The Influence of Water and Nitrogen Availability on the Expression of End-Use Quality Parameters of Spring Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
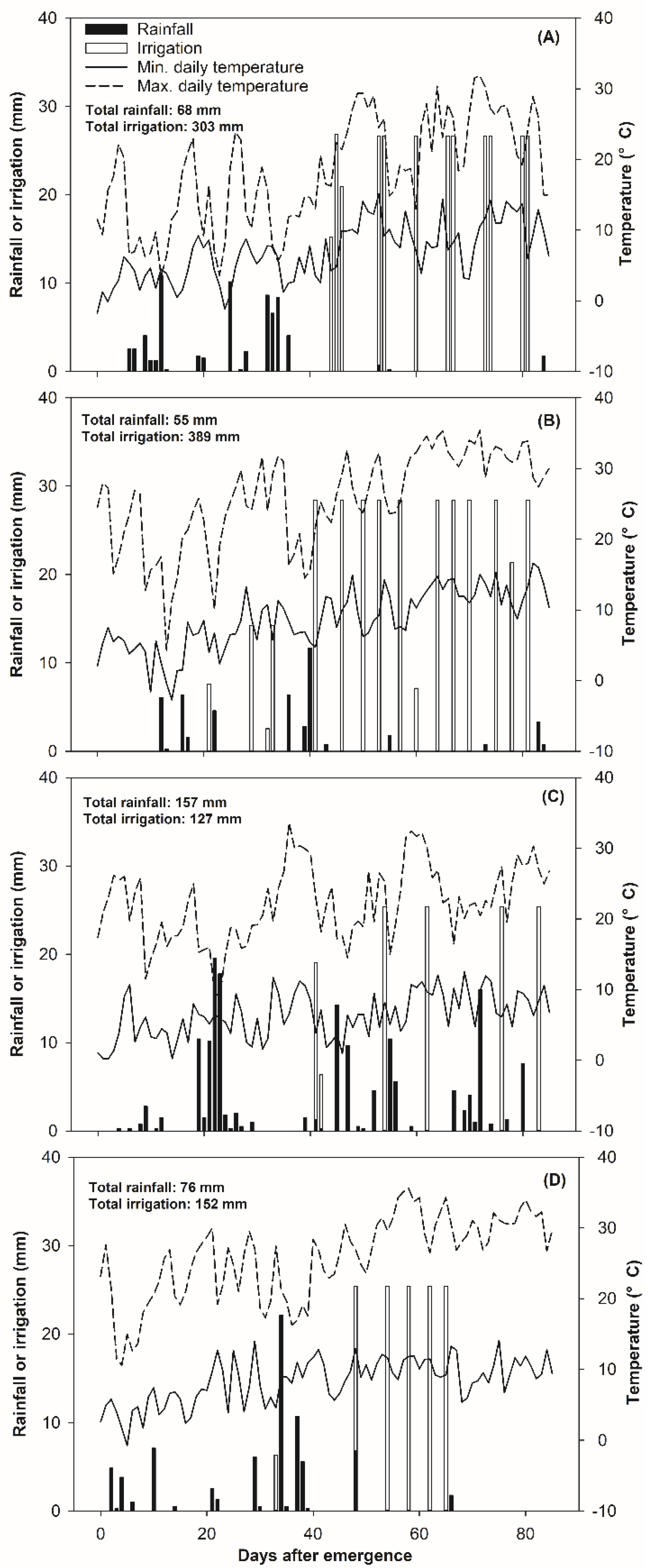
2.2. Experimental Design
2.3. Grain Yield, Test Weight, and End-Use Quality Analysis
2.4. Statistical Analysis
3. Results
3.1. Grain Yield and Test Weight
3.2. Milling Quality
3.3. Flour Protein, Wet Gluten, and Gluten Index
3.4. Dough Rheological Properties
3.5. Solvent Retention Capacity
3.6. Correlations among End-Use Quality Traits
4. Discussion
4.1. Effects of Drought Stress on Grain Yield and Flour End-Use Quality
4.2. Effects of N Application on Grain Yield and Flour End-Use Quality
4.3. Relationships among End-Use Quality Parameters
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Database. Available online: http://faostat3.fao.org/ (accessed on 15 February 2018).
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw, B.; Smale, M.; Braun, H.J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Pasha, I.; Anjum, F.M.; Morris, C.F. Grain Hardness: A major determinant of wheat quality. Food Sci. Technol. Int. 2010, 16, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Caffe-Treml, M.; Glover, K.D.; Krishnan, P.G.; Hareland, G.A.; Bondalapati, K.D.; Stein, J. Effect of wheat genotype and environment on relationships between dough extensibility and breadmaking quality. Cereal Chem. 2011, 88, 201–208. [Google Scholar] [CrossRef]
- Carson, G.R.; Edwards, N.M. Criteria of wheat and flour quality. In Wheat: Chemistry and Technology, 4th ed.; Khan, K., Shewry, P.R., Eds.; AACC International: Saint Paul, MN, USA, 2009; pp. 97–118. ISBN 978. [Google Scholar]
- Dencic, S.; Mladenov, N.; Kobilkjski, B. Effects of genotype and environment on breadmaking quality in wheat. Int. J. Plant Prod. 2011, 5, 71–82. [Google Scholar] [CrossRef]
- Graybosch, R.A.; Peterson, C.J.; Shelton, D.R.; Baezinger, P.S. Genotypic and environmental modification of wheat flour protein composition in relation to end-use quality. Crop Sci. 1996, 36, 296–300. [Google Scholar] [CrossRef]
- Ma, W.; Appels, R.; Békés, F.; Larroque, O.R.; Morell, M.K.; Gale, K.R. Genetic characterization of dough rheological properties in a wheat doubled haploid population: Additive genetic effects and epistatic interactions. Theor. Appl. Genet. 2005, 111, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.J.; Graybosch, R.A.; Shelton, D.R.; Baezinger, P.S. Baking quality of hard winter wheat: Response of cultivars to environment in the Great Plains. Euphytica 1998, 100, 157–162. [Google Scholar] [CrossRef]
- Yong, Z.; He, Z.; Ye, G.; Aimin, Z.; Van Ginkel, M. Effect of environment and genotype on bread-making quality of spring-sown spring wheat cultivars in China. Euphytica 2004, 139, 75–83. [Google Scholar] [CrossRef]
- Brevis, J.C.; Morris, C.F.; Manthey, F.; Dubcovsky, J. Effect of the grain protein content locus Gpc-B1 on bread and pasta quality. J. Cereal Sci. 2010, 51, 357–365. [Google Scholar] [CrossRef]
- Blake, N.K.; Stougaard, R.N.; Bohannon, B.; Weaver, D.K.; Heo, H.-Y.; Lamb, P.F.; Nash, D.; Wichman, D.M.; Kephart, K.D.; Miller, J.H.; et al. Registration of ‘Egan’ wheat with resistance to orange wheat blossom midge. J. Plant Regist. 2014, 8, 298–302. [Google Scholar] [CrossRef]
- Payne, P.I.; Nigtingale, M.A.; Krattiger, A.F.; Holt, L.M. The relationship between HMW glutenin subunit composition and the bread-making quality of British-grown wheat varieties. J. Sci. Food Agric. 1987, 40, 51–65. [Google Scholar] [CrossRef]
- Guttieri, M.J.; McLean, R.; Stark, J.C.; Souza, E. Managing irrigation and nitrogen fertility of hard spring wheats for optimum bread and noodle quality. Crop Sci. 2005, 45, 2049–2059. [Google Scholar] [CrossRef]
- Johansson, E.; Prieto-Linde, M.L.; Jonsson, J.O. Effects of wheat cultivar and nitrogen application on storage protein composition and breadmaking quality. Cereal Chem. 2001, 78, 19–25. [Google Scholar] [CrossRef]
- Johansson, E.; Prieto-Linde, M.L.; Svensson, G. Influence of nitrogen application rate and timing on grain protein composition and gluten strength in Swedish wheat cultivars. J. Plant Nutr. Soil Sci. 2004, 167, 345–350. [Google Scholar] [CrossRef]
- Saint Pierre, C.; Peterson, C.J.; Ross, A.S.; Ohm, J.-B.; Verhoeven, M.C.; Larson, M.; Hoefer, B. White wheat grain quality changes with genotype, nitrogen fertilization, and water stress. Agron. J. 2008, 100, 414–420. [Google Scholar] [CrossRef]
- Saint Pierre, C.; Peterson, C.J.; Ross, A.S.; Ohm, J.-B.; Verhoeven, M.C.; Larson, M.; Hoefer, B. Winter wheat genotypes under different levels of nitrogen and water stress: Changes in grain protein composition. J. Cereal Sci. 2008, 47, 407–416. [Google Scholar] [CrossRef]
- Guttieri, M.J.; Ahmad, R.; Stark, J.C.; Souza, E. End-use quality of six hard red spring wheat cultivars at different irrigation levels. Crop Sci. 2000, 40, 631–635. [Google Scholar] [CrossRef]
- Guzmán, C.; Autrique, J.E.; Mondal, S.; Singh, R.P.; Govindan, V.; Morales-Dorantes, A.; Posadas-Romano, G.; Crossa, J.; Ammar, K.; Peña, R.J. Response to drought and heat stress on wheat quality, with special emphasis on bread-making quality, in durum wheat. Field Crop. Res. 2016, 186, 157–165. [Google Scholar] [CrossRef]
- Zeleke, K.T.; Nendel, C. Analysis of options for increasing wheat (Triticum aestivum L.) yield in south-eastern Australia: The role of irrigation, cultivar choice and time of sowing. Agric. Water Manag. 2016, 166, 139–148. [Google Scholar] [CrossRef]
- Ozturk, A.; Aydin, F. Effect of water stress at various growth stages on some quality characteristics of winter wheat. J. Agron. Crop Sci. 2004, 190, 93–99. [Google Scholar] [CrossRef]
- Wieser, H.; Seilmeier, W. The influence of nitrogen fertilization on quantities and proportions of different protein types in wheat flour. J. Sci. Food Agric. 1998, 76, 49–55. [Google Scholar] [CrossRef]
- Brown, B.; Stark, J.C.; Westermann, D.T. Southern Idaho Fertilizer Guide-Irrigated Spring Wheat; University of Idaho: Moscow, Russia, 2001. [Google Scholar]
- Jacobsen, J.; Jackson, G.; Jones, C. Fertilizer Guidelines for Montana Crops; Montana State University Extension Services: Bozeman, MT, USA, 2005. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements; FAO: Rome, Italy, 1998. [Google Scholar]
- Torrion, J.A.; Stougaard, R.N. Impacts and limits of irrigation water management on wheat yield and quality. Crop Sci 2018, 57, 3239–3251. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- AACC International. Approved Methods of Analysis, 10th ed.; AACC International: Saint Paul, MN, USA, 2000; ISBN 9781891127120. [Google Scholar]
- Guttieri, M.J.; Becker, C.; Souza, E.J. Application of wheat meal solvent retention capacity tests within soft wheat breeding populations. Cereal Chem. 2004, 81, 261–266. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, L.; Ling, Y.; Hu, X.; Cai, H.; Gu, B. Effects of limited irrigation on yield and water use efficiency of winter wheat in the loess plateau of China. Agric. Water Manag. 2002, 55, 203–216. [Google Scholar] [CrossRef]
- Brevis, J.C.; Dubcovsky, J. Effects of the chromosome region including the grain protein content locus Gpc-B1 on wheat grain and protein yield. Crop Sci. 2010, 50, 93–104. [Google Scholar] [CrossRef]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J. A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 2006, 314, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- Uauy, C.; Brevis, J.C.; Dubcovsky, J. The high grain protein content gene GPC-B1 accelerated senescence and has pleiotropic effects on protein content in wheat. J. Exp. Bot. 2006, 57, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Sadras, V.O.; Hayman, P.; Rodriguez, D.; Monjardino, M.; Bielich, M.; Unkovich, M.; Mudge, B.; Wang, E. Interactions between water and nitrogen in Australian cropping systems: Physiological, agronomic, economic, breeding and modelling perspectives. Crop Pasture Sci. 2014, 67, 1019–1053. [Google Scholar] [CrossRef]
- Hoogmoed, M.; Sadras, V.O. Water stress scatters nitrogen dilution curves in wheat. Front. Plant Sci. 2018, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Klikocka, H.; Cybulska, M.; Barczak, B.; Narolski, B.; Szostak, B.; Kobiałka, A.; Nowak, A.; Wójcik, E. The effect of sulphur and nitrogen on grain yield and technological quality of spring wheat. Plant Soil Environ. 2016, 62, 230–236. [Google Scholar] [CrossRef]
- Boehm, D.J.; Berzonsky, W.A.; Bhattacharya, M. Influence of nitrogen fertilizer treatments on spring wheat (Triticum aestivum L.) flour characteristics and effect on fresh and frozen dough quality. Cereal Chem. 2004, 81, 51–54. [Google Scholar] [CrossRef]
- Greer, E.N.; Stewart, B.A. The water absorption of wheat flour: Relative effects of protein and starch. J. Sci. Food Agric. 1959, 10, 248–252. [Google Scholar] [CrossRef]
- Eliasson, A.C.; Larsson, K. Cereals in Breadmaking: A Molecular Colloidal Approach; CRC Press: Boca Raton, FL, USA, 1993; ISBN 9780824788162. [Google Scholar]
- Kulkarni, R.G.; Ponte, J.G., Jr.; Kulp, K., Jr. Significance of gluten content as an index of flour quality. Cereal Chem. 1987, 64, 1–3. [Google Scholar]
- Gaines, C.S. Collaborative study of methods for solvent retention capacity profiles (AACC Method 56−11). Cereal Food World 2000, 45, 303–306. [Google Scholar]
- Mutwali, N.I.A.; Mustafa, A.I.; Gorafi, Y.S.A.; Ahmed, I.A.M. Effect of environment and genotypes on the physicochemical quality of the grains of newly developed wheat inbred lines. Food Sci. Nutr. 2016, 4, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Bonfil, D.J.; Posner, E.S. Can bread wheat quality be determined by gluten index? J. Cereal Sci. 2012, 56, 115–118. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, N.; Ahlawat, A.K.; Kaur, S.; Singh, A.M.; Chauhan, H.; Singh, G.P. Diversity in grain, flour, dough and gluten properties amongst Indian wheat cultivars varying in high molecular weight subunits (HMW-GS). Food Res. Int. 2013, 53, 63–72. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Hernandez-Espinosa, N.; Peña, R.J. The influence of drought and heat stress on the expression of end-use quality parameters of common wheat. J. Cereal Sci. 2013, 57, 73–78. [Google Scholar] [CrossRef]
- Xiao, Z.S.; Park, S.H.; Chung, O.K.; Caley, M.S.; Seib, P.A. Solvent retention capacity values in relation to hard winter wheat and flour properties and straight-dough breadmaking quality. Cereal Chem. 2006, 83, 465–471. [Google Scholar] [CrossRef]
- Rharrabti, Y.; Garcia del Moral, L.F.; Villegas, D.; Royo, C. Durum wheat quality in Mediterranean environments III. Stability and comparative methods in analyzing G × E interaction. Field Crop. Res. 2003, 80, 141–146. [Google Scholar] [CrossRef]

| Site-Year | pH 1 | OM 2 | Soil Residual N 3 | P 4 | K | SO42−-S | ||
|---|---|---|---|---|---|---|---|---|
| Inorganic N | Mineralized N from OM | Mineralized N from Preceding Legume | ||||||
| % | kg N ha−1 | mg kg−1 | ||||||
| AREC | ||||||||
| 2016 | 8.3 | 1.1 | 119 | 0 | N/A5 | 21 | 175 | 20 |
| 2017 | 8.3 | 1.3 | 156 | 0 | N/A | 15 | 175 | 16 |
| NWARC | ||||||||
| 2016 | 7.6 | 2.7 | 36.6 | 11.8 | 39.2 | 10 | 95 | 6 |
| 2017 | 7.8 | 2.5 | 19.5 | 8.4 | N/A | 10 | 112 | 9 |
| Treatment 2 | End-Use Quality Trait 1 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GY | TW | GH | FY | FA | FP | WG | GI | MPT | MPH | WaterSRC | Na2CO3 SRC | Lactic acid SRC | SucroseSRC | LV | |
| Mg ha−1 | kg hL−1 | % | min | cm | % | cm 3 | |||||||||
| Drought stress | |||||||||||||||
| Severe | 2.1 c 3 | 70.5 b | 77.4 | 58.7 b | 0.38 a | 16.4 a | 39.1 a | 85.8 | 4.5 a | 7.8 a | 59.4 | 71.2 | 140 a | 98.6 a | 1363 a |
| Moderate | 5.2 b | 78.3 a | 77.2 | 60.8 a | 0.34 b | 12.9 b | 32.7 b | 89.0 | 3.7 b | 7.1 b | 56.7 | 68.4 | 127 ab | 92.5 ab | 1173 b |
| Mild | 6.3 a | 78.8 a | 76.8 | 60.2 a | 0.34 b | 11.5 c | 30.4 b | 91.0 | 3.2 bc | 6.8 b | 55.7 | 68.2 | 115 b | 89.9 b | 1054 c |
| Well-watered | 6.9 a | 78.3 a | 79.1 | 59.8 ab | 0.34 b | 11.3 c | 29.8 b | 81.3 | 2.9 c | 6.8 b | 56.1 | 69.1 | 103 b | 89.7 b | 1040 c |
| p-value | <0.001 | <0.001 | 0.483 | 0.002 | <0.001 | <0.001 | 0.002 | 0.121 | <0.001 | <0.001 | 0.074 | 0.154 | <0.001 | 0.018 | <0.001 |
| Soil N rate | |||||||||||||||
| Zero | 4.9 | 78.3 a | 76.0 b | 59.5 | 0.34 b | 12.0 b | 30.2 b | 87.5 | 3.5 | 6.8 b | 55.3 | 69.0 | 116 | 91.4 | 1105 b |
| Low | 5.0 | 76.8 ab | 76.7 b | 60.0 | 0.35 ab | 13.1 ab | 31.8 ab | 89.0 | 3.6 | 7.1 ab | 56.4 | 69.1 | 123 | 91.3 | 1160 ab |
| Medium | 5.5 | 75.8 ab | 78.3 ab | 60.0 | 0.36 a | 13.3 a | 33.7 a | 84.6 | 3.5 | 7.1 ab | 57.1 | 69.6 | 119 | 93.3 | 1168 ab |
| High | 5.3 | 75.4 b | 79.4 a | 60.0 | 0.36 a | 13.6 a | 34.7 a | 86.3 | 3.6 | 7.3 a | 58.3 | 68.8 | 123 | 93.0 | 1186 a |
| p-value | 0.317 | 0.043 | 0.005 | 0.418 | <0.001 | 0.003 | 0.003 | 0.392 | 0.901 | 0.018 | 0.109 | 0.484 | 0.101 | 0.054 | 0.049 |
| Treatment 2 | End-Use Quality Trait 1 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GY | TW | GH | FY | FA | FP | WG | GI | MPT | MPH | Water SRC | Na2CO3 SRC | Lactic acid SRC | Sucrose SRC | LV | |
| Mg ha−1 | kg hL−1 | % | min | cm | % | cm 3 | |||||||||
| Drought stress | |||||||||||||||
| Severe | 3.6 c 3 | 74.7 c | 84.2 ab | 53.3 b | 0.65 | 15.5 | 39.7 | 83.5 | 5.4 a | 6.9 b | 70.1 a | 93.1 | 118 b | 111 | 1069 c |
| Moderate | 4.4 b | 76.0 b | 85.0 a | 54.7 ab | 0.64 | 15.7 | 40.5 | 85.6 | 4.5 b | 7.4 a | 69.6 ab | 92.5 | 124 ab | 111 | 1130 b |
| Mild | 4.7 b | 76.2 ab | 83.7 ab | 54.4 a | 0.64 | 15.6 | 41.4 | 88.0 | 4.3 b | 7.7 a | 69.2 ab | 92.4 | 129 a | 112 | 1182 a |
| Well-watered | 5.2 a | 76.4 a | 82.9 b | 55.3 a | 0.64 | 15.5 | 40.5 | 86.5 | 3.9 b | 7.6 a | 68.8 b | 92.8 | 131 a | 112 | 1162 ab |
| P value | <0.001 | <0.001 | 0.030 | <0.001 | 0.058 | 0.658 | 0.148 | 0.064 | <0.001 | <0.001 | 0.002 | 0.925 | 0.005 | 0.329 | <0.001 |
| Soil N rate | |||||||||||||||
| Zero | 4.4 | 76.3 a | 76.4 b | 57.1 a | 0.62 c | 14.2 b | 35.9 b | 95.6 a | 4.7 | 7.3 b | 68.6 b | 93.9 | 136 a | 113 a | 1113 b |
| Low | 4.4 | 75.9 b | 85.6 a | 53.7 b | 0.64 b | 15.7 a | 41.1 a | 85.2 b | 4.6 | 7.6 a | 69.0 b | 91.8 | 124 b | 111 b | 1123 b |
| Medium | 4.5 | 75.7 bc | 87.1 a | 53.6 b | 0.66 a | 16.2 a | 42.5 a | 81.0 b | 4.5 | 7.4 ab | 70.1 a | 93.0 | 121 b | 111 ab | 1129 b |
| High | 4.6 | 75.5 c | 86.7 a | 53.4 b | 0.65 a | 16.2 a | 42.7 a | 81.8 b | 4.4 | 7.4 ab | 69.9 a | 92.1 | 123 b | 111 b | 1178 a |
| p-value | 0.293 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.233 | 0.004 | <0.001 | 0.101 | <0.001 | 0.005 | 0.002 |
| End-Use Quality Traits 1 | GH 2 | FY | FA | FP | WG | GI | MPT | MPH | Water SRC | Na2CO3 SRC | Lactic Acid SRC | Sucrose SRC | LV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TW | −0.04 | 0.47 ** | −0.48 ** | −0.47 ** | −0.80 ** | 0.22 | −0.31 ** | −0.39 ** | −0.37 ** | −0.39 ** | −0.59 ** | −0.64 ** | −0.32 ** |
| GH | 0.09 | 0.29 ** | 0.24 ** | 0.08 | −0.36 ** | −0.25 ** | 0.29 ** | 0.11 | −0.13 | −0.10 | 0.00 | 0.20 * | |
| FY | −0.24 ** | −0.14 | −0.27 * | 0.21 | −0.11 | 0.02 | −0.36 ** | −0.6 1** | −0.14 | −0.55 ** | −0.02 | ||
| FA | 0.89 ** | 0.65 ** | −0.39 ** | 0.56 ** | 0.67 ** | 0.39 ** | 0.33 ** | 0.41 ** | 0.48 ** | 0.75 ** | |||
| FP | 0.88 ** | −0.24 | 0.68 ** | 0.80 ** | 0.43 ** | 0.30 * | 0.81 ** | 0.65 ** | 0.91 ** | ||||
| WG | −0.43 ** | 0.55 ** | 0.77 ** | 0.42 ** | 0.27 * | 0.72 ** | 0.65 ** | 0.71 ** | |||||
| GI | 0.26 * | −0.30 * | −0.17 | −0.22 | 0.03 | −0.21 | −0.11 | ||||||
| MPT | 0.44 ** | 0.10 | 0.02 | 0.65 ** | 0.33 ** | 0.62 ** | |||||||
| MPH | 0.32 ** | 0.09 | 0.56 ** | 0.46 ** | 0.79 ** | ||||||||
| Water SRC | 0.47 ** | 0.43 ** | 0.55 ** | 0.33 ** | |||||||||
| Na2CO3 SRC | 0.36 ** | 0.73 ** | 0.26 * | ||||||||||
| Lactic acid SRC | 0.73 ** | 0.72 ** | |||||||||||
| Sucrose SRC | 0.61 ** |
| End-Use Quality Traits 1 | GH 2 | FY | FA | FP | WG | GI | MPT | MPH | Water SRC | Na2CO3 SRC | Lactic Acid SRC | Sucrose SRC | LV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TW | −0.38 ** | 0.49 ** | 0.87 ** | −0.10 | 0.24 ** | 0.44 ** | −0.88 ** | 0.79 ** | 0.70 ** | 0.85 ** | −0.73 ** | 0.85 ** | −0.71 ** |
| GH | −0.66 ** | 0.59 ** | 0.79 ** | 0.75 ** | −0.85 ** | −0.03 | 0.14 | 0.65 ** | −0.41 ** | −0.71 ** | −0.58 ** | 0.08 | |
| FY | −0.34 ** | −0.61 ** | −0.59 ** | 0.60 ** | −0.34 ** | 0.26 * | −0.43 ** | 0.59 ** | 0.55 ** | 0.48 ** | −0.06 | ||
| FA | 0.14 | 0.41 ** | 0.24 ** | −0.81 ** | 0.70 ** | 0.88 ** | 0.93 ** | −0.93 ** | 0.89 ** | −0.86 ** | |||
| FP | 0.73 ** | −0.63 ** | −0.10 | 0.13 | 0.22 * | 0.00 | −0.25 ** | −0.04 | 0.05 | ||||
| WG | −0.53 ** | −0.41 ** | 0.46 ** | 0.48 ** | 0.29 ** | −0.46 ** | 0.31 ** | −0.22 * | |||||
| GI | −0.26 ** | 0.25 ** | 0.09 | 0.32 ** | −0.02 | 0.40 ** | −0.18 | ||||||
| MPT | −0.88 ** | −0.69 ** | −0.77 ** | 0.68 ** | −0.82 ** | 0.62 ** | |||||||
| MPH | 0.60 ** | 0.65 ** | −0.58 ** | 0.74 ** | −0.46 ** | ||||||||
| Water SRC | 0.82 ** | −0.88 ** | 0.72 ** | −0.78 ** | |||||||||
| Na2CO3 SRC | −0.86 ** | 0.86 ** | −0.83 ** | ||||||||||
| Lactic acid SRC | −0.72 ** | 0.87 ** | |||||||||||
| Sucrose SRC | −0.75 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Liang, X.; Torrion, J.A.; Walsh, O.S.; O’Brien, K.; Liu, Q. The Influence of Water and Nitrogen Availability on the Expression of End-Use Quality Parameters of Spring Wheat. Agronomy 2018, 8, 257. https://doi.org/10.3390/agronomy8110257
Yang R, Liang X, Torrion JA, Walsh OS, O’Brien K, Liu Q. The Influence of Water and Nitrogen Availability on the Expression of End-Use Quality Parameters of Spring Wheat. Agronomy. 2018; 8(11):257. https://doi.org/10.3390/agronomy8110257
Chicago/Turabian StyleYang, Rui, Xi Liang, Jessica A. Torrion, Olga S. Walsh, Katherine O’Brien, and Qian Liu. 2018. "The Influence of Water and Nitrogen Availability on the Expression of End-Use Quality Parameters of Spring Wheat" Agronomy 8, no. 11: 257. https://doi.org/10.3390/agronomy8110257
APA StyleYang, R., Liang, X., Torrion, J. A., Walsh, O. S., O’Brien, K., & Liu, Q. (2018). The Influence of Water and Nitrogen Availability on the Expression of End-Use Quality Parameters of Spring Wheat. Agronomy, 8(11), 257. https://doi.org/10.3390/agronomy8110257







