Development of High Yielding Glutinous Cytoplasmic Male Sterile Rice (Oryza sativa L.) Lines through CRISPR/Cas9 Based Mutagenesis of Wx and TGW6 and Proteomic Analysis of Anther
Abstract
1. Introduction
2. Materials and Methods
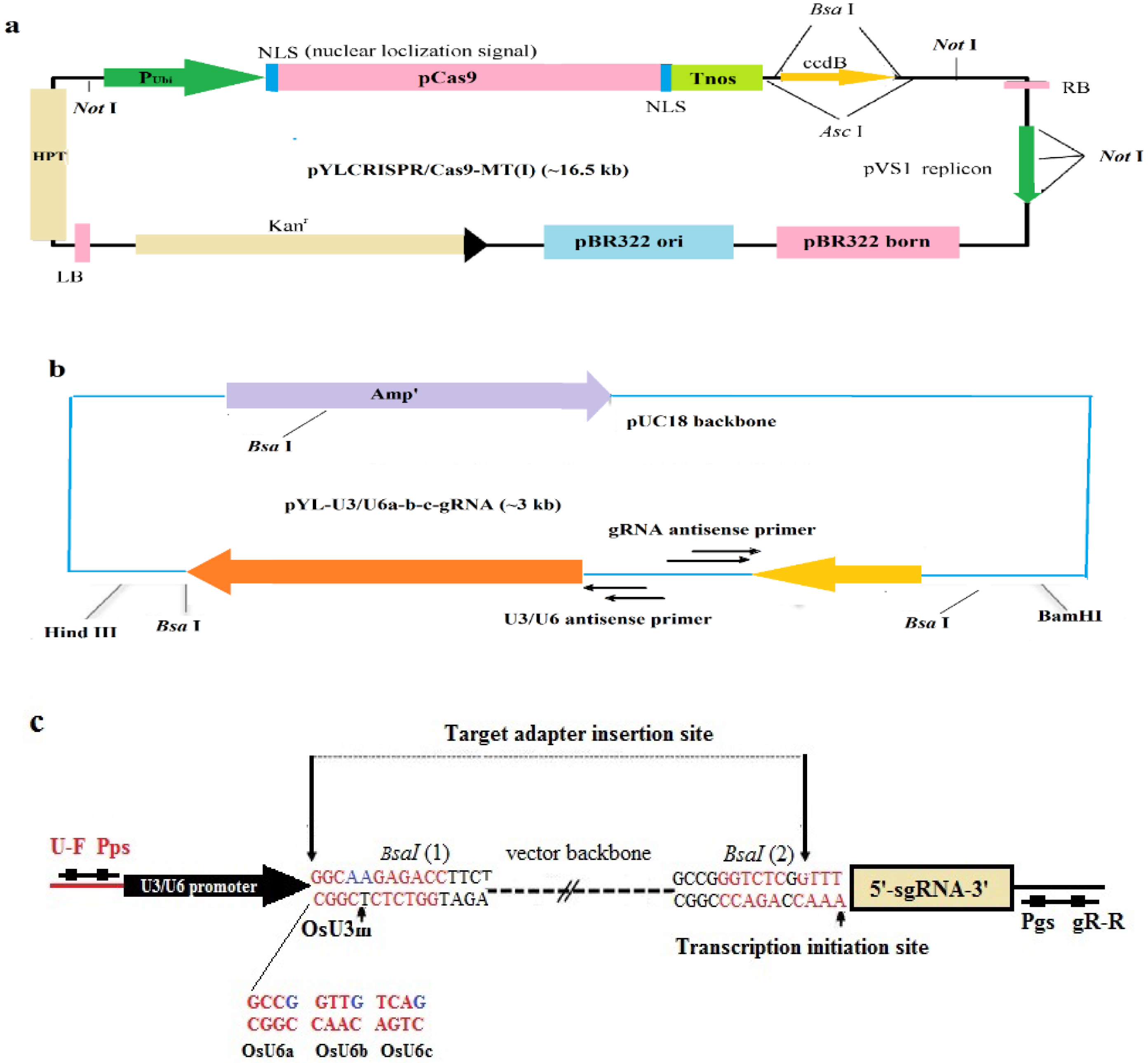
2.1. Rice Material, CRISPR/Cas9, and gRNA Vectors
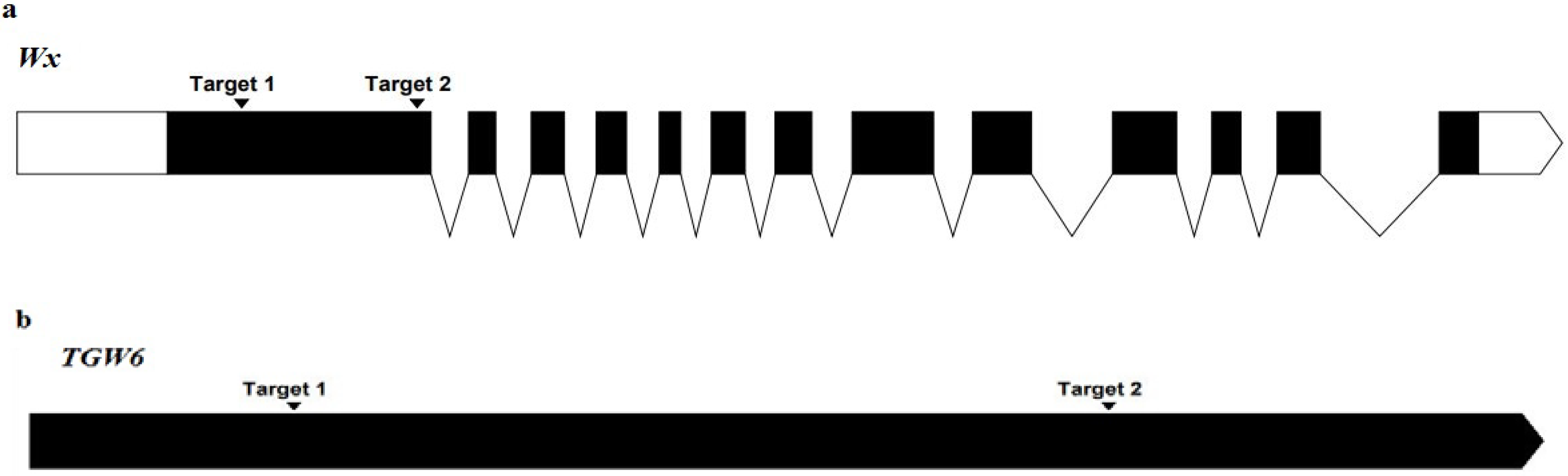
2.2. gRNA Target Selection and Synthesis of Oligonucleotide Strands
2.3. Vector Construction
2.4. Agrobacterium-Mediated Transformation of Rice Callus
2.5. T0 Genotypinng
2.6. Identification of T-DNA Free Mutant Lines and Cross Section Analysis of Grain Endosperm
2.7. Expression Analysis
2.8. Determination of AC, SC, GC, and GT
2.9. Phenotyping
2.10. Backcrossing and Observation of Pollen and Spikelet Fertility
2.11. Pollen Protein Analysis
2.12. Statistical Analysis
3. Results
3.1. gRNA Design and Vector Construction
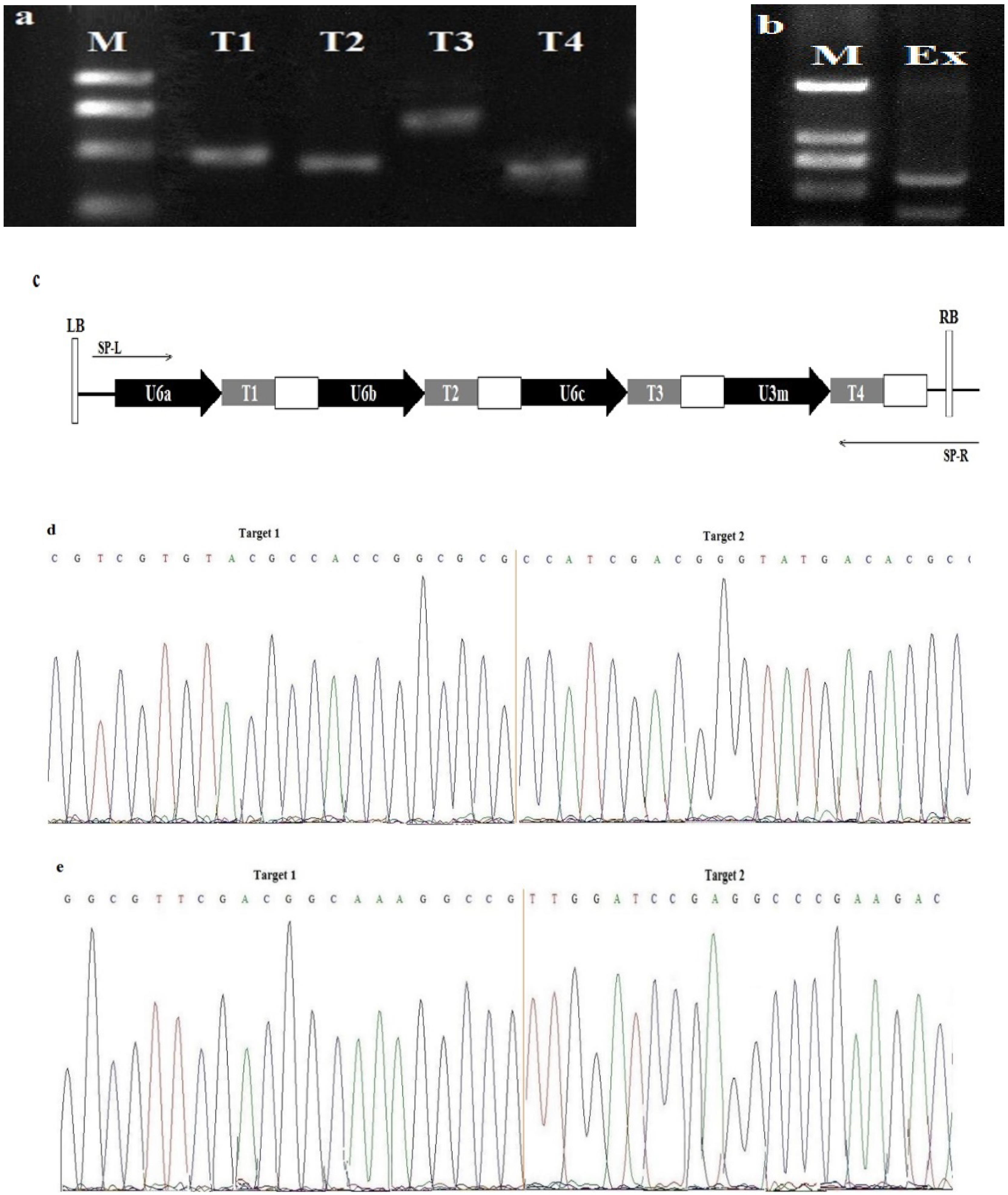
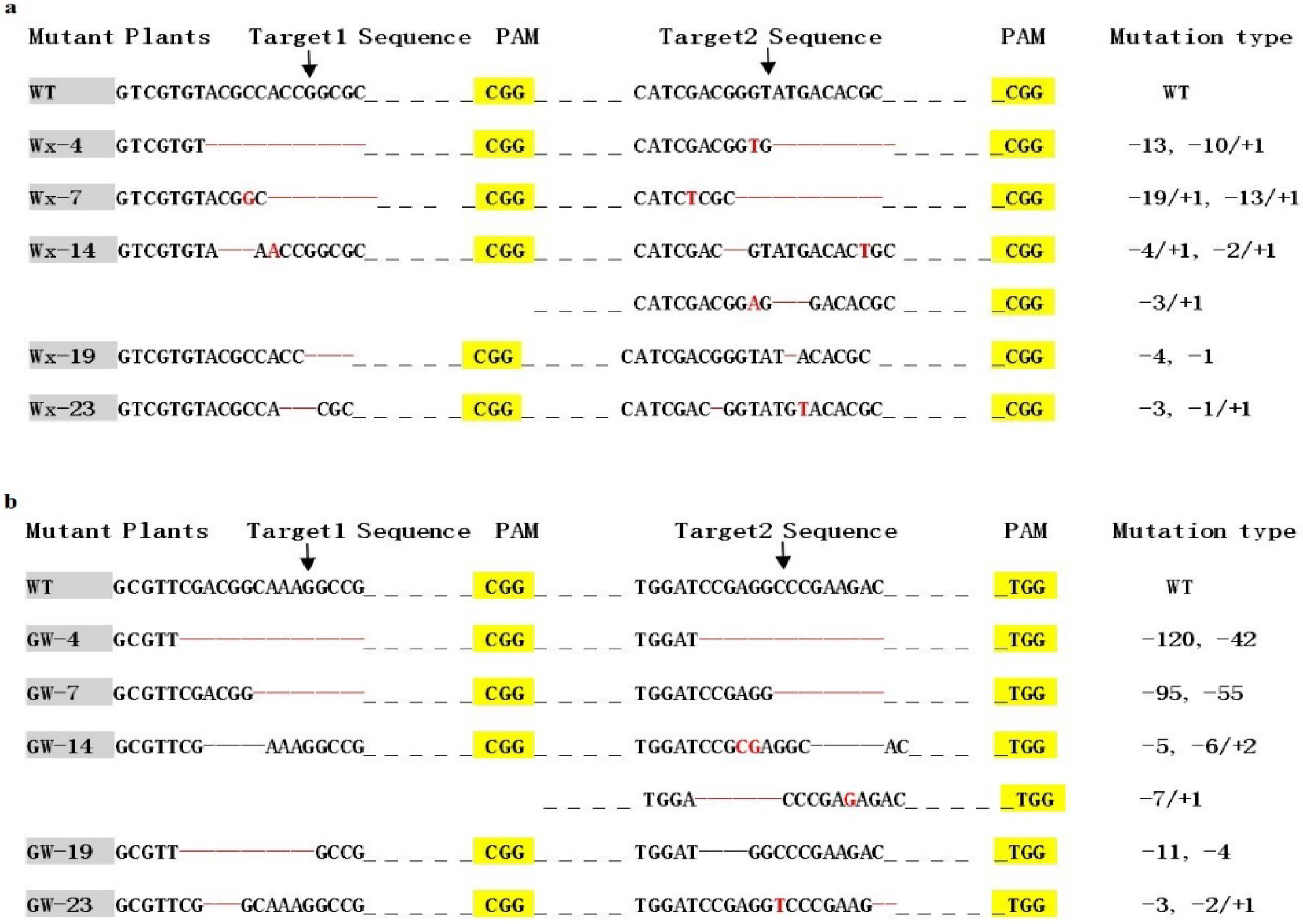
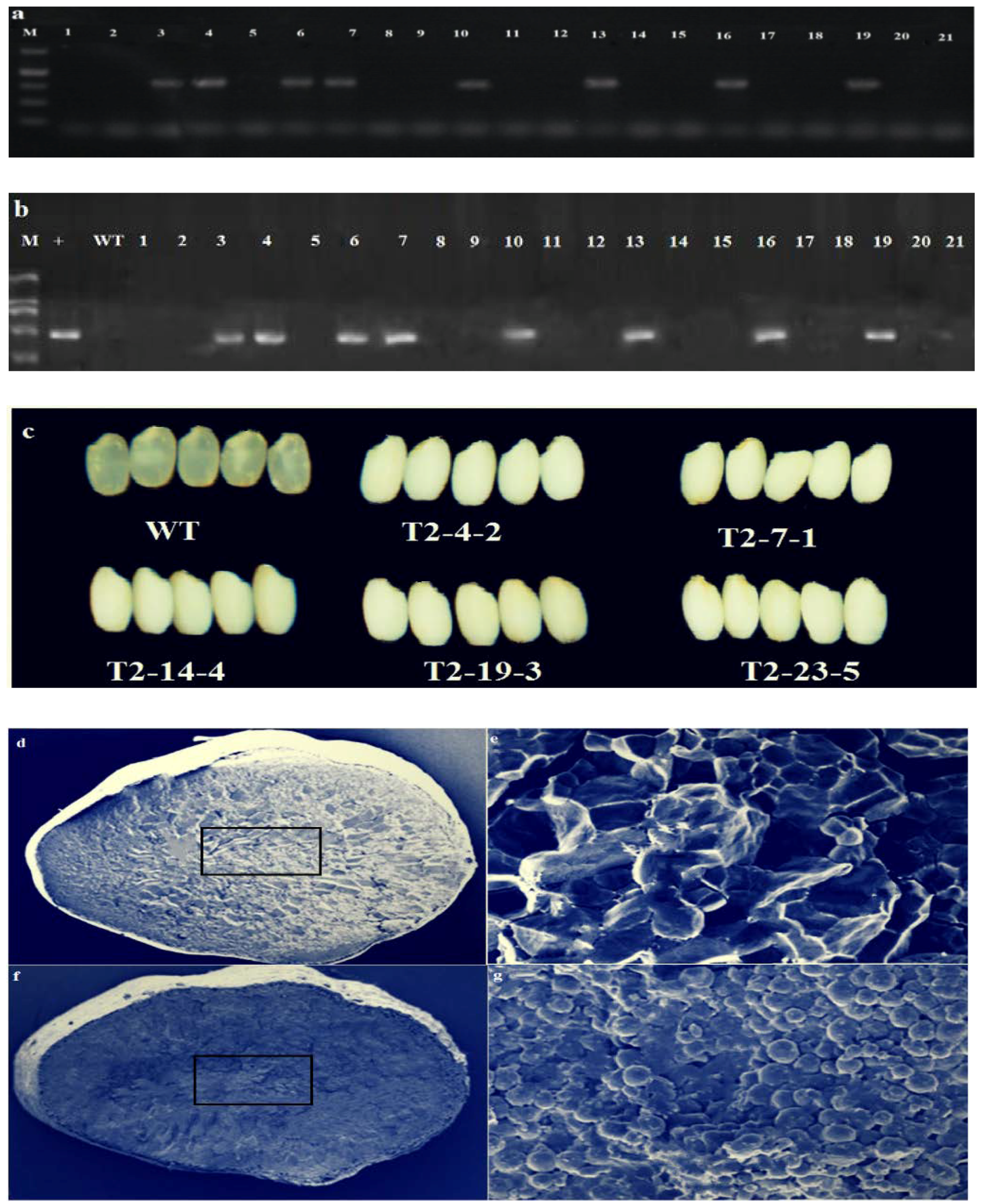
3.2. T0 Genotyping and Off-Target Analysis
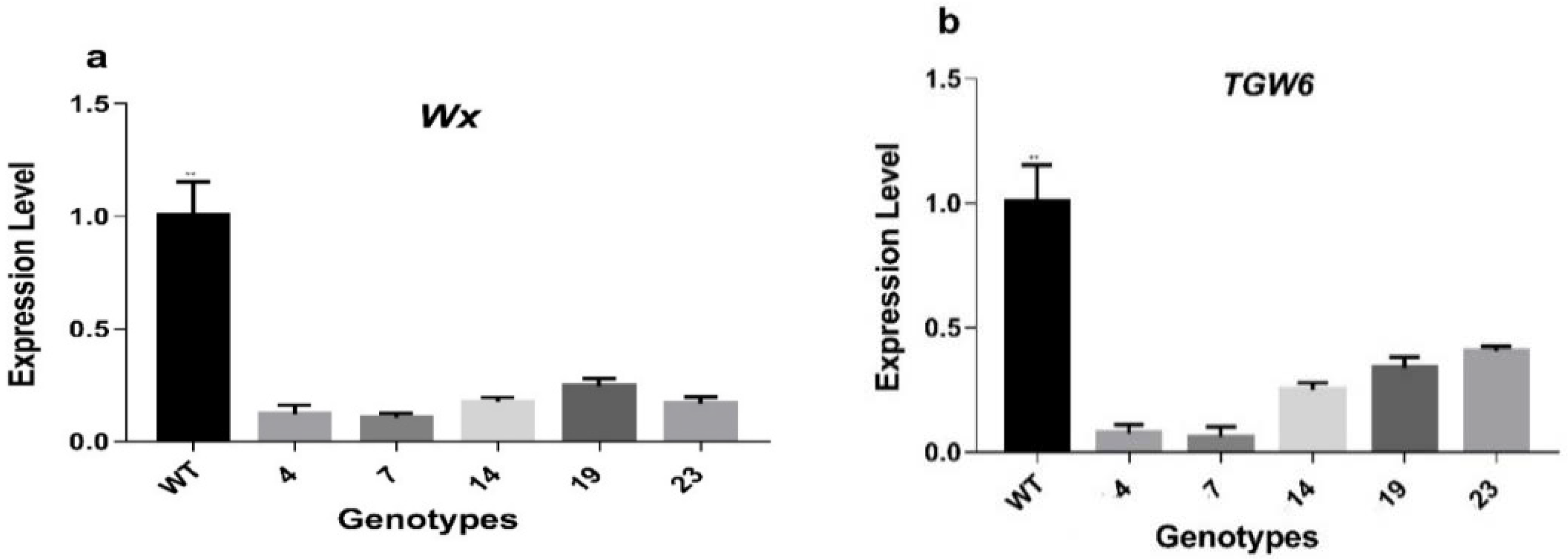
3.3. Expression Level of Target Genes in WT and Mutant Lines
3.4. Screening of T-DNA Free T1 Generation and Seed Cross-Section Analysis
3.5. Transmission of Mutations in T1 and T2 Generations
3.6. AC, GC, GT, and SC
3.7. Yield and Yield Contributing Traits
3.8. Pollen Fertility Status
3.9. Pollen Protein Identification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Accession Numbers
References
- Birla, D.S.; Malik, K.; Sainger, M.; Chaudhary, D.; Jaiwal, R.; Jaiwal, P.K. Progress and challenges in improving the nutritional quality of rice (Oryza sativa L.). Crit. Rev. Food Sci. Nutr. 2017, 57, 2455–2481. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.; Del Cerro, P.; Espuny, M.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, V.; Smutka, L. Asian Countries in the Global Rice Market. Acta Univ. Agric. Silvic. Mendel. Brun. 2017, 65, 679–688. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Tseng, M.C.; Wu, Y.P.; Lin, M.Y.; Wei, F.J.; Hwu, K.K.; Hsing, Y.I.; Lin, Y.R. Genetic factors responsible for eating and cooking qualities of rice grains in a recombinant inbred population of an inter-subspecific cross. Mol. Breed. 2014, 34, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Tang, C. Retrospect, current status and prospect of hybrid rice. Rice in China 1999, 4, 3–6. [Google Scholar]
- Cheng, S.H.; Zhuang, J.Y.; Fan, Y.Y.; Du, J.H.; Cao, L.Y. Progress in research and development on hybrid rice: a super-domesticate in China. Ann. Bot. 2007, 100, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, S.; Nandan, R.; Kumar, M. Identification of restorers and maintainers for CMS lines of rice (Oryza sativa L.). Ind. J. Plant Gen. Res. 2012, 25, 186–188. [Google Scholar] [CrossRef]
- Umesh, S.; Lal, J.; Kumar, H. Isolation and evaluation of restorers and examining possibility of developing new version of CMS lines for upland rainfed rice hybrids. Environ. Ecol. 2012, 30, 872–876. [Google Scholar]
- Xing, Y.; Zhang, Q. Genetic and molecular bases of rice yield. Annu. Rev. Plant Biol. 2010, 61, 421–442. [Google Scholar] [CrossRef]
- You, A.; Lu, X.; Jin, H.; Ren, X.; Liu, K.; Yang, G.; Yang, H.; Zhu, L.; He, G. Identification of quantitative trait loci across recombinant inbred lines and testcross populations for traits of agronomic importance in rice. Genetics 2006, 172, 1287–1300. [Google Scholar] [CrossRef]
- Wani, A.A.; Singh, P.; Shah, M.A.; Schweiggert-Weisz, U.; Gul, K.; Wani, I.A. Rice starch diversity: Effects on structural, morphological, thermal, and physicochemical properties—A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 417–436. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zheng, F.Q.; Shen, G.Z.; Gao, J.P.; Snustad, D.P.; Li, M.G.; Zhang, J.L.; Hong, M.M. The amylose content in rice endosperm is related to the post-transcriptional regulation of the waxy gene. Plant J. 1995, 7, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Isshiki, M.; Morino, K.; Nakajima, M.; Okagaki, R.J.; Wessler, S.R.; Izawa, T.; Shimamoto, K. A naturally occurring functional allele of the rice waxy locus has a GT to TT mutation at the 5′ splice site of the first intron. Plant J. 1998, 15, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y. Differential regulation of waxy gene expression in rice endosperm. Theor. Appl. Genet. 1984, 68, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Larkin, P.D.; Park, W.D. Association of waxy gene single nucleotide polymorphisms with starch characteristics in rice (Oryza sativa L.). Mol. Breed. 2003, 12, 335–339. [Google Scholar] [CrossRef]
- Bligh, H.F.J.; Larkin, P.D.; Roach, P.S.; Jones, C.A.; Fu, H.; Park, W.D. Use of alternate splice sites in granule-bound starch synthase mRNA from low-amylose rice varieties. Plant Mol. Biol. 1998, 38, 407–415. [Google Scholar] [CrossRef]
- Sato, H.; Suzuki, Y.; Sakai, M.; Imbe, T. Molecular characterization of Wx-mq, a novel mutant gene for low-amylose content in endosperm of rice (Oryza sativa L.). Breed. Sci. 2002, 52, 131–135. [Google Scholar] [CrossRef]
- Bergman, C.; Delgado, J.; McClung, A.; Fjellstrom, R. An improved method for using a microsatellite in the rice waxy gene to determine amylose class. Cereal Chem. 2001, 78, 257–260. [Google Scholar] [CrossRef]
- Inukai, T.; Sako, A.; Hirano, H.Y.; Sano, Y. Analysis of intragenic recombination at wx in rice: correlation between the molecular and genetic maps within the locus. Genome 2000, 43, 589–596. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Yang, Z.; Li, H.; Lin, Y. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant. 2015, 8, 1274–1284. [Google Scholar] [CrossRef]
- Terada, R.; Nakajima, M.; Isshiki, M.; Okagaki, R.J.; Wessler, S.R.; Shimamoto, K. Antisense waxy genes with highly active promoters effectively suppress waxy gene expression in transgenic rice. Plant Cell Physiol. 2000, 41, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Z.; Chen, X.; Cai, X.; Tang, S.; Yu, H.; Zhang, J.; Hong, M.; Gu, M. Stable inheritance of the antisense Waxy gene in transgenic rice with reduced amylose level and improved quality. Transgenic Res. 2003, 12, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, H.; Chen, X.; Cai, X.; Tang, S.; Wang, Z.; Gu, M. Field performance of transgenic indica hybrid rice with improved cooking and eating quality by down-regulation of Wx gene expression. Mol. Breed. 2005, 16, 199–208. [Google Scholar] [CrossRef]
- Itoh, K.; Ozaki, H.; Okada, K.; Hori, H.; Takeda, Y.; Mitsui, T. Introduction of Wx transgene into rice wx mutants leads to both high-and low-amylose rice. Plant Cell Physiol. 2003, 44, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Shomura, A.; Izawa, T.; Ebana, K.; Ebitani, T.; Kanegae, H.; Konishi, S.; Yano, M. Deletion in a gene associated with grain size increased yields during rice domestication. Nat. Genet. 2008, 40, 1023. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Gu, S.; Wan, X.; Gao, H.; Guo, T.; Su, N.; Lei, C.; Zhang, X.; Cheng, Z.; Guo, X. Isolation and initial characterization of GW5, a major QTL associated with rice grain width and weight. Cell Res. 2008, 18, 1199. [Google Scholar] [CrossRef]
- Ishimaru, K.; Hirotsu, N.; Madoka, Y.; Murakami, N.; Hara, N.; Onodera, H.; Kashiwagi, T.; Ujiie, K.; Shimizu, B.-i.; Onishi, A. Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat. Genet. 2013, 45, 707. [Google Scholar] [CrossRef]
- Fan, C.; Xing, Y.; Mao, H.; Lu, T.; Han, B.; Xu, C.; Li, X.; Zhang, Q. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 2006, 112, 1164–1171. [Google Scholar] [CrossRef]
- Li, Y.; Fan, C.; Xing, Y.; Jiang, Y.; Luo, L.; Sun, L.; Shao, D.; Xu, C.; Li, X.; Xiao, J. Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat. Genet. 2011, 43, 1266–1269. [Google Scholar] [CrossRef]
- Song, X.J.; Huang, W.; Shi, M.; Zhu, M.Z.; Lin, H.X. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat. Genet. 2007, 39, 623. [Google Scholar] [CrossRef]
- Wang, S.; Wu, K.; Yuan, Q.; Liu, X.; Liu, Z.; Lin, X.; Zeng, R.; Zhu, H.; Dong, G.; Qian, Q. Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 2012, 44, 950. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; He, H.; Zhang, S.; Sun, F.; Xin, X.; Wang, W.; Qian, X.; Yang, J.; Luo, X. A Kelch motif-containing serine/threonine protein phosphatase determines the large grain QTL trait in rice. J. Integr. Plant Biol. 2012, 54, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Lin, Y.S.; Song, X.J.; Shen, J.B.; Huang, W.; Shan, J.X.; Zhu, M.Z.; Jiang, L.; Gao, J.P.; Lin, H.X. The novel quantitative trait locus GL3. 1 controls rice grain size and yield by regulating Cyclin-T1; 3. Cell Res. 2012, 22, 1666–1680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Huang, J.; Lan, H.; Wang, C.; Yin, C.; Wu, Y.; Tang, H.; Qian, Q.; Li, J. Rare allele of OsPPKL1 associated with grain length causes extra-large grain and a significant yield increase in rice. Proc. Natl. Acad. Sci. USA 2012, 109, 21534–21539. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, S.; Liu, Q.; Wu, K.; Zhang, J.; Wang, S.; Wang, Y.; Chen, X.; Zhang, Y.; Gao, C. The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat. Genet. 2015, 47, 949. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Chen, J.; Huang, X.; Gong, H.; Luo, J.; Hou, Q.; Zhou, T.; Lu, T.; Zhu, J.; Shangguan, Y. OsSPL13 controls grain size in cultivated rice. Nat. Genet. 2015, 47, 447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Lang, Z.; Botella, J.R.; Zhu, J.K. Genome editing—Principles and applications for functional genomics research and crop improvement. Crit. Rev. Plant Sci. 2017, 36, 291–309. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Zhou, Z.; Wu, P.; Fang, M.; Pan, X.; Lin, Q.; Luo, W.; Wu, G.; Li, H. Reassessment of the four yield-related genes Gn1a, DEP1, GS3, and IPA1 in rice using a CRISPR/Cas9 system. Front. Plant Sci. 2016, 7, 377. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Y.; Qin, R.; Li, H.; Qiu, C.; Li, L.; Wei, P.; Yang, J. Rapid improvement of grain weight via highly efficient CRISPR/Cas9-mediated multiplex genome editing in rice. J. Genet. Genom. 2016, 43, 529–532. [Google Scholar] [CrossRef]
- Bortesi, L.; Fischer, R. The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol. Adv. 2015, 33, 41–52. [Google Scholar] [CrossRef]
- Baltes, N.J.; Gil-Humanes, J.; Cermak, T.; Atkins, P.A.; Voytas, D.F. DNA replicons for plant genome engineering. Plant Cell. 2014, 26, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Miglani, G.S. Genome editing in crop improvement: Present scenario and future prospects. J. Crop Improv. 2017, 31, 453–559. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, B.; Ding, W.; Liu, X.; Yang, D.L.; Wei, P.; Cao, F.; Zhu, S.; Zhang, F.; Mao, Y. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 2013, 23, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Voytas, D.F.; Gao, C. Precision genome engineering and agriculture: opportunities and regulatory challenges. PLoS Biol. 2014, 12, e1001877. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, K.; Chaparro-Garcia, A.; Kamoun, S.; Patron, N.J.; Nekrasov, V. Editing plant genomes with CRISPR/Cas9. Curr. Opin. Biotechnol. 2015, 32, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Weeks, D.P.; Spalding, M.H.; Yang, B. Use of designer nucleases for targeted gene and genome editing in plants. Plant Biotechnol. J. 2016, 14, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Khatodia, S.; Bhatotia, K.; Passricha, N.; Khurana, S.; Tuteja, N. The CRISPR/Cas genome-editing tool: Application in improvement of crops. Front. Recent Dev. Plant Sci. 2016, 7, 506. [Google Scholar] [CrossRef]
- Nekrasov, V.; Staskawicz, B.; Weigel, D.; Jones, J.D.; Kamoun, S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 2013, 31, 691. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, H.; Bi, H.; Fromm, M.; Yang, B.; Weeks, D.P. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 2013, 41, e188. [Google Scholar] [CrossRef]
- Miao, J.; Guo, D.; Zhang, J.; Huang, Q.; Qin, G.; Zhang, X.; Wan, J.; Gu, H.; Qu, L.J. Targeted mutagenesis in rice using CRISPR-Cas system. Cell Res. 2013, 23, 1233–1236. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.L. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947. [Google Scholar] [CrossRef] [PubMed]
- Char, S.N.; Neelakandan, A.K.; Nahampun, H.; Frame, B.; Main, M.; Spalding, M.H.; Becraft, P.W.; Meyers, B.C.; Walbot, V.; Wang, K.; et al. An Agrobacterium-delivered CRISPR/Cas9 system for high-frequency targeted mutagenesis in maize. Plant Biotech. J. 2017, 15, 257–268. [Google Scholar] [CrossRef]
- Butler, N.M.; Atkins, P.A.; Voytas, D.F.; Douches, D.S. Generation and inheritance of targeted mutations in potato (Solanum tuberosum L.) using the CRISPR/Cas system. PLoS ONE 2015, 10, e0144591. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Ye, L.; Qin, L.; Liu, X.; He, Y.; Wang, J.; Chen, L.; Lu, G. CRISPR/Cas9-mediated efficient and heritable targeted mutagenesis in tomato plants in the first and later generations. Sci. Rep. 2016, 6, 24765. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, Y.G. CRISPR/Cas9-based multiplex genome editing in monocot and dicot plants. Curr. Protoc. Mol. Biol. 2016, 115, 31.6.1–31.6.21. [Google Scholar] [CrossRef]
- Moore, D.; Dowhan, D. Purification and concentration of DNA from aqueous solutions. Curr. Protoc. Mol. Biol. 2002, 59, 2.1.1–2.1.10. [Google Scholar] [CrossRef]
- Hiei, Y.; Ohta, S.; Komari, T.; Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994, 6, 271–282. [Google Scholar] [CrossRef]
- Kang, H.G.; Park, S.; Matsuoka, M.; An, G. White-core endosperm floury endosperm-4 in rice is generated by knockout mutations in the C4-type pyruvate orthophosphate dikinase gene (OsPPDKB). Plant J. 2005, 42, 901–911. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cagampang, G.B.; Perez, C.M.; Juliano, B.O. A gel consistency test for eating quality of rice. J. Sci. Food Agric. 1973, 24, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Little, R.R. Differential effect of dilute alkali on 25 varieties of milled white rice. Cereal Chem. 1958, 35, 111–126. [Google Scholar]
- Virmani, S.S. Hybrid Rice Breeding Manual; International Rice Research Institute: Laguna, Philippines, 1997. [Google Scholar]
- Sarhadi, E.; Bazargani, M.M.; Sajise, A.G.; Abdolahi, S.; Vispo, N.A.; Arceta, M.; Nejad, G.M.; Singh, R.K.; Salekdeh, G.H. Proteomic analysis of rice anthers under salt stress. Plant Physiol. Biochem. 2012, 58, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Mathesius, U.; Imin, N.; Chen, H.; Djordjevic, M.A.; Weinman, J.J.; Natera, S.H.; Morris, A.C.; Kerim, T.; Paul, S.; Menzel, C.; et al. Evaluation of proteome reference maps for cross-species identification of proteins by peptide mass fingerprinting. Proteomics 2002, 2, 1288–1303. [Google Scholar] [CrossRef]
- Dreier, B.; Beerli, R.R.; Segal, D.J.; Flippin, J.D.; Barbas, I. Development of zinc finger domains for recognition of the 5′-ANN-3′family of DNA sequences and their use in the construction of artificial transcription factors. J. Biol. Chem. 2001, 276, 29466–29478. [Google Scholar] [CrossRef] [PubMed]
- Tesson, L.; Usal, C.; Ménoret, S.; Leung, E.; Niles, B.J.; Remy, S.; Santiago, Y.; Vincent, A.I.; Meng, X.; Zhang, L.; et al. Knockout rats generated by embryo microinjection of TALENs. Nat. Biotechnol. 2011, 29, 695. [Google Scholar] [CrossRef]
- Huang, P.; Xiao, A.; Zhou, M.; Zhu, Z.; Lin, S.; Zhang, B. Heritable gene targeting in zebrafish using customized TALENs. Nat. Biotechnol. 2011, 29, 699. [Google Scholar] [CrossRef]
- Juliano, B. Varietal impact on rice quality. Cereal Foods World 1998, 43, 207–222. [Google Scholar]
- Zhang, H.; Zhang, J.; Wei, P.; Zhang, B.; Gou, F.; Feng, Z.; Mao, Y.; Yang, L.; Zhang, H.; Xu, N.; et al. The CRISPR/C as9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnol. J. 2014, 12, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Qian, Q.; Liu, Q.; Yan, M.; Liu, X.; Yan, C.; Liu, G.; Gao, Z.; Tang, S.; Zeng, D.; et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc. Natl. Acad. Sci. USA 2009, 106, 21760–21765. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.F.; Li, H.; Qin, R.Y.; Li, J.; Qiu, C.H.; Yang, Y.C.; Ma, H.; Li, L.; Wei, P.C.; Yang, J.B. Generation of inheritable and “transgene clean” targeted genome-modified rice in later generations using the CRISPR/Cas9 system. Sci. Rep. 2015, 5, 11491. [Google Scholar] [CrossRef] [PubMed]
- Elkonin, L.A.; Tsvetova, M.I. Heritable effect of plant water availability conditions on restoration of male fertility in the “9E” CMS-inducing cytoplasm of sorghum. Front. Plant Sci. 2012, 3, 91. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Liu, G.; Li, S.Q.; Wan, C.X.; Tao, J.; Xu, K.Y.; Zhang, Z.J.; Zhu, Y.G. Proteomic analysis of anthers from Honglian cytoplasmic male sterility line rice and its corresponding maintainer and hybrid. Bot. Stud. 2007, 48, 293–309. [Google Scholar]
- Qi, J.; Ma, H.; Xu, J.; Chen, M.; Zhou, D.; Wang, T.; Chen, S. Proteomic analysis of bud differentiation between cytoplasmic male-sterile line and maintainer in tobacco. Acta Agron. Sin. 2012, 38, 1232–1239. [Google Scholar] [CrossRef]
- Sheoran, I.S.; Ross, A.R.; Olson, D.J.; Sawhney, V.K. Differential expression of proteins in the wild type and 7B-1 male-sterile mutant anthers of tomato (Solanum lycopersicum): A proteomic analysis. J. Proteomics 2009, 71, 624–636. [Google Scholar] [CrossRef]
- Zheng, R.; Yue, S.; Xu, X.; Liu, J.; Xu, Q.; Wang, X.; Han, L.; Yu, D. Proteome analysis of the wild and YX-1 male sterile mutant anthers of wolfberry (Lycium barbarum L.). PLoS ONE 2012, 7, e41861. [Google Scholar] [CrossRef]
- Ivanov, M.; Dymshits, G. Cytoplasmic male sterility and restoration of pollen fertility in higher plants. Russ. J. Genet. 2007, 43, 354–368. [Google Scholar] [CrossRef]
- Teixeira, R.T.; Knorpp, C.; Glimelius, K. Modified sucrose, starch, and ATP levels in two alloplasmic male-sterile lines of B. napus. J. Exp. Bot. 2005, 56, 1245–1253. [Google Scholar] [CrossRef]
- Warmke, H.; Lee, S.L.J. Pollen abortion in T cytoplasmic male-sterile corn (Zea mays): A suggested mechanism. Science 1978, 200, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Edqvist, J.; Farbos, I.; Glimelius, K. Male-sterile tobacco displays abnormal mitochondrial atp1 transcript accumulation and reduced floral ATP/ADP ratio. Plant Mol. Biol. 2000, 42, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.R.; Bentolila, S. Interactions of mitochondrial and nuclear genes that affect male gametophyte development. Plant Cell. 2004, 16, S154–S169. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, R.; Forchioni, A.; Chetrit, P.; Vedel, F. Specific mitochondrial proteins in pollen: Presence of an additional ATP synthase beta subunit. Proc. Natl. Acad. Sci. USA 1993, 90, 5934–5938. [Google Scholar] [CrossRef]
- Sassa, H.; Oguchi, S.; Inoue, T.; Hirano, H. Primary structural features of the 20S proteasome subunits of rice (Oryza sativa). Gene 2000, 250, 61–66. [Google Scholar] [CrossRef]
- Benedetti, M.; Verrascina, I.; Pontiggia, D.; Locci, F.; Mattei, B.; De Lorenzo, G.; Cervone, F. Four Arabidopsis berberine bridge enzyme-like proteins are specific oxidases that inactivate the elicitor-active oligogalacturonides. Plant J. 2018, 94, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.B.; Morgan, R.O.; Fernandez, M.P.; Roux, S.J. Evolutionary adaptation of plant annexins has diversified their molecular structures, interactions and functional roles. New Phytol. 2012, 196, 695–712. [Google Scholar] [CrossRef]
- Schnurr, J.; Shockey, J. The acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis. Plant Cell. 2004, 16, 629–642. [Google Scholar] [CrossRef]
- Lallemand, B.; Erhardt, M.; Heitz, T.; Legrand, M. Sporopollenin biosynthetic enzymes interact and constitute a metabolon localized to the endoplasmic reticulum of tapetum cells. Plant Physiol. 2013, 162, 616–625. [Google Scholar] [CrossRef]
- Shi, J.; Cui, M.; Yang, L.; Kim, Y.J.; Zhang, D. Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci. 2015, 20, 741–753. [Google Scholar] [CrossRef]
- Kubo, A.; Fujita, N.; Harada, K.; Matsuda, T.; Satoh, H.; Nakamura, Y. The starch–debranching enzymes isoamylase and pullulanase are both involved in amylopectin biosynthesis in rice endosperm. Plant Physiol. 1999, 121, 399–410. [Google Scholar] [CrossRef]


| Mutation Type | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gene | Target Site | Bi-Allelic | Homozygous | Heterozygous | Chimeric | WT | Total | |
| Wx | Target 1 | No. of plants | 7 | 10 | 5 | 1 | 2 | 25 |
| Mutation rate (%) | 28 | 40 | 20 | 4 | 8 | 100 | ||
| Target 2 | No. of plants | 10 | 4 | 6 | 1 | 4 | 25 | |
| Mutation rate (%) | 40 | 16 | 24 | 4 | 16 | 100 | ||
| TGW6 | Target 1 | No. of plants | 6 | 9 | 7 | 1 | 2 | 25 |
| Mutation rate (%) | 24 | 36 | 28 | 4 | 8 | 100 | ||
| Target 2 | No. of plants | 9 | 5 | 8 | 1 | 2 | 25 | |
| Mutation rate (%) | 36 | 20 | 32 | 4 | 8 | 100 | ||
| T0 plant | AC (%) | T1 plants | AC (%) | T2 Plants | AC (%) | GC (mm) | GT (ASV) | SC (%) |
|---|---|---|---|---|---|---|---|---|
| 4 | 2.6 ± 0.5 ** | 4-2 | 1.7 ± 0.1 ** | T2-4-2 | 1.8 ± 0.1 ** | 138.62 ± 2.8 ** | 3.12 ± 0.9 ** | 62.5 ± 1.4 ns |
| 7 | 3.6 ± 0.3 ** | 7-1 | 2.2 ± 0.5 ** | T2-7-1 | 2.1 ± 0.3 ** | 129.65 ± 3.9 ** | 3.09 ± 1.1 ** | 63.2 ± 2.3 ns |
| 14 | 10.5 ± 0.2 ** | 14-4 | 2.4 ± 0.1 ** | T2-14-4 | 2.6 ± 0.2 ** | 125.32 ± 4.6 ** | 3.21 ± 0.2 ** | 63.2 ± 1.9 ns |
| 19 | 12.2 ± 0.1 ** | 19-3 | 2.8 ± 0.3 ** | T2-19-3 | 2.7 ± 0.2 ** | 114.22 ± 2.6 ** | 3.19 ± 0.8 ** | 62.8 ± 2.9 ns |
| 23 | 9.5 ± 0.6 ** | 23-5 | 3.2 ± 0.5 ** | T2-23-5 | 3.1 ± 0.1 ** | 111.56 ± 5.2 ** | 3.24 ± 0.6 ** | 64.5 ± 3.4 ns |
| WT | 18.2 ± 1.2 | WT | 17.6 ± 1.3 | WT | 18.1 ± 2.1 | 58.65 ± 3.7 | 5.67 ± 1.4 | 62.97 ± 2.7 |
| T0 plants | GW (g) | T1 Plants | GW (g) | T2 Plants | GW (g) |
|---|---|---|---|---|---|
| 4 | 23.6 ± 0.50 * | 4-2 | 23.9 ± 0.50 * | T2-4-2 | 24.0 ± 0.50 * |
| 7 | 23.1 ± 0.57 * | 7-1 | 23.8 ± 0.57 * | T2-7-1 | 23.1 ± 0.57 * |
| 14 | 23.4 ± 0.50 * | 14-4 | 23.7 ± 0.50 * | T2-14-4 | 23.4 ± 0.50 * |
| 19 | 23.1 ± 0.47 * | 19-3 | 23.3 ± 0.47 * | T2-19-3 | 23.1 ± 0.47 * |
| 23 | 23.7 ± 0.38 * | 23-5 | 23.7 ± 0.38 * | T2-23-5 | 23.7 ± 0.38 * |
| WT | 21.0 ± 0.35 | WT | 21.3 ± 0.35 | WT | 21.1 ± 0.35 |
| T2 Plants | PH (cm) | NOP | FLL (cm) | FLW (cm) | PL (cm) | GPS | SSR (%) |
|---|---|---|---|---|---|---|---|
| T2-4-2 | 84 ± 3.4 ns | 9.5 ± 5.4 ns | 42.3 ± 4.3 ns | 1.7 ± 0.2 ns | 25.1 ± 1.2 ns | 191 ± 6.7 ns | 87.8 ± 2.3 ns |
| T2-7-1 | 83 ± 4.5 ns | 8.8 ± 2.6 ns | 39.5 ± 5.2 ns | 1.6 ± 0.1 ns | 24.6 ± 2.3 ns | 195 ± 6.9 ns | 87.9 ± 4.5 ns |
| T2-14-4 | 85 ± 2.7 ns | 9.5 ± 1.6 ns | 43.2 ± 2.1 ns | 1.5 ± 0.3 ns | 23.9 ± 1.5 ns | 196 ± 4.5 ns | 88.9 ± 6.2 ns |
| T2-19-3 | 86 ± 3.6 ns | 9.3 ± 3.4 ns | 44.6 ± 1.9 ns | 1.8 ± 0.4 ns | 25.1 ± 2.4 ns | 188 ± 7.6 ns | 88.4 ± 1.3 ns |
| T2-23-5 | 85 ± 2.8 ns | 9.2 ± 4.6 ns | 43.3 ± 2.5 ns | 1.6 ± 0.5 ns | 23.9 ± 3.1 ns | 193 ± 5.8 ns | 86.9 ± 2.8 ns |
| WT | 83 ± 4.6 | 9.5 ± 2.9 | 44.5 ± 3.6 | 1.7 ± 0.2 | 25.4 ± 1.9 | 192 ± 4.9 | 87.6 ± 4.6 |
| S. No | Symbol | Fertility Status | Genotypes |
|---|---|---|---|
| 1 | CS | Completely Sterile | 4-2A, 19-5A, 19-3B |
| 2 | S | Sterile | 4-8A, 7-5B, 7-3A |
| 3 | PS | Partially Sterile | 4-1A, 4-4B |
| 4 | PF | Partially fertile | 23-5B, 23-7A |
| 5 | F | Fertile | 4-5C, 4-7A, |
| 6 | FF | Highly/fully Fertile | 4-3A, 14-4C, 19-3C, 14-4A |
| Sr. No. | Matched Protein | Organism | Accession No. | Mr/pl * | Spot Regulation | |
|---|---|---|---|---|---|---|
| GX-B1 | 4-2A | |||||
| 1 | 20S proteasome beta 4 subunit | O. sativa | Q9LST6 | 23.6/5.42 | + | − |
| 2 | Putative RNA-binding protein | O. sativa | Q852C0 | 97.3/9.34 | + | − |
| 3 | Putative berberine bridge enzyme | O. sativa | Q84pv5 | 60.10/6.0 | + | − |
| 4 | Putative mitochondrial NAD+ -dependent malic enzyme | O. sativa | Q9FVY8 | 57.34/8.2 | − | + |
| 5 | Putative calcium-binding protein annexin | O. sativa | Q84Q48 | 35.5/9.44 | + | − |
| 6 | UDP-glucuronic acid decarboxylase | O. sativa | Q8W3J0 | 39.5/7.16 | − | + |
| 7 | Putative phosphoribosyl pyrophosphate synthase | O. sativa | Q8S2E5 | 44.17/6.9 | + | ++ |
| 8 | Putative RNA binding protein | O. sativa | Q7XC34 | 48.4/5.21 | − | + |
| 9 | H+ -transporting two-sector ATPase alpha chain–rice mitochondria | O. sativa | P15998 | 55.53/7.9 | + | - |
| 10 | Glucose-1-phosphate adenylyltransferase large subunit 3 | O. sativa | Q688T8 | 56.2/6.48 | − | + |
| 11 | Putative membrane-associated salt-inducible protein | O. sativa | Q8W2V6 | 78.02/9.2 | + | − |
| 12 | Putative leucine-rich repeat protein | O. sativa | Q6I5I5 | 29.58/9.6 | ++ | + |
| 13 | Putative acetyl-CoA synthetase | O. sativa | Q6H798 | 78.5/5.69 | + | ++ |
| 14 | Putative lipoamide dehydrogenase | O. sativa | Q94GU7 | 58.8/6.35 | − | + |
| 15 | Isoamylase (fragrant) | O. sativa | D0TZF0 | 82.1/5.46 | ++ | + |
| 16 | DNA binding protein | O. sativa | Q40691 | 33.0/8.96 | + | + |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Luo, D.; Usman, B.; Nawaz, G.; Zhao, N.; Liu, F.; Li, R. Development of High Yielding Glutinous Cytoplasmic Male Sterile Rice (Oryza sativa L.) Lines through CRISPR/Cas9 Based Mutagenesis of Wx and TGW6 and Proteomic Analysis of Anther. Agronomy 2018, 8, 290. https://doi.org/10.3390/agronomy8120290
Han Y, Luo D, Usman B, Nawaz G, Zhao N, Liu F, Li R. Development of High Yielding Glutinous Cytoplasmic Male Sterile Rice (Oryza sativa L.) Lines through CRISPR/Cas9 Based Mutagenesis of Wx and TGW6 and Proteomic Analysis of Anther. Agronomy. 2018; 8(12):290. https://doi.org/10.3390/agronomy8120290
Chicago/Turabian StyleHan, Yue, Dengjie Luo, Babar Usman, Gul Nawaz, Neng Zhao, Fang Liu, and Rongbai Li. 2018. "Development of High Yielding Glutinous Cytoplasmic Male Sterile Rice (Oryza sativa L.) Lines through CRISPR/Cas9 Based Mutagenesis of Wx and TGW6 and Proteomic Analysis of Anther" Agronomy 8, no. 12: 290. https://doi.org/10.3390/agronomy8120290
APA StyleHan, Y., Luo, D., Usman, B., Nawaz, G., Zhao, N., Liu, F., & Li, R. (2018). Development of High Yielding Glutinous Cytoplasmic Male Sterile Rice (Oryza sativa L.) Lines through CRISPR/Cas9 Based Mutagenesis of Wx and TGW6 and Proteomic Analysis of Anther. Agronomy, 8(12), 290. https://doi.org/10.3390/agronomy8120290







