Characterization of Interspecific Hybrids between Flowering Chinese Cabbage and Chinese Kale
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Methods
2.2.1. Embryo Rescue and Colchicine Doubling
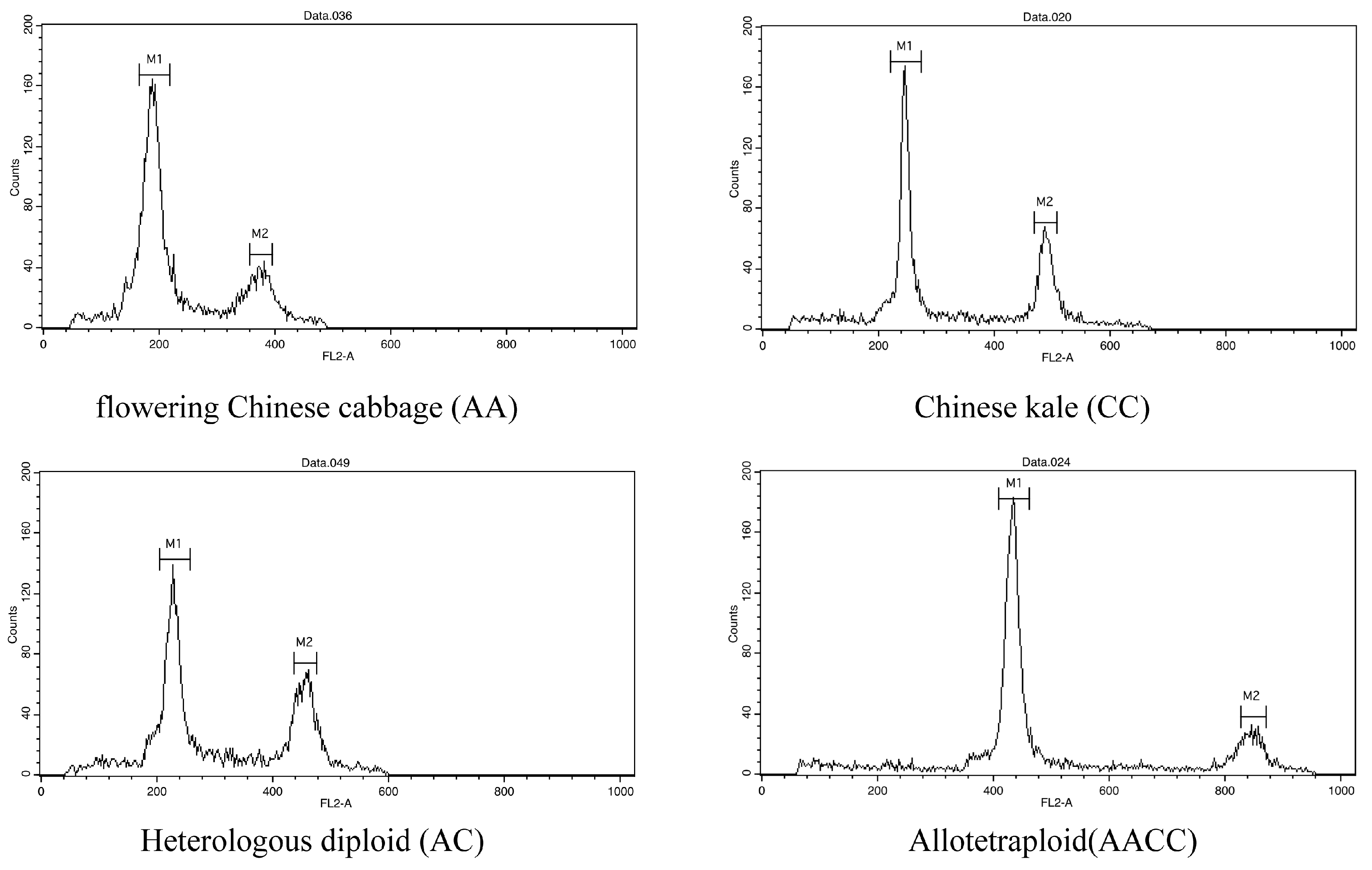
2.2.2. Ploidy Identification and Assessment of Plant Characteristics
2.2.3. Genomic DNA Isolation and Molecular Analysis
3. Results
3.1. Generation of Hybrid Offspring
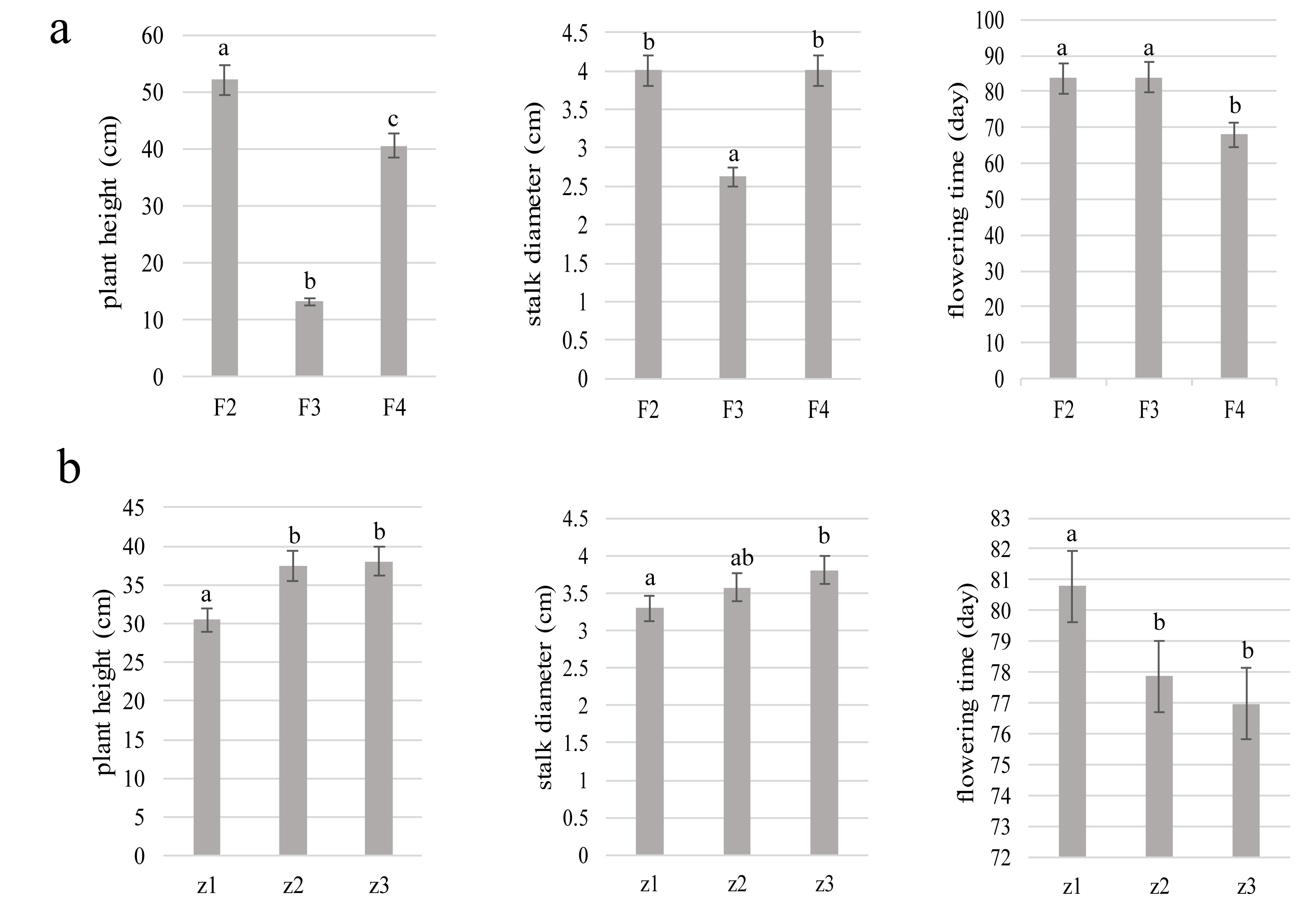
3.2. Morphological Characteristics
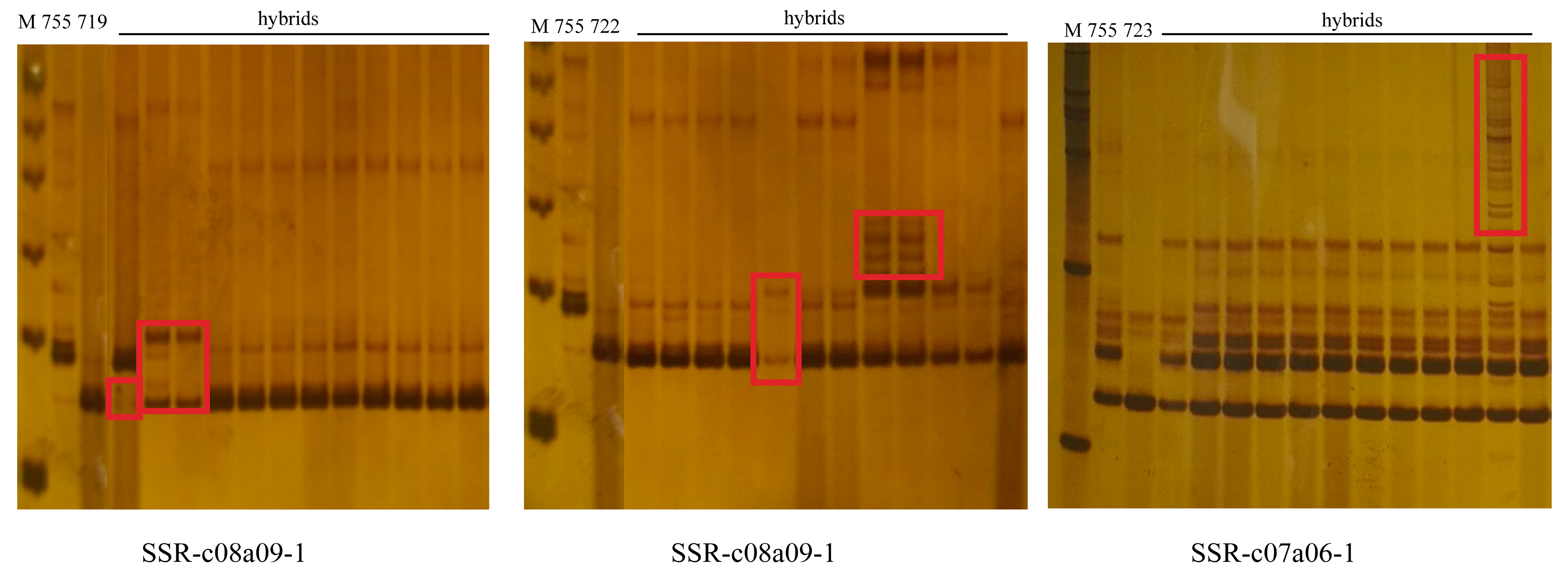
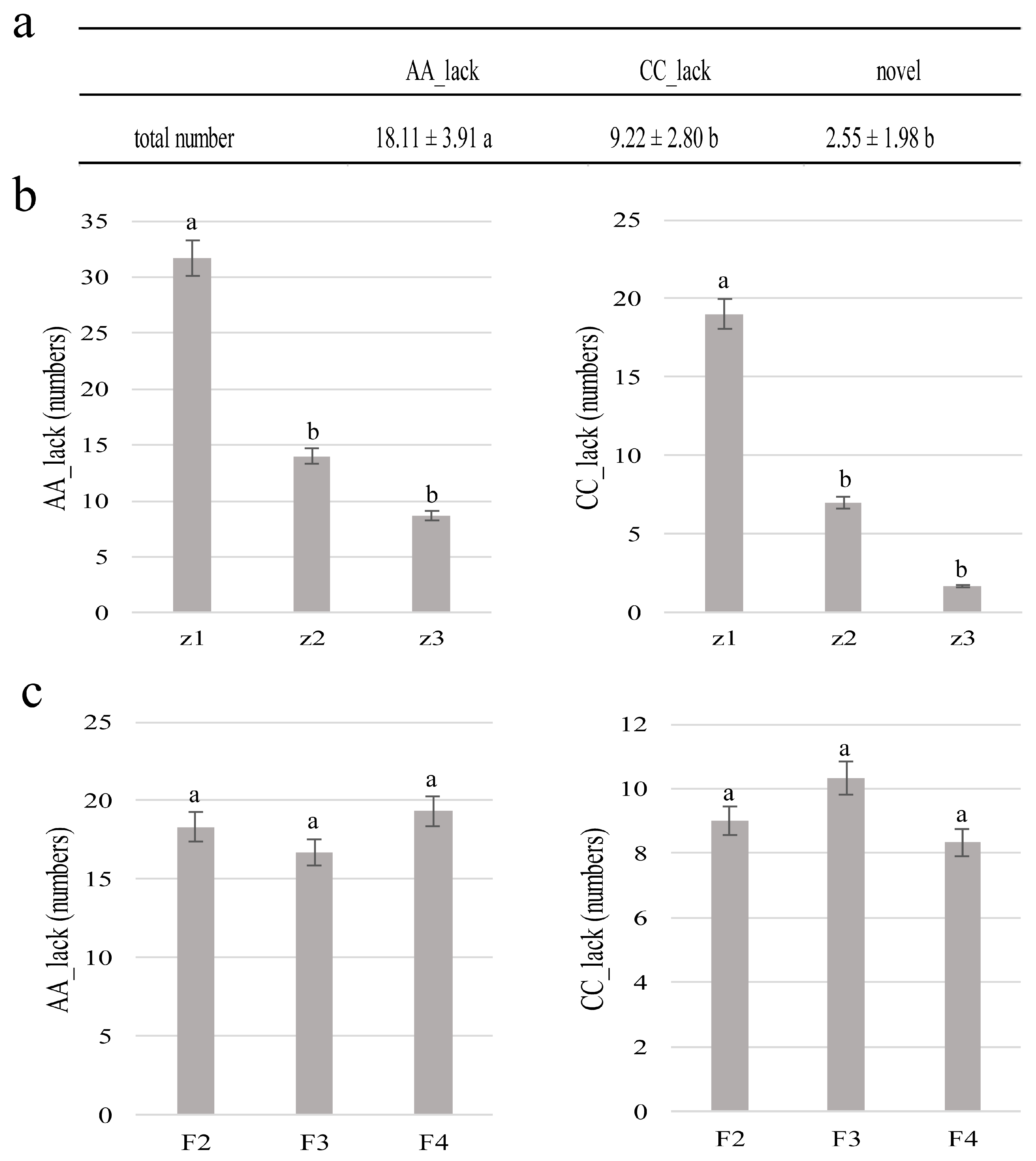
3.3. Molecular Characterization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peng, Y.; Shi, D.; Zhang, T.; Li, X.; Fu, T.; Xu, Y.; Wan, Z. Development and utilization of an efficient cytoplasmic male sterile system for Cai-xin (Brassica rapa L.). Sci. Hortic. 2015, 190, 36–42. [Google Scholar] [CrossRef]
- Sun, B.; Yan, H.; Liu, N.; Wei, J.; Wang, Q. Effect of 1-MCP treatment on postharvest quality characters, antioxidants and glucosinolates of Chinese kale. Food Chem. 2012, 131, 519–526. [Google Scholar] [CrossRef]
- Deng, M.; Qian, H.; Chen, L.; Sun, B.; Chang, J.; Miao, H.; Cai, C.; Wang, Q. Influence of pre-harvest red light irradiation on main phytochemicals and antioxidant activity of Chinese kale sprouts. Food Chem. 2017, 222, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Liu, N.; Zhao, Y.; Yan, H.; Wang, Q. Variation of glucosinolates in three edible parts of Chinese kale (Brassica alboglabra Bailey) varieties. Food Chem. 2011, 124, 941–947. [Google Scholar] [CrossRef]
- Li, G.G.; Zhang, H.; Huang, H.D.; Qiao, Y.C.; Zheng, Y.S. Research progress on flowering Chinese cabbage breeding in Guangdong province. Chin. Veg. 2010, 20, 9–14. [Google Scholar]
- Yan, R.L. The Origin and Variety Selection and Cultivation of Flowering Chinses Cabbage; China Agriculture Press: Beijing, China, 2014. [Google Scholar]
- Zhang, H.H.; Liu, Z.Z. Market demand and breeding status of flowering Chinese cabbage. Chin. Veg. 2010, 3, 10–12. [Google Scholar]
- Qin, Y.G.; Yang, C.Q.; Cao, B.H.; Chen, G.J.; Lei, J.J. Advances in Research on Genetic Breeding and Biotechnology of Chinese Kale. Chin. Agric. Sci. Bull. 2009, 25, 296–299. [Google Scholar]
- Cicin, N.V. Distant Hybridization in Plants; Moskva: Moscow, Russia, 1954. [Google Scholar]
- Chen, J.; Luo, M.; Li, S.; Tao, M.; Ye, X.; Duan, W.; Zhang, C.; Qin, Q.; Xiao, J.; Liu, S. A comparative study of distant hybridization in plants and animals. Sci. Chin. Life Sci. 2018, 61, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Whitney, K.D.; Ahern, J.R.; Campbell, L.G.; Albert, L.P.; King, M.S. Patterns of hybridization in plants. Perspect. Plant Ecol. Evol. Systemat. 2010, 12, 175–182. [Google Scholar] [CrossRef]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.P.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Wincker, P.; et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, R.T.; Pires, J.C.; Iniguez-Luy, F.; Leon, E.; Osborn, T.C. Genomic changes in resynthesized Brassica napus and their effect on gene expression and phenotype. Plant Cell 2007, 19, 3403–3417. [Google Scholar] [CrossRef] [PubMed]
- He, L.Q.; Tang, R.H.; Jiang, J.; Xiong, F.Q.; Huang, Z.P.; Wu, H.N.; Gao, Z.K.; Zhong, R.C.; He, X.H.; Han, Z.Q. Rapid gene expression change in a novel synthesized allopolyploid population of cultivated peanut × Arachis doigoi cross by cDNA-SCoT and HFO-TAG technique. J. Integr. Agric. 2017, 16, 1093–1102. [Google Scholar] [CrossRef]
- Mestiri, I.; Chague, V.; Tanguy, A.M.; Huneau, C.; Huteau, V.; Belcram, H.; Coriton, O.; Chalhoub, B.; Jahier, J. Newly synthesized wheat allohexaploids display progenitor-dependent meiotic stability and aneuploidy but structural genomic additivity. New Phytol. 2010, 186, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Tian, E.; Jiang, Y.; Chen, L.; Zou, J.; Liu, F.; Meng, J. Synthesis of a Brassica trigenomic allohexaploid (B. carinata × B. rapa) de novo and its stability in subsequent generations. Theor. Appl. Genet. 2010, 121, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Baldwin, S.J.; Jacobs, J.M.; Linden, C.G.; Voorrips, R.E.; Leunissen, J.A.; Eck, H.; Vosman, B. Large-scale identification of polymorphic microsatellites using an in silico approach. BMC Bioinform. 2008, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, T.; Li, X.; Duan, M.; Wang, J.; Qiu, Y.; Wang, H.; Song, J.; Shen, D. Interspecific hybridization, polyploidization, and backcross of Brassica oleracea var. alboglabra with B. rapa var. purpurea morphologically recapitulate the evolution of Brassica vegetables. Sci. Rep. 2016, 6, 18618. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Fu, S.; Ren, Z.; Zou, Y. Rapid evolution of simple sequence repeat induced by allopolyploidization. J. Mol. Evol. 2009, 69, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.M.; Siddika, A.; Tonu, N.N.; Hossain, D.M.; Meah, M.B.; Kawanabe, T.; Fujimoto, R.; Okazaki, K. Production of high yield short duration Brassica napus by interspecific hybridization between B. oleracea and B. rapa. Breed. Sci. 2014, 63, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Sun, S.; Hua, S.; Shen, E.; Ye, C.Y.; Cai, D.; Timko, M.P.; Zhu, Q.H.; Fan, L. Analysis of transcriptional and epigenetic changes in hybrid vigor of allopolyploid Brassica napus uncovers key roles for small RNAs. Plant J. 2017, 91, 874–893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Tang, G.X.; Song, W.J.; Zhou, W.J. Resynthesizing Brassica napus from interspecific hybridization between Brassica rapa and B. oleracea through ovary culture. Euphytica 2004, 140, 181–187. [Google Scholar] [CrossRef]
- Ayotte, R.; Harney, P.M.; Machado, V.S. The transfer of triazine resistance from Brassica napus L. to B. oleracea L. I. Production of F1 hybrids through embryo rescue. Euphytica 1987, 36, 615–624. [Google Scholar] [CrossRef]
- Sharma, D.R.; Kaur, R.; Kumar, K. Embryo rescue in plants—A review. Euphytica 1996, 89, 325–337. [Google Scholar] [CrossRef]
- Zhao, J.; Simmonds, D.H.; Newcomb, W. High frequency production of doubled haploid plants of Brassica napus cv. Topas derived from colchicine-induced microspore embryogenesis without heat shock. Plant Cell Rep. 1996, 15, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Dolezel, J.; Greilhuber, J.; Suda, J. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2007, 2, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Fang, Z.Y.; Liu, Y.M.; Yang, L.M.; Zhuang, M.; Lv, H.H.; Li, Z.S.; Han, F.Q.; Liu, X.P.; Zhang, Y.Y. Development of a novel allele-specific Rfo marker and creation of Ogura CMS fertility-restored interspecific hybrids in Brassica oleracea. Theor. Appl. Genet. 2016, 129, 1625–1637. [Google Scholar] [CrossRef] [PubMed]
- Kidwell, K.K.; Osborn, T.C. Simple plant DNA isolation procedures. In Plant Genomes: Methods for Genetic and Physical Mapping; Beckmann, J.S., Osborn, T.C., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1992; pp. 1–13. [Google Scholar] [CrossRef]
- Ozminkowski, R.H.; Jourdan, P. Comparing the resynthesis of Brassica napus L. by interspecific somatic and sexual hybridization. I. Producing and identifying hybrids. J. Am. Soc. Hortic. Sci. 1994, 119, 808–815. [Google Scholar]
- Eickermann, M.; Ulber, B.; Vidal, S. Resynthesized lines and cultivars of Brassica napus L. provide sources of resistance to the cabbage stem weevil (Ceutorhynchus pallidactylus (Mrsh.)). Bull. Entomol. Res. 2011, 101, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Gemayel, R.; Vinces, M.D.; Legendre, M.; Verstrepen, K.J. Variable tandem repeats accelerate evolution of coding and regulatory sequences. Ann. Rev. Genet. 2010, 44, 445–477. [Google Scholar] [CrossRef] [PubMed]
- Yaakov, B.; Kashkush, K. Massive alterations of the methylation patterns around DNA transposons in the first four generations of a newly formed wheat allohexaploid. Genome 2011, 54, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zuo, K.; Zhang, F.; Cao, Y.; Wang, J.; Zhang, Y.; Sun, X.; Tang, K. Conservation of noncoding microsatellites in plants: Implication for gene regulation. BMC Genom. 2006, 7, 323. [Google Scholar] [CrossRef]
- Grover, A.; Sharma, P.C. Is spatial occurrence of microsatellites in the genome a determinant of their function and dynamics contributing to genome evolution? Curr. Sci. 2011, 100, 859–869. [Google Scholar]
- Yotoko, K.S.; Dornelas, M.C.; Togni, P.D.; Fonseca, T.C.; Salzano, F.M.; Bonatto, S.L.; Freitas, L.B. Does variation in genome sizes reflect adaptive or neutral processes? New clues from Passiflora. PLoS ONE 2011, 6, e18212. [Google Scholar] [CrossRef] [PubMed]
- Raskina, O.; Barber, J.C.; Nevo, E.; Belyayev, A. Repetitive DNA and chromosomal rearrangements: Speciation-related events in plant genomes. Cytogenet. Genome Res. 2008, 120, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, S.D.; Leflon, M.; Liu, Z.; Eber, F.; Chelysheva, L.; Coriton, O.; Chèvre, A.M.; Jenczewski, E. Chromosome ‘speed dating’ during meiosis of polyploid Brassica hybrids and haploids. Cytogenet. Genome Res. 2008, 120, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J. Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu. Rev. Plant Biol. 2007, 58, 377–406. [Google Scholar] [CrossRef] [PubMed]

| Species | Genome Type | Code | Material Original | Name |
|---|---|---|---|---|
| Flowering Chinese cabbage | AA | 755 | Inbred line | flowering Chinese cabbage A |
| Chinese kale | CC | 719 | Inbred line | Cujie |
| Chinese kale | CC | 722 | Commercial variety | Sijicutiao Chinese kale |
| Chinese kale | CC | 723 | Commercial variety | Xianggangbaihuatian Chinese kale |
| Code | Cross Combination | Ovule Culture Number | Obtain Seedlings Number | Rate of Obtaining the Hybrids (%) | Colchicine Treatment Number | Doubled Number | Rate of Double (%) |
|---|---|---|---|---|---|---|---|
| z1 | 755 × 719 | 190 | 70 | 0.37 | 15 | 6 | 0.40 |
| z2 | 755 × 722 | 140 | 52 | 0.37 | 15 | 7 | 0.47 |
| z3 | 755 × 723 | 80 | 29 | 0.36 | 15 | 8 | 0.53 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Li, F.; Zhang, S.; Zhang, S.; Zhang, H.; Sun, R. Characterization of Interspecific Hybrids between Flowering Chinese Cabbage and Chinese Kale. Agronomy 2018, 8, 258. https://doi.org/10.3390/agronomy8110258
Wei Y, Li F, Zhang S, Zhang S, Zhang H, Sun R. Characterization of Interspecific Hybrids between Flowering Chinese Cabbage and Chinese Kale. Agronomy. 2018; 8(11):258. https://doi.org/10.3390/agronomy8110258
Chicago/Turabian StyleWei, Yunxiao, Fei Li, Shujiang Zhang, Shifan Zhang, Hui Zhang, and Rifei Sun. 2018. "Characterization of Interspecific Hybrids between Flowering Chinese Cabbage and Chinese Kale" Agronomy 8, no. 11: 258. https://doi.org/10.3390/agronomy8110258
APA StyleWei, Y., Li, F., Zhang, S., Zhang, S., Zhang, H., & Sun, R. (2018). Characterization of Interspecific Hybrids between Flowering Chinese Cabbage and Chinese Kale. Agronomy, 8(11), 258. https://doi.org/10.3390/agronomy8110258





