants. J. Arid. Environ. 2014, 103, 107–113. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Extreme temperature responses, oxidative stress and antioxidant defense in plants. In Abiotic Stress—Plant Responses and Applications in Agriculture; Vahdati, D.K., Ed.; InTech: London, UK, 2013; pp. 169–205. [Google Scholar]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Fazeli, F.; Ghorbanli, M.; Niknam, V. Effect of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biol. Plant. 2007, 51, 98–103. [Google Scholar] [CrossRef]
- Ren, S.; Lyle, C.; Jiang, G.-L.; Penumala, A. Soybean salt tolerance 1 (GmST1) reduces ROS production, enhances aba sensitivity, and abiotic stress tolerance in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 445. [Google Scholar] [CrossRef] [PubMed]
- Avramova, V.; AbdElgawad, H.; Vasileva, I.; Petrova, A.S.; Holek, A.; Mariën, J.; Asard, H.; Beemster, G.T.S. High antioxidant activity facilitates maintenance of cell division in leaves of drought tolerant maize hybrids. Front. Plant Sci. 2017, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Navarre, C.; Goffeau, A. Membrane hyperpolarization and salt sensitivity induced by PMP3 a highly conserved small protein of yeast plasma membrane. EMBO J. 2000, 19, 2515–2524. [Google Scholar] [CrossRef] [PubMed]
- Nylander, M.; Heino, P.; Helenius, E.; TapioPalva, E.; Ronne, H.; Welin, B.V. The low-temperature- and salt-induced RCI2A gene of arabidopsis complements the sodium sensitivity caused by a deletion of the homologous yeast gene SNA1. Plant Mol. Biol. 2001, 45, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Sivankalyani, V.; Geetha, M.; Subramanyam, K.; Girija, S. Ectopic expression of arabidopsis RCI2A gene contributes to cold tolerance in tomato. Transgenic Res. 2014, 24, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Farooq, M.; Xie, X.-Y.; Liu, X.-J.; Ijaz, M.F. Antioxidant defense system and proline accumulation enables hot pepper to perform better under drought. Sci. Hortic. 2012, 140, 66–73. [Google Scholar] [CrossRef]
- Tabot, P.T.; Adams, J.B. Salt secretion, proline accumulation and increased branching confer tolerance to drought and salinity in the endemic halophyte Limoniumlinifolium. S. Afr. J. Bot. 2014, 94, 64–73. [Google Scholar] [CrossRef]
- Zhu, H.S.; Yu, X.J.; Zhao, X.; Dong, K.H.; Yang, W.D. The activity and gene expression levels of p5cs and delta-oat in Medicago sativa cvpianguan under drought stress. Pak. J. Bot. 2016, 48, 137–142. [Google Scholar]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Krannich, C.T.; Maletzki, L.; Kurowsky, C.; Horn, R. Network candidate genes in breeding for drought tolerant crops. Int. J. Mol. Sci. 2015, 16, 16378–16400. [Google Scholar] [CrossRef]
- Liang, C.e.a. GhABF2, a bzip transcription factor, confers drought and salinity tolerance in cotton (Gossypiumhirsutum L.). Sci. Rep. 2016, 6, 35040. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mao, B.; Ou, S.; Wang, W.; Liu, L.; Wu, Y.; Chu, C.; Wang, X. OsbZIP71, a bZIP transcription factor, confers salinity and drought tolerance in rice. Plant Mol. Biol. 2014, 84, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Abogadallah, G.M.; Nada, R.M.; Malinowski, R.; Quick, P. Overexpression of HARDY, an AP2/ERF gene from arabidopsis, improves drought and salt tolerance by reducing transpiration and sodium uptake in transgenic Trifoliumalexandrinum L. Planta 2011, 233, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Marcolino-Gomes, J.; Rodrigues, F.A.; Oliveira, M.C.N.; Farias, J.R.B.; Neumaier, N.; Abdelnoor, R.V.; Marcelino-Guimarães, F.C.; Nepomuceno, A.L. Expression patterns of GMAP2/EREB-like transcription factors involved in soybean responses to water deficit. PLoS ONE 2013, 8, e62294. [Google Scholar] [CrossRef] [PubMed]
- Mun, B.-G.; Lee, S.-U.; Park, E.-J.; Kim, H.-H.; Hussain, A.; Imran, Q.M.; Lee, I.-J.; Yun, B.-W. Analysis of transcription factors among differentially expressed genes induced by drought stress in Populusdavidiana. 3 Biotech 2017, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Y.; Chao, D.-Y.; Gao, J.-P.; Zhu, M.-Z.; Shi, M.; Lin, H.-X. A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev. 2009, 23, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Kiegerl, S.; Cardinale, F.; Siligan, C.; Gross, A.; Baudouin, E.; Liwosz, A.; Eklöf, S.; Till, S.; Bögre, L.; Hirt, H.; et al. SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress–induced MAPK, SIMK. Plant Cell 2000, 12, 2247–2258. [Google Scholar] [CrossRef]
- Ning, J.; Li, X.; Hicks, L.M.; Xiong, L. A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol. 2010, 152, 876–890. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef]
- Sinha, A.K.; Jaggi, M.; Raghuram, B.; Tuteja, N. Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal. Behav. 2011, 6, 196–203. [Google Scholar] [CrossRef]
- Shivakumara, T.N.; Sreevathsa, R.; Dash, P.K.; Sheshshayee, M.S.; Papolu, P.K.; Rao, U.; Tuteja, N.; UdayaKumar, M. Overexpression of pea DNA helicase 45 (PDH45) imparts tolerance to multiple abiotic stresses in chili (Capsicum annuum L.). Sci. Rep. 2017, 7, 2760. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Heterosis, stress, and the environment: A possible road map towards the general improvement of crop yield. J. Exp. Bot. 2013, 64, 4829–4837. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, V.K.; Mikkilineni, V.; Zehr, U.B.; Tyagi, A.K.; Kapoor, S. Heterosis: Emerging ideas about hybrid vigour. J. Exp. Bot. 2012, 63, 6309–6314. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Xiao, M.; Hayward, A.; Jiang, G.; Zhu, L.; Zhou, Q.; Li, J.; Zhang, M. What is crop heterosis: New insights into an old topic. J. Appl. Genet. 2015, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Barnaby, J.Y.; Kim, M.; Bauchan, G.; Bunce, J.; Reddy, V.; Sicher, R.C. Drought responses of foliar metabolites in three maize hybrids differing in water stress tolerance. PLoS ONE 2013, 8, e77145. [Google Scholar] [CrossRef] [PubMed]

| Sequence Name | Genbank Number | Primer Sequence | Amplicon Size |
|---|---|---|---|
| Ms-actin2-F | JQ028730.1 | TTCTCACCACACTTCTCGCC | 173bp |
| Ms-actin2-R | CCAGCCTTCACCATTCCAGT | ||
| Ms-AP2/EREB-F | Not deposited | AATGGGTGGGGAAACGGAAC | 95bp |
| Ms- AP2/EREB-R | TTTGGTGGTGGAGTGTGGTT | ||
| Ms-NHX1-F | AY513732.1 | GCCATGAAATTCACCGACCG | 118bp |
| Ms-NHX1-R | CTGCCACCAAAAACAGGACG | ||
| Ms- P5CS1-F | X98421.1 | TTTGCGGTCGGAAGGTGTTA | 119bp |
| Ms- P5CS1-R | CGATTTCCAAGGTGCAAGCC | ||
| Ms-ZFN-F | JX131368.1 | CCCAAGCTGCAAGTTTGACC | 154bp |
| Ms-ZFN-R | TGAGCCCGACTCAACAAGTC | ||
| Ms-SIMKK-F | AJ293274.1 | ACCAGAAGCTCCAACGACTG | 94bp |
| Ms-SIMKK-R | CCTCGAAGCAGTCCATCTCC | ||
| Ms-RCI2-F | JQ665271.1 | GTTGTCAGGGGCGTCATTCT | 169bp |
| Ms-RCI2-R | TCCAAGCAGGACAAAACGGA | ||
| Ms-Helicase-F | EF011022.1 | CCGGATCTTCAGGTTTGCCT | 131bp |
| Ms-Helicase-R | TGCTTGATGCCCTCCAATGT | ||
| Ms-bZIP-F | HQ911778.1 | GGTGACAGTGGTTCAGAGGG | 109bp |
| Ms-bZIP-R | CGTTGGCTCCATCAACAAGC |
| Height (cm) | SER | RWC | Pn (μmol CO2/m2s) | Gs (mol CO2/m2s) | E (mmol H2O/m2s) | WUE (μmol (CO2) mmol (H2O)−1 | ||
|---|---|---|---|---|---|---|---|---|
| Drought | Control | 23 ± 0.75a * | 0.8 ± 0.06a | 0.9 ± 0.09a | 13.2 ± 0.5a | 0.14 ± 0.01a | 1.7 ± 0.12a | 8.2 ± 0.3b |
| Drought | 21 ± 0.52b | 0.4 ± 0.04b | 0.8 ± 0.01b | 9.3 ± 0.4b | 0.08 ± 0.006b | 1.0 ± 0.08b | 10.2 ± 0.5a | |
| Entries | M. arborea | 16 ± 0.21c | 0.3 ± 0.03b | 0.87 ± 0.01a | 9.7 ± 0.5b | 0.07 ± 0.004b | 0.9 ± 0.07c | 11.1 ± 0.50a |
| M. sativa | 19 ± 0.41b | 0.4 ± 0.04b | 0.85 ± 0.01a | 10.6 ± 0.5b | 0.11 ± 0.009a | 1.3 ± 0.08b | 8.3 ± 0.34b | |
| Alborea | 31 ± 0.77a | 1.0 ± 0.09a | 0.8 ± 0.02b | 13.4 ± 0.9a | 0.14 ± 0.015a | 1.8 ± 0.20a | 8.1 ± 0.60b | |
| Dates | 1 (20/11/15) | 17 ± 0.59e | ||||||
| 2 (23/11/15) | 20 ± 0.78d | 0.94 ± 0.10a | ||||||
| 3 (26/11/15) | 22 ± 1.04c | 0.84 ± 0.10a | 0.87 ± 0.01a | 11.8 ± 0.5a | 0.11 ± 0.008 | 1.4 ± 0.11 | 9.1 ± 0.4 | |
| 4 (30/11/15) | 24 ± 1.13bc | 0.51 ± 0.05b | ||||||
| 5 (3/12/15) | 25 ± 1.25ab | 0.46 ± 0.07b | 0.85 ± 0.01b | 10.7 ± 0.7b | 0.10 ± 0.010 | 1.3 ± 0.14 | 9.2 ± 0.5 | |
| 6 (7/12/15) | 26 ± 1.33a | 0.26 ± 0.04b | 0.8 ± 0.02c | |||||
| Source of | Drought (A) | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 |
| Variation | Entries (B) | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 |
| Dates (C) | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | ns | ns | ns | |
| AXB | ns ** | p ˂ 0.05 | ns | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | ns | |
| AXC | ns | ns | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | ns | |
| BXC | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | ns | ns | ns | ns | |
| AXBXC | ns | ns | ns | ns | ns | ns | ns |
| ABTS | FRAP | SOD | POD | MDA | Total Proteins | |
|---|---|---|---|---|---|---|
| Entries | ||||||
| M. arborea | 1.7 ± 0.33ab * | 2.4 ± 0.4 | 1.1 ± 0.2 | 0.7 ± 0.20b | 1.3 ± 0.31b | 2.3 ± 0.2a |
| M. sativa | 2.1 ± 0.18a | 2.1 ± 0.2 | 0.8 ± 0.1 | 0.5 ± 0.04b | 1.6 ± 0.13ab | 2.0 ± 0.6a |
| Alborea | 1.2 ± 0.22b | 1.9 ± 0.4 | 1.5 ± 0.1 | 1.4 ± 0.10a | 1.8 ± 0.36a | 0.8 ± 0.1b |
| Organs | ||||||
| Leaves | 1.9 ± 0.23a | 2.0 ± 0.3 | 1.3 ± 0.1 | 0.8 ± 0.2 | 2.2 ± 0.2a | 1.9 ± 0.4 |
| Roots | 1.4 ± 0.21b | 2.3 ± 0.3 | 1.0 ± 0.2 | 1.2 ± 0.1 | 0.9 ± 0.1b | 1.4 ± 0.3 |
| Source of variation | ||||||
| Entries (A) | p < 0.05 | ns | ns | p < 0.05 | p < 0.05 | p < 0.05 |
| Tissues (B) | p < 0.05 | ns | ns | ns | p < 0.05 | ns |
| AXB (Interaction) | ns ** | ns | ns | p < 0.05 | ns | p < 0.05 |
| AREB | bZIP | P5CS1 | ZFG | NHX | MH1 | SIMKK | RCI2A | |
|---|---|---|---|---|---|---|---|---|
| Source of variation | ||||||||
| Drought (A) | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 |
| Entries (B) | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | ns | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 |
| Organs (C) | p ˂ 0.05 | p ˂ 0.05 | ns * | p ˂ 0.05 | p ˂ 0.05 | ns | ns | p ˂ 0.05 |
| AXB (Interaction) | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | ns | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 |
| AXC (Interaction) | p ˂ 0.05 | p ˂ 0.05 | ns | p ˂ 0.05 | ns | ns | ns | p ˂ 0.05 |
| BXC (Interaction) | p ˂ 0.05 | p ˂ 0.05 | ns | ns | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 |
| AXBXC(Interaction) | p ˂ 0.05 | p ˂ 0.05 | ns | ns | ns | p ˂ 0.05 | p ˂ 0.05 | p ˂ 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tani, E.; Chronopoulou, E.G.; Labrou, N.E.; Sarri, E.; Goufa, Μ.; Vaharidi, X.; Tornesaki, A.; Psychogiou, M.; Bebeli, P.J.; Abraham, Ε.M. Growth, Physiological, Biochemical, and Transcriptional Responses to Drought Stress in Seedlings of Medicago sativa L., Medicago arborea L. and Their Hybrid (Alborea). Agronomy 2019, 9, 38. https://doi.org/10.3390/agronomy9010038
Tani E, Chronopoulou EG, Labrou NE, Sarri E, Goufa Μ, Vaharidi X, Tornesaki A, Psychogiou M, Bebeli PJ, Abraham ΕM. Growth, Physiological, Biochemical, and Transcriptional Responses to Drought Stress in Seedlings of Medicago sativa L., Medicago arborea L. and Their Hybrid (Alborea). Agronomy. 2019; 9(1):38. https://doi.org/10.3390/agronomy9010038
Chicago/Turabian StyleTani, Eleni, Evangelia G. Chronopoulou, Nikolaos E. Labrou, Effie Sarri, Μaria Goufa, Xristina Vaharidi, Alexia Tornesaki, Maria Psychogiou, Penelope J. Bebeli, and Εleni M. Abraham. 2019. "Growth, Physiological, Biochemical, and Transcriptional Responses to Drought Stress in Seedlings of Medicago sativa L., Medicago arborea L. and Their Hybrid (Alborea)" Agronomy 9, no. 1: 38. https://doi.org/10.3390/agronomy9010038
APA StyleTani, E., Chronopoulou, E. G., Labrou, N. E., Sarri, E., Goufa, Μ., Vaharidi, X., Tornesaki, A., Psychogiou, M., Bebeli, P. J., & Abraham, Ε. M. (2019). Growth, Physiological, Biochemical, and Transcriptional Responses to Drought Stress in Seedlings of Medicago sativa L., Medicago arborea L. and Their Hybrid (Alborea). Agronomy, 9(1), 38. https://doi.org/10.3390/agronomy9010038









