Nitrogen Use Efficiency of Watermelon Grafted onto 10 Wild Watermelon Rootstocks under Low Nitrogen Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rootstocks and Scion Utilized, and Experimental Details
2.2. Plant Growth
2.3. Relative Chlorophyll Content (SPAD Index) and Leaf Photosynthetic Assessment
2.4. Nitrogen Estimation and Nitrogen Use Efficiency
2.5. Statistical Analysis
3. Results
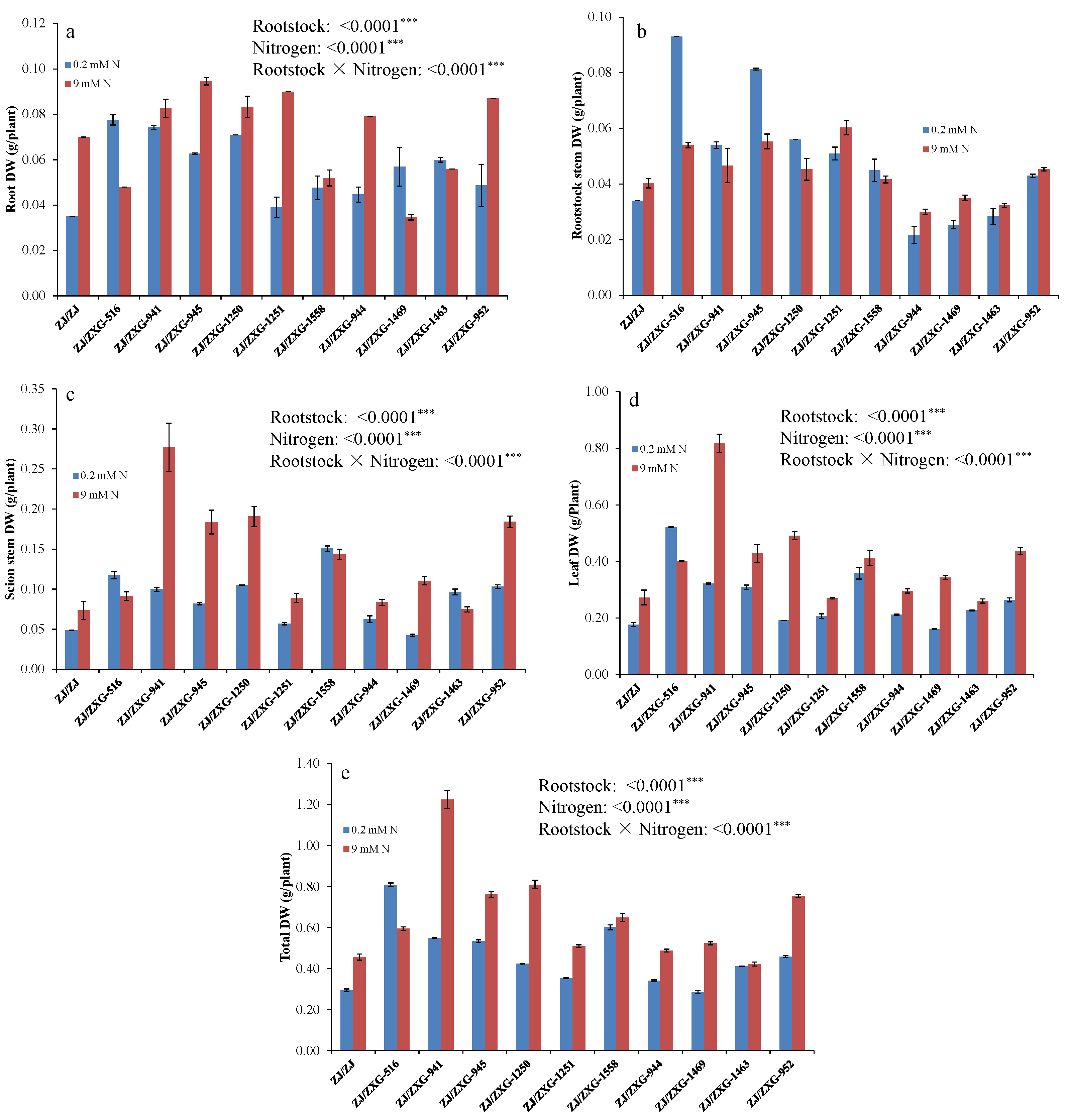
3.1. Wild Watermelon Rootstocks Improve the Growth of Watermelon Scion
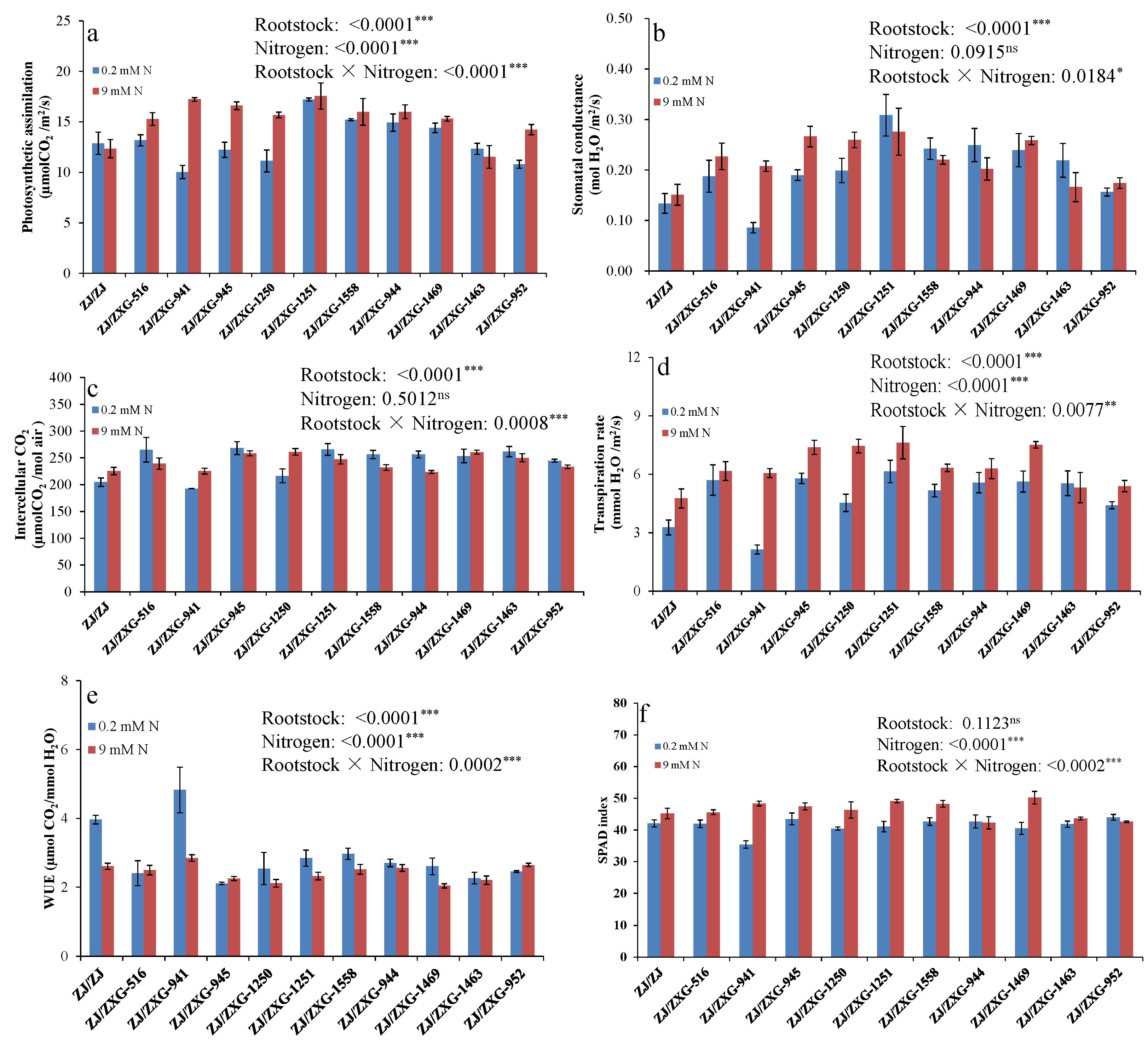
3.2. Wild Watermelon Rootstocks Improve the Photosynthetic Responses of Watermelon Scion
3.3. Wild Watermelon Rootstocks Improve the NUE
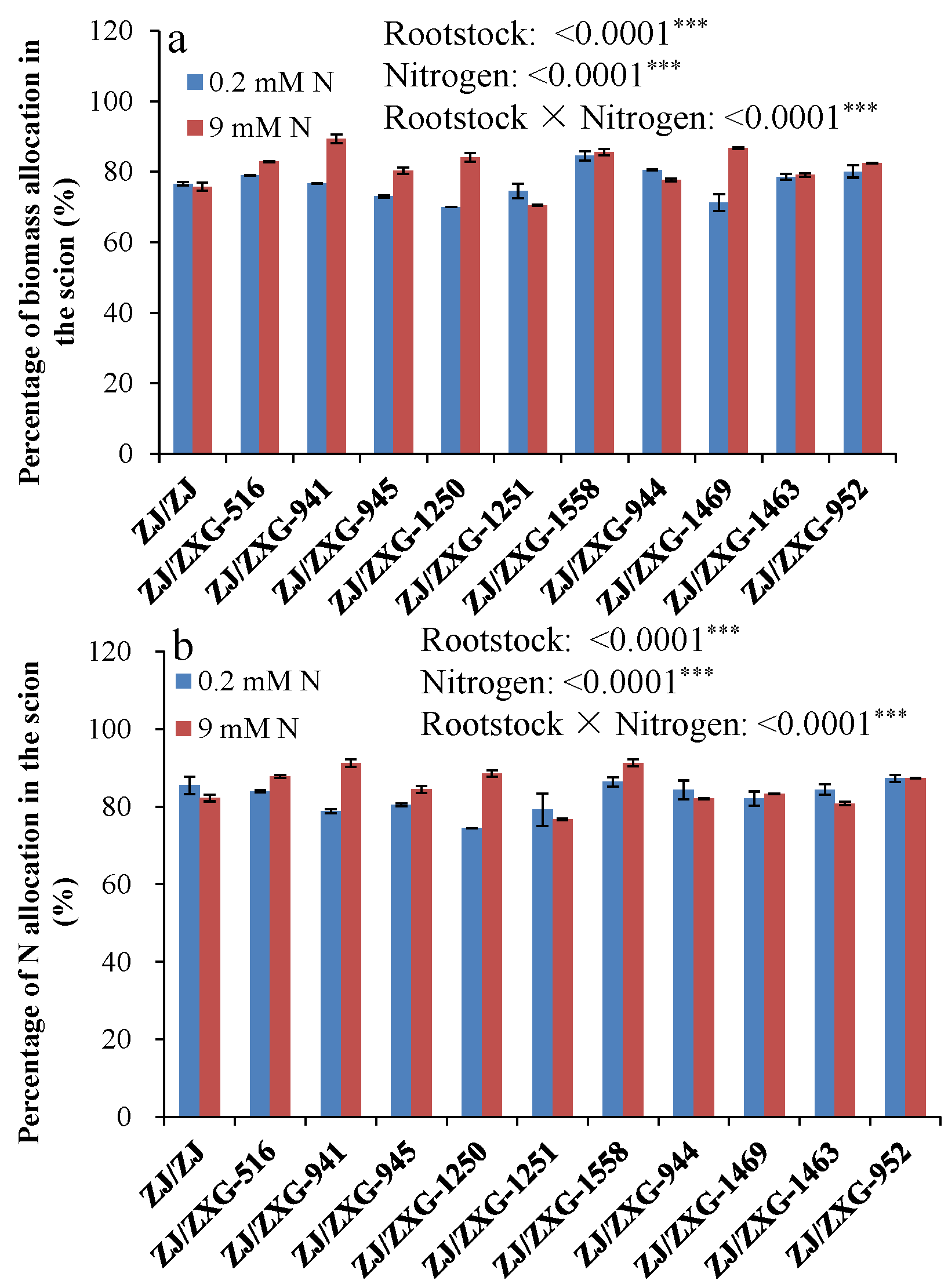
3.4. Percentage Biomass and N Allocation in the Scion
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Prinsi, B.; Negri, A.S.; Pesaresi, P.; Cocucci, M.; Espen, L. Evaluation of protein pattern changes in roots and leaves of Zea mays plants in response to nitrate availability by two-dimensional gel electrophoresis analysis. BMC Plant Biol. 2009, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Curci, P.L.; Cigliano, R.A.; Zuluaga, D.L.; Janni, M.; Sanseverino, W.; Sonnante, G. Transcriptomic response of durum wheat to nitrogen starvation. Sci. Rep. 2017, 7, 1176. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Wang, L.; Jiao, Y.Y.; Chen, C.; Zhao, L.; Mei, M.; Yu, Y.; Bie, Z.; Huang, Y. Pumpkin rootstock improves nitrogen use efficiency of watermelon scion by enhancing nutrient uptake, cytokinin content, and expression of nitrate reductase gene. Plant Growth Regul. 2017, 82, 233–246. [Google Scholar] [CrossRef]
- Luo, J.; Li, H.; Liu, T.; Polle, A.; Peng, C.; Luo, Z.B. Nitrogen metabolism of two contrasting poplar species during acclimation to limiting nitrogen availability. J. Exp. Bot. 2013, 64, 4207–4224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, M.; Wang, L.; Liu, Y.; Feng, Z.; Zhou, X.; Qin, Z. Transcriptome profiling of cucumber genome expression in response to long-term low nitrogen stress. Acta Physiologiae Plantarum. 2017, 39, 130. [Google Scholar] [CrossRef]
- Hakeem, K.R.; Ahmad, A.; Iqbal, M.; Gucel, S.; Ozturk, M. Nitrogen-efficient rice cultivars can reduce nitrate pollution. Environ. Sci. Poll. Res. 2011, 18, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Sattelmacher, B.; Horst, W.J.; Becker, H.C. Factors that contribute to genetic variation for nutrient efficiency of crop plants. J. Plant Nutr. Soil Sci. 1994, 157, 215–224. [Google Scholar] [CrossRef]
- Gerendás, J.; Abbadi, J.; Sattelmacher, B. Potassium efficiency of safflower (Carthamus tinctorius L.) and sunflower (Helianthus annuus L.). J. Plant Nut Soil Sci. 2008, 171, 431–439. [Google Scholar] [CrossRef]
- Meister, R.; Rajani, M.S.; Ruzicka, D.; Schachtman, D.P. Challenges of modifying root traits in crops for agriculture. Trends Plant Sci. 2014, 19, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.D.; Benfey, P.N. Regulation of plant root system architecture: implications for crop advancement. Curr. Opin. Biotechnol. 2015, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Wissuwa, M.; Kretzschmar, T.; Rose, T.J. From promise to application: Root traits for enhanced nutrient capture in rice breeding. J. Exp. Bot. 2016, 67, 3605–3615. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Suarez, C.M.C.; Cardarelli, M. Improving Nitrogen Use Efficiency in Melon by Grafting. HortScience 2010, 45, 559–565. [Google Scholar]
- Nawaz, M.A.; Imtiaz, M.; Kong, Q.; Fei, C.; Ahmed, W.; Huang, Y.; Bie, Z. Grafting: a technique to modify ion accumulation in horticultural crops. Front. Plant Sci. 2016, 7, 1457. [Google Scholar] [CrossRef] [PubMed]
- Bie, Z.; Nawaz, M.A.; Huang, Y.; Lee, J.M.; Colla, G. Introduction of vegetable grafting. In Vegetable Grafting: Principles and Practices; Colla, G., Alfocea, F.P., Schwarz, D., Eds.; CABI Publishing: Oxfordshire, UK, 2017; pp. 1–21. [Google Scholar]
- Shireen, F.; Nawaz, M.A.; Chen, C.; Zhang, Q.; Zheng, Z.; Sohail, H.; Sun, J.; Cao, H.; Huang, Y.; Bie, Z. Boron: Functions and approaches to enhance its availability in plants for sustainable agriculture. Int. J. Mol. Sci. 2018, 19, 1856. [Google Scholar] [CrossRef] [PubMed]
- Pulgar, G.; Villora, G.; Moreno, D.A.; Romero, L. Improving the mineral nutrition in grafted watermelon: Nitrogen metabolism. Biol. Plant. 2000, 43, 607–609. [Google Scholar] [CrossRef]
- Zen, Y.A.; Zhu, Y.L.; Huang, B.J.; Yang, L.F. Effects of Cucurbita ficifolia as rootstock on growth, fruit setting, disease resistance and leaf nutrient element contents in Cucumis sativus. J. Plant Resour. Environ. 2004, 13, 15–19. [Google Scholar]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current status of vegetable grafting: diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Schwarz, D.; Öztekin, G.B.; Tüzel, Y.; Brückner, B.; Krumbein, A. Rootstocks can enhance tomato growth and quality characteristics at low potassium supply. Sci. Hortic. 2013, 149, 70–79. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Shireen, F.; Huang, Y.; Zhilong, B.; Ahmed, W.; Saleem, B.A. Perspectives of vegetable grafting in Pakistan, current status, challenges and opportunities. Int. J. Agric. Biol. 2017, 19, 1165–1174. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Chen, C.; Shireen, F.; Zheng, Z.; Jiao, Y.; Sohail, H.; Afzal, M.; Imtiaz, M.; Ali, M.A.; Huang, Y.; et al. Improving vanadium stress tolerance of watermelon by grafting onto bottle gourd and pumpkin rootstock. Plant Growth Regul. 2018, 85, 41–56. [Google Scholar] [CrossRef]
- Gregory, P.J.; Atkinson, C.J.; Bengough, A.G.; Else, M.A.; Fernandez-Fernandez, F.; Harrison, R.J.; Schmidt, S. Contributions of roots and rootstocks to sustainable, intensified crop production. J. Exp. Bot. 2013, 64, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Albacete, A.; Martinez-Anujar, C.; Marinez-Pérez, A.; Thompson, A.J.; Dodd, I.C.; Pérez-Alfocea, F. Unravelling rootstock×scion interactions to improve food Security. J. Exp. Bot. 2015, 66, 2211–2226. [Google Scholar] [CrossRef] [PubMed]
- Venema, J.H.; Elzenga, J.T.M.; Bouwmeester, H.J. Selection and breeding of robust rootstocks as a tool to improve nutrient-use efficiency and abiotic stress tolerance in tomato. In Proceedings of the First International Conference on Organic Greenhouse Horticulture, Bleiswijk, The Netherlands, 11–14 October 2010; Dorais, M., Bishop, S.D., Eds.; Acta Horticulturae: Leuven, Belgium, 2011. [Google Scholar]
- Hassell, R.L.; Memmott, F.; Liere, D.G. Grafting methods for watermelon production. Hort. Sci. 2008, 43, 1677–1679. [Google Scholar]
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis; Part 2, 9; Black, C.A., Evans, D.D., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Rengel, Z.; Damon, P.M. Crops and genotypes differ in efficiency of potassium uptake and use. Physiol. Plant. 2008, 133, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.R.; Perkins-Veazie, P.; Sakata, Y.; López-Galarza, S.; Maroto, J.V.; Lee, S.G.; Huh, Y.C.; Sun, Z.; Miguel, A.; King, S.R; et al. Cucurbit grafting. Critic. Rev. Plant Sci. 2008, 27, 50–74. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Mirabelli, C.; Cardarelli, M. Nitrogen-use efficiency traits of mini-watermelon in response to grafting and nitrogen-fertilization doses. J. Plant Nutr. Soil Sci. 2011, 174, 933–941. [Google Scholar] [CrossRef]
- Gao, Y.; Tian, Y.; Liang, X.; Gao, L. Effects of single-root-grafting, double-root grafting and compost application on microbial properties of rhizosphere soils in Chinese protected cucumber (Cucumissativus L.) production systems. Sci. Hortic. 2015, 186, 190–200. [Google Scholar] [CrossRef]
- Goncalves, B.; Correia, C.M.; Silva, A.P.; Bacelar, E.A.; Santos, A. Leaf structure and function of sweet cherry tree (Prunusavium L.) cultivars with open and dense canopies. Sci. Hortic. 2008, 116, 381–387. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia J. 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.R.; Kaldenhoff, R.; Genty, B.; Terashima, I. Resistances along the CO2 diffusion pathway inside leaves. J. Exp. Bot. 2009, 60, 2235–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.X.; Xu, Z.G.; Liu, X.Y.; Tang, C.M.; Wang, L.W.; Han, X.L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Hua, B.; Liu, Z.; Fan, M.; Bie, Z. Grafting onto different rootstocks as a means to improve watermelon tolerance to low potassium stress. Sci. Hortic. 2013, 149, 80–85. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Belakbir, A.; Lhpez-Cantarero, I.; Romero, L. Leaf-macronutrient content and yield in grafted melon plants. A model to evaluate the influence of rootstock genotype. Sci. Hortic. 1997, 71, 227–234. [Google Scholar] [CrossRef]
- Uygur, V.; Yetisir, H. Effects of rootstocks on some growth parameters, phosphorous and nitrogen uptake by watermelon under salt stress. J. Plant Nutr. 2009, 32, 629–643. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, L.Q.; Kong, Q.S.; Cheng, F.; Niu, M.L.; Xie, J.J.; Nawaz, M.A.; Bie, Z.L. Comprehensive mineral nutrition analysis of watermelon grafted onto two different rootstocks. Hort. Plant J. 2016, 2, 105–113. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, Y.; Nawaz, M.A.; Chen, C.; Liu, L.; Lu, Z.; Kong, Q.; Cheng, F.; Bie, Z. Improving magnesium uptake, photosynthesis and antioxidant enzyme activities of watermelon by grafting onto pumpkin rootstock under low magnesium. Plant Soil. 2016, 409, 229–246. [Google Scholar] [CrossRef]
- Ahmed, W.; Pervez, M.A.; Amjad, M.; Khalid, M.; Ayyub, C.M.; Nawaz, M.A. Effect of stionic combination on the growth and yield of Kinnow mandarin (Citrus reticulate Blanco.). Pak. J. Bot. 2006, 38, 603–612. [Google Scholar]
- Ahmed, W.; Nawaz, M.A.; Iqbal, M.A.; Khan, M.M. Effect of different rootstocks on plant nutrient Status and yield in Kinnow mandarin (Citrus reticulate Blanco). Pak. J. Bot. 2007, 39, 1779–1786. [Google Scholar]
- Nawaz, M.A.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z.; Imtiaz, M.; Bie, Z.; Huang, Y. Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 2018, 220, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, N.; Monte, R.; Varanini, Z.; Cesco, S.; Pinton, R. Induction of nitrate uptake in Sauvignon Blanc and Chardonnay grapevines depends on the scion and is affected by the rootstock. Aust. J. Grape Wine Res. 2015, 21, 331–338. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, X.; Yan, B.; Li, B.; Sun, J.; Guo, S.; Tezuka, T. Bottle gourd rootstock-grafting affects nitrogen metabolism in NaCl-stressed watermelon leaves and enhances short-term salt tolerance. J. Plant Physiol. 2013, 170, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A; Chen, C.; Shireen, F.; Zheng, Z.; Sohail, H.; Afzal, M.; Ali, M.A.; Zhilong, B.; Huang, Y. Genome-wide expression profiling of leaves and root of watermelon in response to low nitrogen. BMC Genomics 2018, 19, 456. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, M.A.; Han, X.; Chen, C.; Zheng, Z.; Shireen, F.; Bie, Z.; Huang, Y. Nitrogen Use Efficiency of Watermelon Grafted onto 10 Wild Watermelon Rootstocks under Low Nitrogen Conditions. Agronomy 2018, 8, 259. https://doi.org/10.3390/agronomy8110259
Nawaz MA, Han X, Chen C, Zheng Z, Shireen F, Bie Z, Huang Y. Nitrogen Use Efficiency of Watermelon Grafted onto 10 Wild Watermelon Rootstocks under Low Nitrogen Conditions. Agronomy. 2018; 8(11):259. https://doi.org/10.3390/agronomy8110259
Chicago/Turabian StyleNawaz, Muhammad Azher, Xiaojie Han, Chen Chen, Zuhua Zheng, Fareeha Shireen, Zhilong Bie, and Yuan Huang. 2018. "Nitrogen Use Efficiency of Watermelon Grafted onto 10 Wild Watermelon Rootstocks under Low Nitrogen Conditions" Agronomy 8, no. 11: 259. https://doi.org/10.3390/agronomy8110259
APA StyleNawaz, M. A., Han, X., Chen, C., Zheng, Z., Shireen, F., Bie, Z., & Huang, Y. (2018). Nitrogen Use Efficiency of Watermelon Grafted onto 10 Wild Watermelon Rootstocks under Low Nitrogen Conditions. Agronomy, 8(11), 259. https://doi.org/10.3390/agronomy8110259





