Architectural Root Responses of Rice to Reduced Water Availability Can Overcome Phosphorus Stress
Abstract
Highlight
Abstract
1. Introduction
2. Material and Methods
2.1. Pot trial with Lowland Soil
2.1.1. Trial Establishment and Management
2.1.2. Data Collection on Shoot Growth and Root Architecture
2.1.3. Data Analyses
2.2. Pot Trial with Upland Soil
2.3. Lowland Field Trial
2.3.1. Trial Establishment and Management
2.3.2. Data Collection
2.4. Upland Field Trial
3. Results
3.1. Pot Trial with Lowland Soil
3.2. Pot Trial with Upland Soil
3.3. Lowland Field Trial
3.4. Upland Field Trial
4. Discussion
4.1. Shoot and Grain Yield Responses of Contrasting Rice Varieties to Combinations of P and Water Availability
4.2. Architectural Responses of Rice Roots to P and Water Availability
4.3. Comparing Root Architecture among Varieties in Relation to Stress (Low P & Drought) Tolerance
4.4. Implications of the Root Responses to Reduced Water Availability for P Acquisition and Agronomic Measures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chauhan, B.S.; Jabran, K.; Mahajan, G. (Eds.) Rice Production Worldwide; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-47514-1. [Google Scholar]
- Seck, P.A.; Tollens, E.; Wopereis, M.C.S.; Diagne, A.; Bamba, I. Rising trends and variability of rice prices: Threats and opportunities for sub-Saharan Africa. Food Policy 2010, 35, 403–411. [Google Scholar] [CrossRef]
- van Oort, P.A.J.; Saito, K.; Tanaka, A.; Amovin-Assagba, E.; Van Bussel, L.G.J.; van Wart, J.; de Groot, H.; van Ittersum, M.K.; Cassman, K.G.; Wopereis, M.C.S. Assessment of rice self-sufficiency in 2025 in eight African countries. Glob. Food Secur. 2015, 5, 39–49. [Google Scholar] [CrossRef]
- Diagne, A.; Alia, D.Y.; Amovin-Assagba, E.; Wopereis, M.C.S.; Saito, K.; Nakelse, T. Farmer perceptions of the biophysical constraints to rice production in sub-Saharan Africa, and potential impact of research. In Realizing Africa’s Rice Promise; CABI: Wallingford, UK, 2013; pp. 46–68. [Google Scholar]
- Nziguheba, G.; Zingore, S.; Kihara, J.; Merckx, R.; Njoroge, S.; Otinga, A.; Vandamme, E.; Vanlauwe, B. Phosphorus in smallholder farming systems of sub-Saharan Africa: Implications for agricultural intensification. Nutr. Cycl. Agroecosystems 2016, 104, 321–340. [Google Scholar] [CrossRef]
- Sahrawat, K.L.; Jones, M.P.; Diatta, S. Plant phosphorus and rice yield in an Ultisol of the humid forest zone in West Africa. Commun. Soil Sci. Plant Anal. 1998, 29, 997–1005. [Google Scholar] [CrossRef]
- Saito, K.; Nelson, A.; Zwart, S.J.; Niang, A.; Sow, A.; Yoshida, H.; Wopereis, M.C.S. Towards a better understanding of biophysical determinants of yield gaps and the potential for expansion of the rice area in Africa. In Realizing Africa’s Rice Promise; CABI: Wallingford, UK, 2013; pp. 188–203. [Google Scholar]
- Serraj, R.; McNally, K.L.; Slamet-Loedin, I.; Kohli, A.; Haefele, S.M.; Atlin, G.; Kumar, A. Drought Resistance Improvement in Rice: An Integrated Genetic and Resource Management Strategy. Plant Prod. Sci. 2011, 14, 1–14. [Google Scholar] [CrossRef]
- Vandamme, E.; Rose, T.; Saito, K.; Jeong, K.; Wissuwa, M. Integration of P acquisition efficiency, P utilization efficiency and low grain P concentrations into P-efficient rice genotypes for specific target environments. Nutr. Cycl. Agroecosystems 2016, 104, 413–427. [Google Scholar] [CrossRef]
- Bishopp, A.; Lynch, J.P. The hidden half of crop yields. Nat. Plants 2015, 1, 15117. [Google Scholar] [CrossRef]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef]
- Ramaekers, L.; Remans, R.; Rao, I.M.; Blair, M.W.; Vanderleyden, J. Strategies for improving phosphorus acquisition efficiency of crop plants. Field Crops Res. 2010, 117, 169–176. [Google Scholar] [CrossRef]
- Zhan, A.; Schneider, H.; Lynch, J.P. Reduced Lateral Root Branching Density Improves Drought Tolerance in Maize. Plant Physiol. 2015, 168, 1603–1615. [Google Scholar] [CrossRef]
- Kano, M.; Inukai, Y.; Kitano, H.; Yamauchi, A. Root plasticity as the key root trait for adaptation to various intensities of drought stress in rice. Plant Soil 2011, 342, 117–128. [Google Scholar] [CrossRef]
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.M.; Testerink, C. Roots Withstanding their Environment: Exploiting Root System Architecture Responses to Abiotic Stress to Improve Crop Tolerance. Front. Plant Sci. 2016, 7, 1335. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J. Root Architecture and Plant Productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef]
- Lynch, J.P. Root Architecture and Nutrient Acquisition. In Nutrient Acquisition by Plants; Springer: Berlin/Heidelberg, Germany, 2005; pp. 147–183. [Google Scholar]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.J.; Impa, S.M.; Rose, M.T.; Pariasca-Tanaka, J.; Mori, A.; Heuer, S.; Johnson-Beebout, S.E.; Wissuwa, M. Enhancing phosphorus and zinc acquisition efficiency in rice: A critical review of root traits and their potential utility in rice breeding. Ann. Bot. 2013, 112, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lynch, J.P. The contribution of lateral rooting to phosphorus acquisition efficiency in maize (Zea mays) seedlings. Funct. Plant Biol. 2004, 31, 949–958. [Google Scholar] [CrossRef]
- Heppell, J.; Talboys, P.; Payvandi, S.; Zygalakis, K.C.; Fliege, J.; Withers, P.J.A.; Jones, D.L.; Roose, T. How changing root system architecture can help tackle a reduction in soil phosphate (P) levels for better plant P acquisition. Plant Cell Environ. 2015, 38, 118–128. [Google Scholar] [CrossRef]
- Gao, Y.; Lynch, J.P. Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). J. Exp. Bot. 2016, 67, 4545–4557. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- White, R.G.; Kirkegaard, J.A. The distribution and abundance of wheat roots in a dense, structured subsoil and implications for water uptake. Plant Cell Environ. 2010, 33, 133–148. [Google Scholar] [CrossRef]
- Kirk, G.J.D.; Yu, T.R.; Choudhury, F.A. Phosphorus chemistry in relation to water regime. In Phosphorus Requirements for Sustainable Agriculture in Asia and Oceania. Proceedings of a Symposium, 6–10 March 1989; International Rice Research Institute: Los Baños, Philippines, 1990; pp. 211–223. [Google Scholar]
- Bünemann, E.; Oberson, A.; Frossard, E. (Eds.) Phosphorus in Action; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26, ISBN 978-3-642-15270-2. [Google Scholar]
- Lal, R.; Stewart, B.A. Soil Phosphorus; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781482257847. [Google Scholar]
- Ho, M.D.; Rosas, J.C.; Brown, K.M.; Lynch, J.P. Root architectural tradeoffs for water and phosphorus acquisition. Funct. Plant Biol. 2005, 32, 737. [Google Scholar] [CrossRef]
- Ahmadi, N.; Audebert, A.; Bennett, M.J.; Bishopp, A.; de Oliveira, A.C.; Courtois, B.; Diedhiou, A.; Diévart, A.; Gantet, P.; Ghesquière, A.; et al. The roots of future rice harvests. Rice 2014, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Donald, C.M. The breeding of crop ideotypes. Euphytica 1968, 17, 385–403. [Google Scholar] [CrossRef]
- Mori, A.; Fukuda, T.; Vejchasarn, P.; Nestler, J.; Pariasca-Tanaka, J.; Wissuwa, M. The role of root size versus root efficiency in phosphorus acquisition in rice. J. Exp. Bot. 2016, 67, 1179–1189. [Google Scholar] [CrossRef]
- Vejchasarn, P.; Lynch, J.P.; Brown, K.M. Genetic Variability in Phosphorus Responses of Rice Root Phenotypes. Rice 2016, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Nestler, J.; Keyes, S.D.; Wissuwa, M. Root hair formation in rice (Oryza sativa L.) differs between root types and is altered in artificial growth conditions. J. Exp. Bot. 2016, 67, 3699–3708. [Google Scholar] [CrossRef] [PubMed]
- Asch, F.; Dingkuhn, M.; Sow, A.; Audebert, A. Drought-induced changes in rooting patterns and assimilate partitioning between root and shoot in upland rice. Field Crops Res. 2005, 93, 223–236. [Google Scholar] [CrossRef]
- Henry, A. IRRI’s drought stress research in rice with emphasis on roots: Accomplishments over the last 50 years. Plant Root 2013, 7, 92–106. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Lee, D.-J.; Ito, O.; Siddique, K.H.M. Advances in Drought Resistance of Rice. CRC Crit. Rev. Plant Sci. 2009, 28, 199–217. [Google Scholar] [CrossRef]
- Saito, K.; Vandamme, E.; Segda, Z.; Fofana, M.; Ahouanton, K. A Screening Protocol for Vegetative-stage Tolerance to Phosphorus Deficiency in Upland Rice. Crop Sci. 2015, 55, 1223–1229. [Google Scholar] [CrossRef]
- Vandamme, E.; Wissuwa, M.; Rose, T.; Dieng, I.; Drame, K.N.; Fofana, M.; Senthilkumar, K.; Venuprasad, R.; Jallow, D.; Segda, Z.; et al. Genotypic Variation in Grain P Loading across Diverse Rice Growing Environments and Implications for Field P Balances. Front. Plant Sci. 2016, 7, 1435. [Google Scholar] [CrossRef] [PubMed]
- Menge, D.M.; Kameoka, E.; Kano-Nakata, M.; Yamauchi, A.; Asanuma, S.; Asai, H.; Kikuta, M.; Suralta, R.R.; Koyama, T.; Tran, T.T.; et al. Drought-induced root plasticity of two upland NERICA varieties under conditions with contrasting soil depth characteristics. Plant Prod. Sci. 2016, 19, 389–400. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Hubick, K.T.; Condon, A.G.; Richards, R.A. Carbon Isotope Fractionation and Plant Water-Use Efficiency; Springer: New York, NY, USA, 1989; pp. 21–40. [Google Scholar]
- Muccio, Z.; Jackson, G.P. Isotope ratio mass spectrometry. Analyst 2009, 134, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, S.; Kaeppler, S.M.; Brown, K.M.; Lynch, J.P. Shovelomics: High throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 2011, 341, 75–87. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Matsumoto, S.; Tsuboi, T.; Asea, G.; Maruyama, A.; Kikuchi, M.; Takagaki, M. Water Response of Upland Rice Varieties Adopted in Sub-Saharan Africa: A Water Application Experiment. J. Rice Res. 2014, 2, 121. [Google Scholar] [CrossRef]
- Diagne, A.; Gnonna Midingoyi, S.-K.; Wopereis, M.; Akintayo, I. The NERICA Success Story: Development, Achievements and Lessons Learned; Africa Rice Center: Cotonou, Benin, 2010. [Google Scholar]
- Somado, E.A. NERICA®: The New Rice for Africa: A Compendium; Africa Rice Center: Bouaké, Ivory Coast, 2008. [Google Scholar]
- Vallino, M.; Fiorilli, V.; Bonfante, P. Rice flooding negatively impacts root branching and arbuscular mycorrhizal colonization, but not fungal viability. Plant. Cell Environ. 2014, 37, 557–572. [Google Scholar] [CrossRef]
- Mitchell, J.; Owusu, M.; Fukai, S. Root development of rice under flooded and aerobic conditions. In Proceedings of the 16th Agronomy Conference, Armidale, Australia, 4–18 October 2012. [Google Scholar]
- Henry, A.; Cal, A.J.; Batoto, T.C.; Torres, R.O.; Serraj, R. Root attributes affecting water uptake of rice (Oryza sativa) under drought. J. Exp. Bot. 2012, 63, 4751–4763. [Google Scholar] [CrossRef]
- Kadam, N.N.; Yin, X.; Bindraban, P.S.; Struik, P.C.; Jagadish, K.S.V. Does Morphological and Anatomical Plasticity during the Vegetative Stage Make Wheat More Tolerant of Water Deficit Stress Than Rice? Plant Physiol. 2015, 167, 1389–1401. [Google Scholar] [CrossRef]
- Bao, Y.; Aggarwal, P.; Robbins, N.E.; Sturrock, C.J.; Thompson, M.C.; Tan, H.Q.; Tham, C.; Duan, L.; Rodriguez, P.L.; Vernoux, T.; et al. Plant roots use a patterning mechanism to position lateral root branches toward available water. Proc. Natl. Acad. Sci. USA 2014, 111, 9319–9324. [Google Scholar] [CrossRef]
- De Kroon, H.; Visser, E.J.W. Root Ecology; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 9783662097847. [Google Scholar]
- Poorter, H.; Nagel, O.; Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Aust. J. Plant Physiol. 2000, 27, 1191. [Google Scholar] [CrossRef]
- Gregory, P.J. (Ed.) Plant Roots; Blackwell Publishing Ltd: Oxford, UK, 2006; ISBN 9780470995563. [Google Scholar]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; ISBN 9780123849052. [Google Scholar]
- Borch, K.; Bouma, T.J.; Lynch, J.P.; Brown, K.M. Ethylene: A regulator of root architectural responses to soil phosphorus availability. Plant Cell Environ. 1999, 22, 425–431. [Google Scholar] [CrossRef]
- Nielsen, K.L.; Eshel, A.; Lynch, J.P. The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. J. Exp. Bot. 2001, 52, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.E. Aerenchyma formation. New Phytol. 2003, 161, 35–49. [Google Scholar] [CrossRef]
- Postma, J.A.; Lynch, J.P. Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiol. 2011, 156, 1190–1201. [Google Scholar] [CrossRef]
- Kamara, A.Y.; Ekeleme, F.; Omoigui, L.O.; Oikeh, S.O.; Chikoye, D.; Tegbaru, A. Response of Upland Rice Cultivars to Nitrogen Fertilizer in the Savannas of Nigeria. Agron. J. 2010, 102, 333–339. [Google Scholar] [CrossRef]
- Oikeh, S.O.; Nwilene, F.; Diatta, S.; Osiname, O.; Touré, A.; Okeleye, K.A. Responses of Upland NERICA Rice to Nitrogen and Phosphorus in Forest Agroecosystems. Agron. J. 2008, 100, 735–741. [Google Scholar] [CrossRef]
- Kaneda, C. Breeding and Dissemination Efforts of “NERICA”. Jpn. J. Trop. 2007, 51, 1–4. [Google Scholar]
- Nielsen, K.L.; Lynch, J.P.; Jablokow, A.G.; Curtis, P.S. Carbon cost of root systems: An architectural approach. In Belowground Responses to Rising Atmospheric CO2: Implications for Plants, Soil Biota, and Ecosystem Processes; Springer: Dordrecht, The Netherlands, 1994; pp. 161–169. [Google Scholar]
- Berntson, G.M. Modelling root architecture: Are there tradeoffs between efficiency and potential of resource acquisition? New Phytol. 1994, 127, 483–493. [Google Scholar] [CrossRef]
- Ge, Z.; Rubio, G.; Lynch, J.P. The importance of root gravitropism for inter-root competition and phosphorus acquisition efficiency: Results from a geometric simulation model. Plant Soil 2000, 218, 159–171. [Google Scholar] [CrossRef]
- Föhse, D.; Claassen, N.; Jungk, A. Phosphorus efficiency of plants. Plant Soil 1991, 132, 261–272. [Google Scholar] [CrossRef]
- Hammond, J.P.; Broadley, M.R.; White, P.J.; King, G.J.; Bowen, H.C.; Hayden, R.; Meacham, M.C.; Mead, A.; Overs, T.; Spracklen, W.P.; et al. Shoot yield drives phosphorus use efficiency in Brassica oleracea and correlates with root architecture traits. J. Exp. Bot. 2009, 60, 1953–1968. [Google Scholar] [CrossRef] [PubMed]
- Wissuwa, M.; Ae, N. Genotypic variation for tolerance to phosphorus deficiency in rice and the potential for its exploitation in rice improvement. Plant Breed. 2001, 120, 43–48. [Google Scholar] [CrossRef]
- Wissuwa, M.; Ae, N. Further characterization of two QTLs that increase phosphorus uptake of rice (Oryza sativa L.) under phosphorus deficiency. Plant Soil 2001, 237, 275–286. [Google Scholar] [CrossRef]
- Wissuwa, M. How do plants achieve tolerance to phosphorus deficiency? Small causes with big effects. Plant Physiol. 2003, 133, 1947–1958. [Google Scholar] [CrossRef] [PubMed]

| Pot Trials | Location | Coordinates | pH | Aloxalate | Feoxalate | Poxalate | ----Texture---- | ||
|---|---|---|---|---|---|---|---|---|---|
| (mg kg−1) | (mg kg−1) | (mg kg−1) | (%sand) | (%silt) | (%clay) | ||||
| (a) Lowland soil | Dakawa, Tanzania | 06°23′56.6′′ S 37°33′47.5′′ E | 6.1 (H2O) | 670 | 2600 | 29 | 6 | 71 | 23 |
| (b) Upland soil | Matombo, Tanzania | 07°02′46.8′′ S 37°47′11.6′′ E | 6.1 (CaCl2) | 1050 | 1400 | 31 | 17 | 53 | 30 |
| Field Trials | |||||||||
| (c) Lowland field April–August 2016 | Ruvu, Tanzania | 06°43′12.0′′ S 38°40′48.0′′ E | 6.4 (H2O) | 1150 | 2800 | 91 | 6 | 70 | 24 |
| (d) Upland field July–November 2016 | Morogoro, Tanzania | 06°50′55.7′′ S 37°39′18.2′′ E | 6.0 (H2O) | 925 | 1410 | 47 | 22 | 61 | 17 |
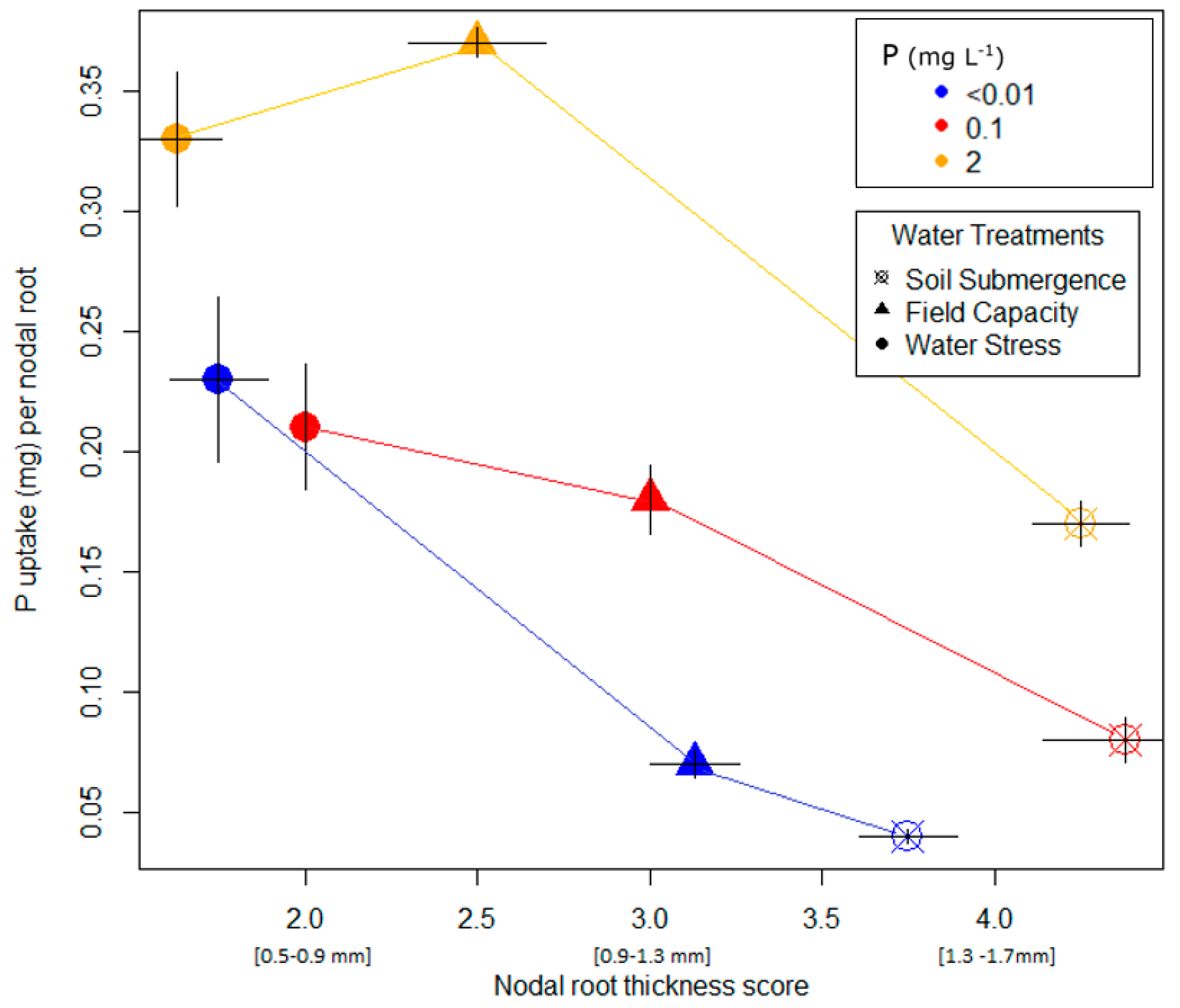
| Trait Score | 1 | 2 | 3 | 4 | 5 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Nodal root thickness | Very Thin <0.5 mm | Thin (0.5–0.9 mm) | Average (0.9–1.3 mm) | Thick (1.3–1.7 mm) | Very Thick >1.7 mm | ||||
| Secondary branching degree | Very Low (almost no branchings) | Low (only few branchings) | Average (average amount of branchings) | High (many branchings) | Very High (lots of branchings) | ||||
| Trait score from shovelomics board | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Basal lateral root density | Very Sparse | - | - | - | - | - | - | - | Highly Dense |
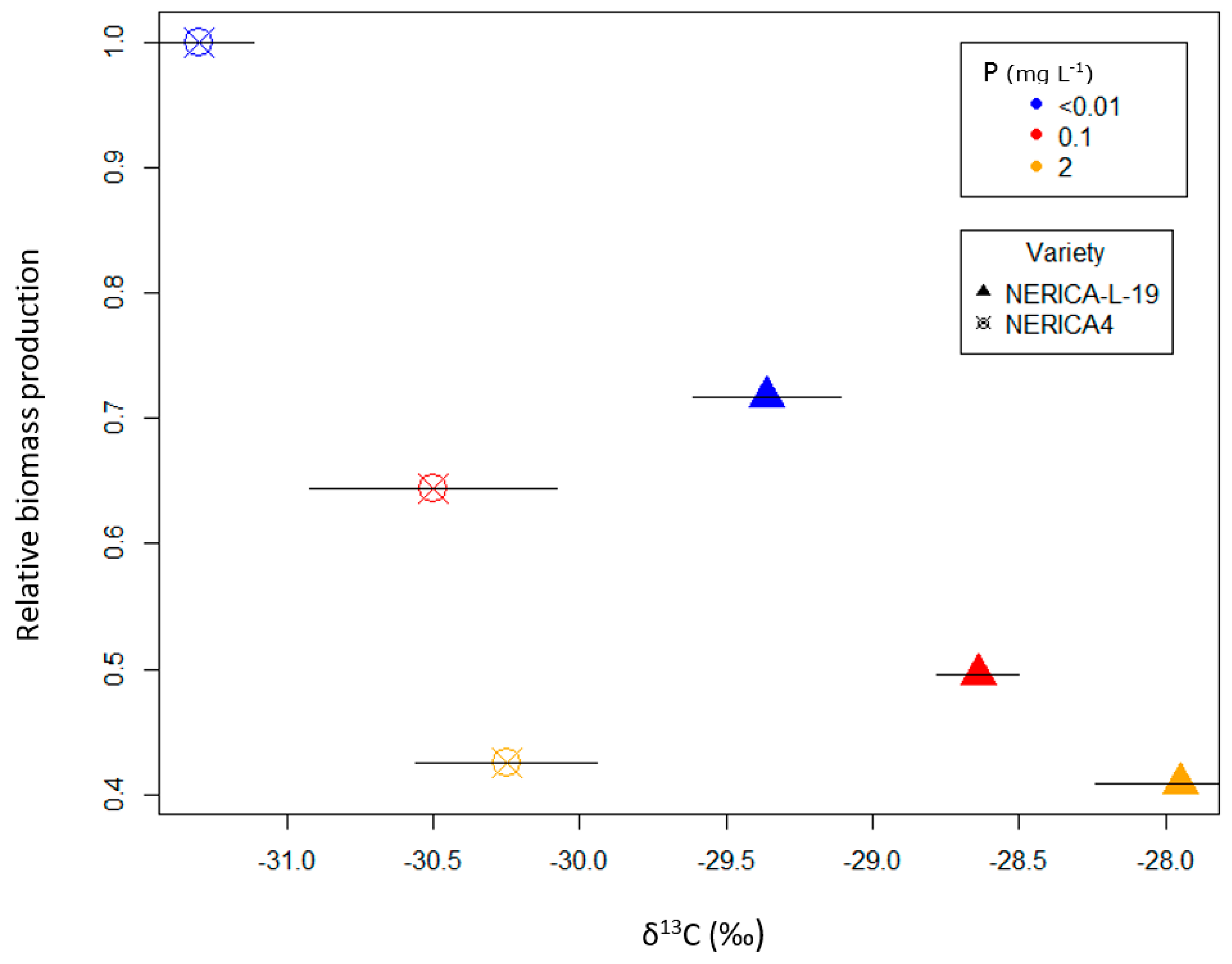
| Variety | Water | P | Shoot Mass | δ13C | Nodal Roots | Root Mass | Root-Shoot Ratio | Pc | Pup | Puproot | Pupnodal |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg L−1) | (g) | (‰) | (number plant−1) | (g) | (mg P kg−1) | (mg P plant−1) | (mg P g−1) | (mg P nodal−1) | |||
| NERICA4 | Sub pF = 0 | 2 | 11.5 (1.9) | −30.99 (0.08) | 146 (16.9) | 2.57 (0.55) | 0.22 (0.02) | 4391 (127) | 50.3 (8.1) | 35.3 (3.0) | 0.34 (0.028) |
| 0.1 | 5.1 (0.9) | −30.70 (0.25) | 64 (8.3) | 1.09 (0.09) | 0.23 (0.03) | 1782 (88) | 9.2 (1.6) | 11.1 (0.7) | 0.15 (0.031) | ||
| <0.01 | 1.5 (0.1) | −31.28 (0.09) | 34 (2.3) | 0.38 (0.02) | 0.26 (0.01) | 1127 (159) | 1.6 (0.2) | 4.9 (0.7) | 0.05 (0.007) | ||
| FC pF = 2 | 2 | 12.7 (0.7) | −30.72 (0.20) | 98 (3.8) | 2.18 (0.31) | 0.17 (0.02) | 4440 (483) | 56.6 (7.2) | 42.1 (5.9) | 0.58 (0.074) | |
| 0.1 | 5.9 (0.7) | −31.20 (0.13) | 64 (8.0) | 0.93 (0.07) | 0.16 (0.01) | 2154 (126) | 12.8 (2.2) | 19.0 (1.2) | 0.22 (0.068) | ||
| <0.01 | 2.4 (0.4) | −31.13 (0.07) | 41 (3.2) | 0.47 (0.07) | 0.20 (0.01) | 2085 (175) | 5.1 (1.0) | 12.4 (1.1) | 0.12 (0.015) | ||
| WS pF =3–3.5 | 2 | 5.4 (0.4) | −30.25 (0.31) | 37 (1.9) | 0.73 (0.06) | 0.14 (0.01) | 3284 (243) | 17.8 (2.0) | 30.2 (3.4) | 0.48 (0.57) | |
| 0.1 | 3.8 (0.1) | −30.50 (0.42) | 36 (3.4) | 0.74 (0.10) | 0.19 (0.03) | 2478 (141) | 9.5 (0.6) | 15.4 (2.1) | 0.27 (0.026) | ||
| <0.01 | 2.4 (0.2) | −31.30 (0.19) | 27 (1.5) | 0.34 (0.02) | 0.14 (0.01) | 2332 (157) | 5.7 (0.8) | 19.8 (3.2) | 0.22 (0.035) | ||
| NERICA-L-19 | Sub pF = 0 | 2 | 21.0 (2.4) | −30.71 (0.20) | 367 (24.2) | 8.11 (1.61) | 0.39 (0.06) | 3031 (163) | 62.7 (4.7) | 18.5 (3.0) | 0.17 (0.009) |
| 0.1 | 9.6 (2.0) | −30.46 (0.10) | 191 (32.4) | 3.48 (0.84) | 0.35 (0.01) | 1510 (134) | 14.2 (2.7) | 6.7 (0.6) | 0.08 (0.009) | ||
| <0.01 | 3.0 (0.5) | −30.92 (0.07) | 88 (9.5) | 0.90 (0.15) | 0.31 (0.02) | 1044 (44) | 3.2 (0.6) | 4.1 (0.4) | 0.04 (0.003) | ||
| FC pF = 2 | 2 | 18.6 (0.7) | −29.77 (0.32) | 153 (5.6) | 3.56 (0.48) | 0.19 (0.02) | 2822 (136) | 55.5 (3.9) | 31.0 (1.9) | 0.37 (0.006) | |
| 0.1 | 12.7 (0.8) | −29.64 (0.35) | 130 (10.1) | 2.14 (0.13) | 0.17 (0.00) | 1856 (115) | 23.2 (0.4) | 17.1 (1.7) | 0.18 (0.014) | ||
| <0.01 | 5.3 (0.2) | −30.67 (0.07) | 108 (4.3) | 1.26 (0.07) | 0.24 (0.01) | 1337 (94) | 7.0 (0.3) | 7.5 (1.1) | 0.07 (0.006) | ||
| WS pF = 3–3.5 | 2 | 7.6 (0.3) | −27.95 (0.29) | 77 (6.3) | 1.11 (0.13) | 0.15 (0.02) | 3263 (77) | 25.0 (1.3) | 50.4 (9.0) | 0.33 (0.028) | |
| 0.1 | 6.3 (0.3) | −28.64 (0.14) | 60 (9.0) | 1.07 (0.18) | 0.17 (0.02) | 1903 (201) | 11.8 (0.8) | 18.1 (2.4) | 0.21 (0.026) | ||
| <0.01 | 3.8 (0.3) | −29.36 (0.25) | 37 (3.5) | 0.43 (0.05) | 0.11 (0.01) | 2115 (271) | 7.9 (0.5) | 28.7 (1.4) | 0.23 (0.034) | ||
| Water | *** | *** | *** | *** | *** | ** | *** | *** | *** | ||
| P | *** | *** | *** | *** | ns | *** | *** | *** | *** | ||
| Variety | *** | *** | *** | *** | *** | *** | ** | ns | *** | ||
| Water × P | *** | * | *** | *** | * | *** | *** | * | ** | ||
| Water × Variety | ** | *** | *** | *** | *** | * | ns | *** | ns | ||
| P × Variety | ** | ns | *** | ** | ns | * | ns | ns | ** | ||
| Water × P × Variety | ns | ns | *** | ** | ns | ** | ns | ** | ns | ||
| Lowland Pot Trial (Dakawa Soil) | Upland Pot Trial (Matombo Soil) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variety | Water | P (mg L−1) | Nodal Root Thickness Score | Secondary Branching Degree | Variety | Water | P (mg L−1) | Nodal Root Thickness Score | Secondary Branching Degree |
| NERICA4 | Submerged pF = 0 | 2 | 4.13 (0.13) | 2.13 (0.13) | NERICA4 | FC pF = 2 | 2 | 4.63 (0.20) | 2.63 (0.24) |
| 0.1 | 3.50 (0.29) | 3.13 (0.32) | 0.1 | 4.63 (0.24) | 3.75 (0.25) | ||||
| <0.01 | 3.25 (0.14) | 3.38 (0.24) | <0.01 | 3.00 (0.20) | 2.63 (0.24) | ||||
| FC pF = 2 | 2 | 2.88 (0.13) | 2.75 (0.14) | Moderate WS pF = 2.7 | 2 | 3.13 (0.13) | 3.75 (0.14) | ||
| 0.1 | 2.75 (0.14) | 2.75 (0.25) | 0.1 | 3.13 (0.13) | 4.00 (0.20) | ||||
| <0.01 | 3.00 (0.00) | 3.25 (0.14) | <0.01 | 2.50 (0.20) | 3.00 (0.20) | ||||
| WS pF = 3–3.5 | 2 | 2.00 (0.00) | 4.50 (0.20) | Severe WS pF = 3–3.5 | 2 | 3.00 (0.20) | 5.00 (0.00) | ||
| 0.1 | 1.63 (0.13) | 4.75 (0.25) | 0.1 | 2.75 (0.14) | 4.88 (0.24) | ||||
| <0.01 | 2.00 (0.00) | 3.63 (0.13) | <0.01 | 1.63 (0.24) | 3.88 (0.43) | ||||
| NERICA-L-19 | Submerged pF = 0 | 2 | 4.25 (0.14) | 2.50 (0.00) | Mudgo | FC pF = 2 | 2 | 4.50 (0.29) | 2.88 (0.43) |
| 0.1 | 4.38 (0.24) | 2.63 (0.13) | 0.1 | 4.38 (0.24) | 2.88 (0.13) | ||||
| <0.01 | 3.75 (0.14) | 3.25 (0.14) | <0.01 | 3.38 (0.43) | 3.13 (0.13) | ||||
| FC pF = 2 | 2 | 2.50 (0.20) | 3.75 (0.32) | Moderate WS pF = 2.7 | 2 | 3.63 (0.13) | 4.50 (0.20) | ||
| 0.1 | 3.00 (0.00) | 3.75 (0.14) | 0.1 | 3.50 (0.20) | 4.38 (0.24) | ||||
| <0.01 | 3.13 (0.13) | 3.00 (0.20) | <0.01 | 2.38 (0.13) | 3.25 (0.14) | ||||
| WS pF = 3–3.5 | 2 | 1.63 (0.13) | 4.50 (0.20) | Severe WS pF = 3–3.5 | 2 | 3.00 (0.00) | 4.88 (0.13) | ||
| 0.1 | 2.00 (0.00) | 5.00 (0.00) | 0.1 | 2.75 (0.14) | 5.13 (0.24) | ||||
| <0.01 | 1.75 (0.14) | 4.00 (0.00) | <0.01 | 1.75 (0.14) | 3.88 (0.32) | ||||
| Water | *** | *** | Water | *** | *** | ||||
| P | ns | * | P | *** | *** | ||||
| Variety | * | * | Variety | ns | ns | ||||
| Water × P | *** | *** | Water × P | ns | ** | ||||
| Water × Variety | *** | * | Water × Variety | ns | ns | ||||
| P × Variety | *** | ns | P × Variety | ns | ns | ||||
| Water × P × Variety | ns | ** | Water × P × Variety | ns | ns | ||||
| Variety | Water | P | Shoot Mass | δ13C | Nodal Roots | Root Mass | Root-Shoot Ratio | Pc | Pup | Puproot | Pupnodal |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg L−1) | (g) | (‰) | (number plant−1) | (g) | (mg P kg−1) | (mg P plant−1) | (mg P g−1) | (mg P nodal−1) | |||
| NERICA4 | FC pF = 2 | 2 | 15.1 (1.3) | −31.35 (0.09) | 118 (9.5) | 2.63 (0.29) | 0.17 (0.01) | 3542 (65) | 53.2 (3.8) | 29.8 (1.5) | 0.45 (0.014) |
| 0.1 | 9.9 (0.8) | −31.36 (0.14) | 88 (8.4) | 1.87 (0.28) | 0.19 (0.02) | 1649 (60) | 16.2 (1.0) | 12.2 (1.7) | 0.18 (0.004) | ||
| <0.01 | 0.9 (0.1) | −30.91 (0.18) | 27 (2.2) | 0.40 (0.05) | 0.44 (0.02) | 832 (49) | 0.8 (0.1) | 2.1 (0.1) | 0.03 (0.003) | ||
| Moderate WS | 2 | 11.9 (0.7) | −30.57 (0.20) | 82 (4.9) | 2.25 (0.17) | 0.19 (0.01) | 3915 (155) | 46.6 (3.6) | 32.1 (2.1) | 0.58 (0.046) | |
| 0.1 | 8.5 (0.9) | −31.02 (0.27) | 71 (5.0) | 1.88 (0.19) | 0.23 (0.02) | 1758 (85) | 14.8 (1.2) | 9.5 (1.1) | 0.21 (0.010) | ||
| <0.01 | 0.9 (0.2) | −31.37 (0.07) | 26 (1.1) | 0.34 (0.06) | 0.42 (0.06) | 1127 (155) | 1.1 (0.3) | 3.3 (1.1) | 0.04 (0.013) | ||
| Severe WS pF =3–3.5 | 2 | 7.1 (0.9) | −29.97 (0.11) | 66 (11.3) | 1.33 (0.19) | 0.19 (0.02) | 3764 (54) | 26.7 (3.1) | 26.6 (2.7) | 0.44 (0.083) | |
| 0.1 | 6.1 (0.7) | −30.30 (0.14) | 48 (4.1) | 1.61 (0.25) | 0.26 (0.02) | 1489 (69) | 9.1 (1.3) | 7.4 (0.7) | 0.19 (0.018) | ||
| <0.01 | 1.0 (0.1) | −31.34 (0.11) | 16 (2.2) | 0.37 (0.05) | 0.37 (0.04) | 771 (21) | 0.8 (0.1) | 2.3 (0.2) | 0.05 (0.005) | ||
| Mudgo | FC pF = 2 | 2 | 17.2 (2.3) | −30.59 (0.19) | 150 (12.2) | 3.02 (0.74) | 0.17 (0.02) | 4188 (276) | 72.6 (12.5) | 35.1 (3.3) | 0.47 (0.047) |
| 0.1 | 14.5 (2.1) | −30.70 (0.06) | 159 (16.7) | 3.40 (0.63) | 0.23 (0.02) | 1599 (27) | 23.2 (3.3) | 9.9 (1.3) | 0.15 (0.009) | ||
| <0.01 | 1.2 (0.2) | −30.84 (0.16) | 37 (2.6) | 0.48 (0.09) | 0.40 (0.03) | 719 (36) | 0.9 (0.1) | 2.0 (0.1) | 0.02 (0.002) | ||
| Moderate WS | 2 | 15.4 (0.5) | −29.33 (0.18) | 127 (4.1) | 2.85 (0.24) | 0.19 (0.02) | 4921 (469) | 76.3 (9.1) | 44.6 (5.6) | 0.60 (0.065) | |
| 0.1 | 12.2 (1.0) | −30.25 (0.40) | 120 (7.9) | 2.53 (0.32) | 0.21 (0.01) | 2054 (72) | 24.9 (1.8) | 13.8 (0.5) | 0.21 (0.010) | ||
| <0.01 | 1.0 (0.1) | −30.85 (0.06) | 28 (2.3) | 0.32 (0.06) | 0.31 (0.04) | 826 (63) | 0.8 (0.1) | 2.9 (0.3) | 0.03 (0.006) | ||
| Severe WS pF = 3–3.5 | 2 | 10.0 (0.6) | −28.94 (0.47) | 93 (3.8) | 1.40 (0.22) | 0.14 (0.01) | 6210 (340) | 62.0 (5.1) | 68.3 (7.3) | 0.66 (0.042) | |
| 0.1 | 9.1 (0.5) | −29.03 (0.25) | 81 (6.9) | 1.70 (0.19) | 0.19 (0.01) | 1853 (61) | 16.9 (1.4) | 14.3 (2.4) | 0.21 (0.009) | ||
| <0.01 | 1.2 (0.1) | −30.20 (0.11) | 25 (2.9) | 0.24 (0.03) | 0.21 (0.01) | 980 (101) | 1.1 (0.1) | 5.4 (0.6) | 0.05 (0.008) | ||
| Water | *** | *** | *** | *** | ** | *** | ** | ** | ** | ||
| P | *** | *** | *** | *** | *** | *** | *** | *** | *** | ||
| Variety | *** | *** | *** | ** | *** | *** | *** | *** | ns | ||
| Water × P | *** | *** | *** | * | ** | *** | * | ** | ns | ||
| Water × Variety | ns | * | ns | ns | ** | *** | ns | *** | * | ||
| P × Variety | ** | ns | *** | ns | ** | *** | *** | *** | * | ||
| Water × P × Variety | ns | ns | ns | ns | ns | ** | ns | *** | ns | ||
| Variety | Water | P | Shoot Mass | δ13C | Nodal Roots | Root Mass | Root-Shoot Ratio | Pc | Pup | Puproot | Pupnodal | Grain Yield |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g) | (‰) | (number plant−1) | (g) | (mg P kg−1) | (mg P plant−1) | (mg P g−1) | (mg P nodal−1) | (kg ha−1) | ||||
| NERICA4 | Sub | +P | 16.45 (0.92) | −29.75 (0.05) | 164 (5.9) | 3.17 (0.21) | 0.19 (0.01) | 2425 (93) | 40.2 (3.0) | 20.5 (1.4) | 0.24 (0.014) | 3960 (1090.2) |
| 0P | 9.23 (0.99) | −29.66 (0.10) | 120 (6.4) | 1.88 (0.19) | 0.21 (0.01) | 1910 (143) | 18.8 (3.6) | 14.4 (1.8) | 0.15 (0.021) | 3170 (741.2) | ||
| FC | +P | 17.40 (1.08) | −29.95 (0.06) | 140 (7.3) | 3.23 (0.24) | 0.19 (0.01) | 2131 (56) | 37.1 (2.6) | 18.1 (1.2) | 0.27 (0.016) | 3947 (233.4) | |
| 0P | 9.41 (1.07) | −29.99 (0.07) | 98 (6.3) | 1.84 (0.20) | 0.20 (0.01) | 2110 (165) | 20.8 (3.0) | 15.8 (1.6) | 0.20 (0.023) | 3044 (405.9) | ||
| NERICA-L-19 | Sub | +P | 21.77 (1.34) | −29.67 (0.09) | 406 (13.9) | 7.17 (0.46) | 0.33 (0.02) | 2099 (89) | 45.3 (2.9) | 12.6 (1.0) | 0.11 (0.007) | 4925 (543.6) |
| 0P | 13.91 (1.06) | −29.99 (0.11) | 314 (15.8) | 4.64 (0.37) | 0.34 (0.02) | 1847 (109) | 26.4 (3.1) | 10.8 (1.8) | 0.08 (0.008) | 4884 (435.6) | ||
| FC | +P | 21.96 (1.92) | −29.28 (0.12) | 276 (14.5) | 5.30 (0.42) | 0.25 (0.01) | 1613 (46) | 35.9 (3.8) | 14.6 (1.3) | 0.13 (0.009) | 4212 (377.6) | |
| 0P | 15.00 (0.70) | −29.45 (0.09) | 274 (14.5) | 3.90 (0.24) | 0.26 (0.01) | 1759 (42) | 26.1 (1.3) | 12.3 (0.8) | 0.10 (0.005) | 3504 (275.7) | ||
| Mudgo | Sub | +P | 22.24 (1.22) | −30.04 (0.07) | 313 (9.1) | 4.40 (0.30) | 0.20 (0.01) | 2925 (67) | 64.6 (3.2) | 28.0 (1.2) | 0.21 (0.011) | 4060 (449.4) |
| 0P | 16.95 (1.38) | −30.12 (0.06) | 232 (8.0) | 3.12 (0.22) | 0.19 (0.01) | 2245 (129) | 38.1 (3.6) | 19.9 (2.1) | 0.16 (0.013) | 3535 (937.7) | ||
| FC | +P | 21.69 (1.46) | −29.53 (0.05) | 240 (16.9) | 3.67 (0.28) | 0.17 (0.01) | 2306 (160) | 50.8 (6.0) | 23.4 (2.8) | 0.21 (0.015) | 3435 (104.5) | |
| 0P | 16.59 (1.35) | −29.64 (0.10) | 196 (6.0) | 2.82 (0.14) | 0.18 (0.01) | 2290 (82) | 38.5 (3.8) | 21.9 (1.7) | 0.19 (0.016) | 2626 (637.5) | ||
| Water | ns | ns | ** | *** | *** | ** | * | ns | ns | p = 0.052 | ||
| P | *** | * | *** | *** | ns | *** | *** | *** | *** | p = 0.052 | ||
| Variety | *** | *** | *** | *** | *** | *** | *** | *** | *** | * | ||
| Water × P | ns | ns | ** | ns | ns | *** | * | 0.058 | ns | ns | ||
| Water × Variety | ns | *** | *** | *** | *** | ns | ns | ns | ns | ns | ||
| P × Variety | ns | ns | ns | ns | ns | ns | ns | ns | * | ns | ||
| Water × P × Variety | ns | ns | * | ns | ns | ns | ns | ns | ns | ns | ||
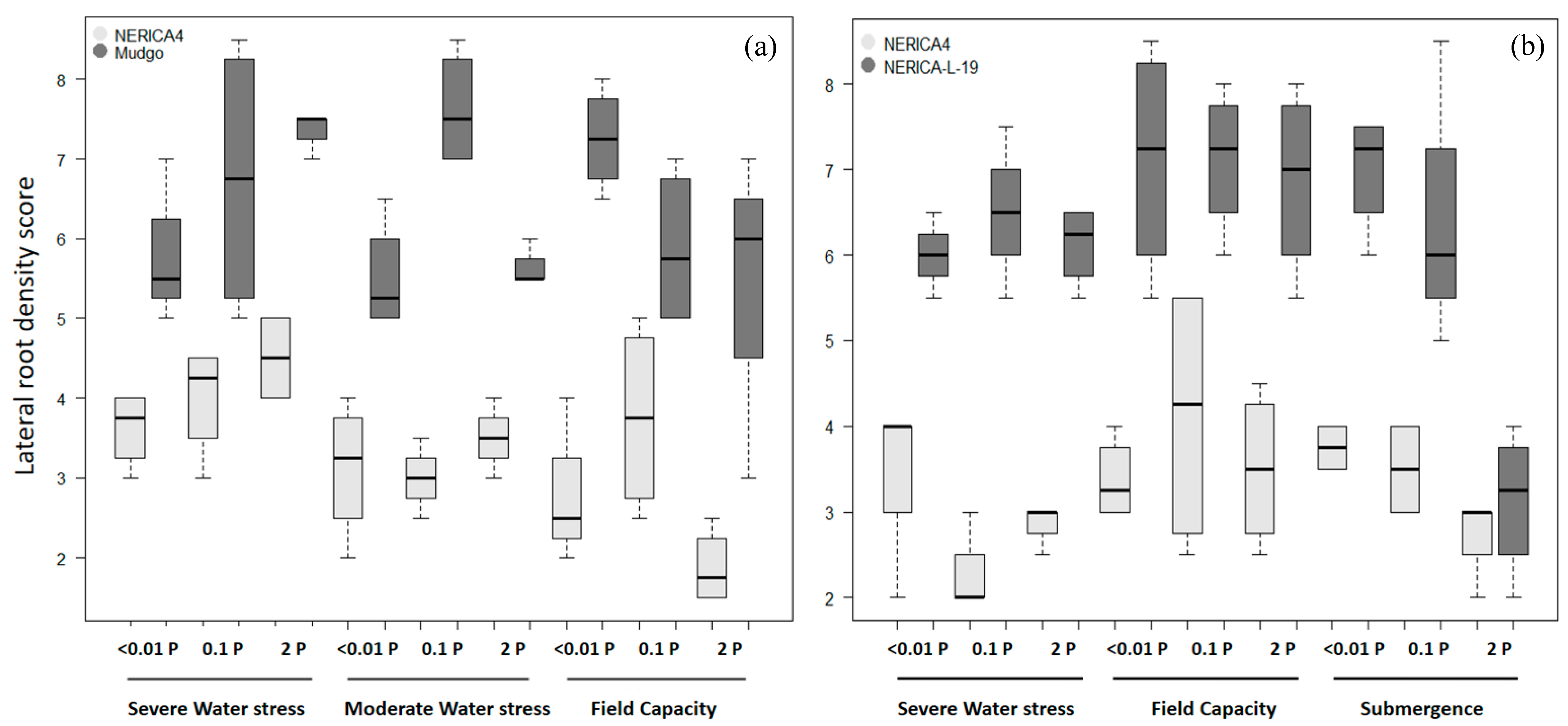
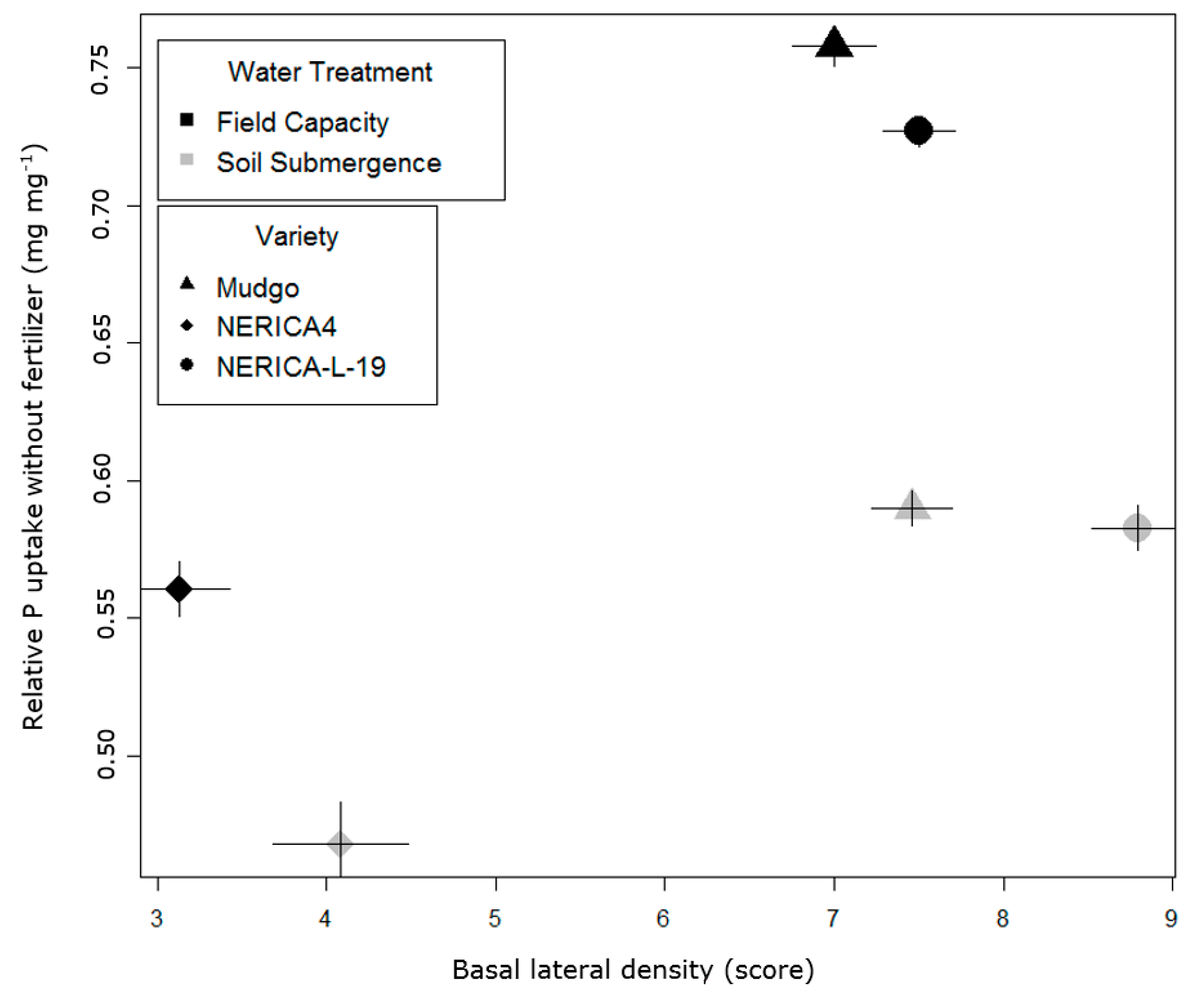
| Lowland Field Trial (Ruvu) | Upland Field Trial (Morogoro) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variety | Water | P | Nodal Root Thickness Score | Basal Lateral Root Density Score | Secondary Branching Degree | Water | P | Nodal Root Thickness Score | Basal Lateral Root Density Score | Secondary Branching Degree |
| NERICA4 | Sub | +P | 4.08 (0.08) | 3.71 (0.29) | 2.42 (0.15) | FC | +P | 3.0 (0.20) | 2.7 (0.40) | 2.4 (0.14) |
| 0P | 3.79 (0.10) | 4.08 (0.40) | 2.54 (0.13) | 0P | 2.6 (0.18) | 2.1 (0.22) | 2.1 (0.08) | |||
| FC | +P | 3.33 (0.07) | 2.46 (0.23) | 3.08 (0.13) | drought | +P | 2.7 (0.12) | 3.5 (0.30) | 2.8 (0.17) | |
| 0P | 3.13 (0.07) | 3.13 (0.30) | 2.96 (0.07) | 0P | 2.4 (0.16) | 2.7 (0.29) | 2.4 (0.14) | |||
| NERICA-L-19 | Sub | +P | 3.50 (0.11) | 7.00 (0.21) | 3.75 (0.13) | FC | +P | 2.4 (0.13) | 4.7 (0.37) | 3.0 (0.13) |
| 0P | 3.25 (0.10) | 8.79 (0.27) | 3.83 (0.18) | 0P | 2.3 (0.10) | 3.9 (0.34) | 2.8 (0.11) | |||
| FC | +P | 2.92 (0.08) | 7.21 (0.22) | 4.17 (0.07) | drought | +P | 2.3 (0.14) | 3.5 (0.52) | 2.5 (0.20) | |
| 0P | 2.79 (0.07) | 7.50 (0.21) | 4.04 (0.07) | 0P | 2.3 (0.12) | 4.7 (0.46) | 3.0 (0.21) | |||
| Mudgo | Sub | +P | 3.50 (0.11) | 6.69 (0.23) | 2.83 (0.13) | FC | +P | 2.8 (0.13) | 4.1 (0.65) | 2.6 (0.26) |
| 0P | 3.41 (0.12) | 7.46 (0.24) | 2.83 (0.18) | 0P | 2.7 (0.14) | 3.5 (0.63) | 2.8 (0.19) | |||
| FC | +P | 3.00 (0.06) | 6.71 (0.32) | 3.92 (0.10) | drought | +P | 2.6 (0.15) | 4.8 (0.38) | 3.0 (0.12) | |
| 0P | 2.83 (0.07) | 7.00 (0.25) | 3.83 (0.13) | 0P | 2.4 (0.11) | 4.3 (0.46) | 2.8 (0.18) | |||
| Water | *** | ns | ns | Water | * | ns | ns | |||
| P | ns | ns | ns | P | * | ns | ns | |||
| Variety | ns | *** | ** | Variety | *** | *** | *** | |||
| Water × P | ns | ns | ns | Water × P | ns | ns | ns | |||
| Water × Variety | ns | ns | ** | Water× Variety | ns | ns | ns | |||
| P × Variety | ns | ns | ns | P× Variety | ns | ns | ns | |||
| Water × P × Variety | ns | ns | ns | Water × P× Variety | ns | ns | * | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bauw, P.; Vandamme, E.; Lupembe, A.; Mwakasege, L.; Senthilkumar, K.; Merckx, R. Architectural Root Responses of Rice to Reduced Water Availability Can Overcome Phosphorus Stress. Agronomy 2019, 9, 11. https://doi.org/10.3390/agronomy9010011
De Bauw P, Vandamme E, Lupembe A, Mwakasege L, Senthilkumar K, Merckx R. Architectural Root Responses of Rice to Reduced Water Availability Can Overcome Phosphorus Stress. Agronomy. 2019; 9(1):11. https://doi.org/10.3390/agronomy9010011
Chicago/Turabian StyleDe Bauw, Pieterjan, Elke Vandamme, Allen Lupembe, Leah Mwakasege, Kalimuthu Senthilkumar, and Roel Merckx. 2019. "Architectural Root Responses of Rice to Reduced Water Availability Can Overcome Phosphorus Stress" Agronomy 9, no. 1: 11. https://doi.org/10.3390/agronomy9010011
APA StyleDe Bauw, P., Vandamme, E., Lupembe, A., Mwakasege, L., Senthilkumar, K., & Merckx, R. (2019). Architectural Root Responses of Rice to Reduced Water Availability Can Overcome Phosphorus Stress. Agronomy, 9(1), 11. https://doi.org/10.3390/agronomy9010011




