Non-Structural Carbohydrate Metabolism, Growth, and Productivity of Maize by Increasing Plant Density
Abstract
1. Introduction
2. Materials and Methods
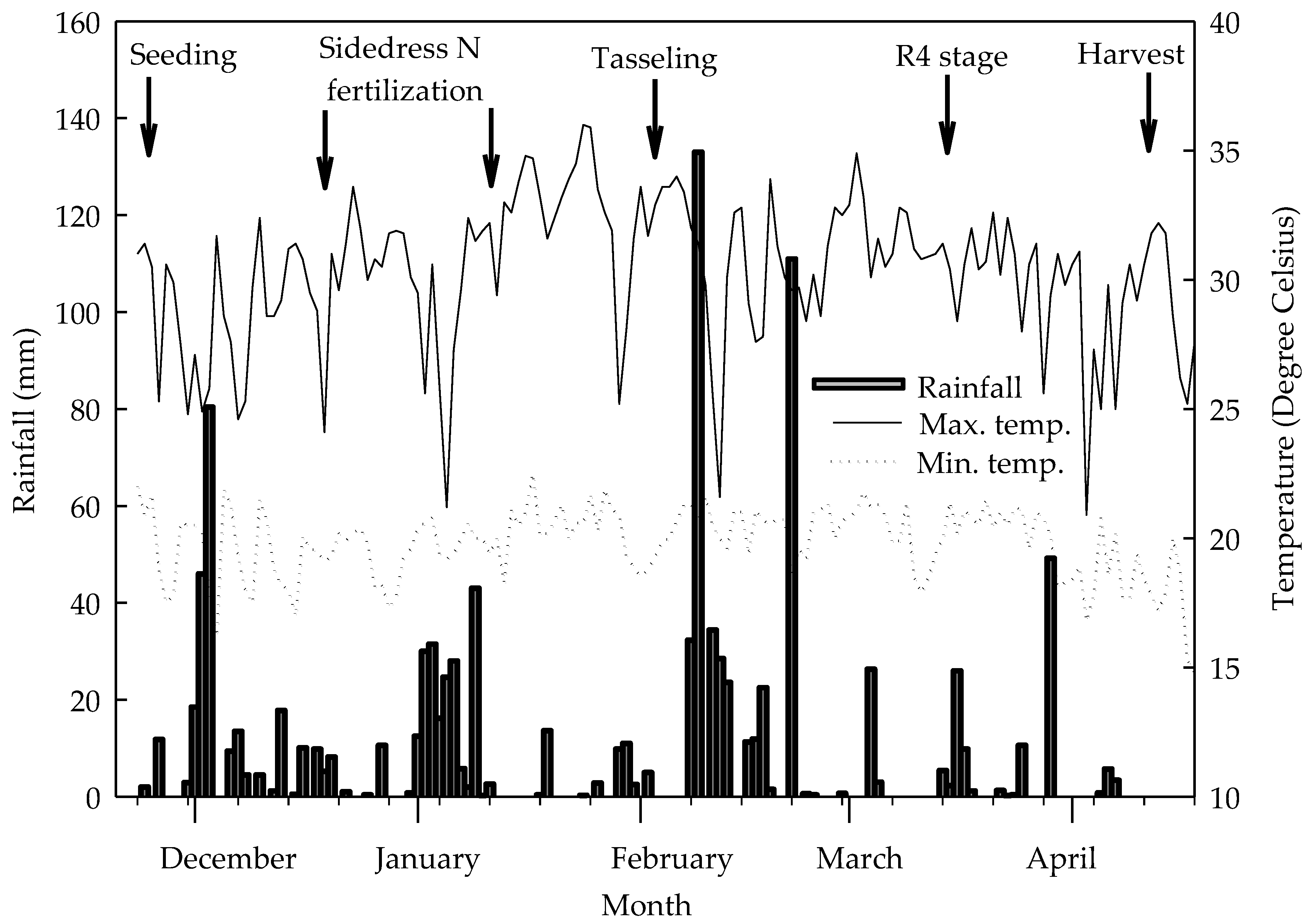
2.1. Characterization of Experimental Conditions, Treatments, and Assay Design
2.2. Biometric Determinations in Plants Evaluated at the R4 Stage
2.3. Non-Structural Carbohydrate Determinations in Plants at the R4 Stage
2.4. Enzymatic Activities Determination in Plants at the R4 Stage
2.5. Agronomic Determinations in Plants Evaluated at Final Harest Time
2.6. Statistical Analysis
3. Results
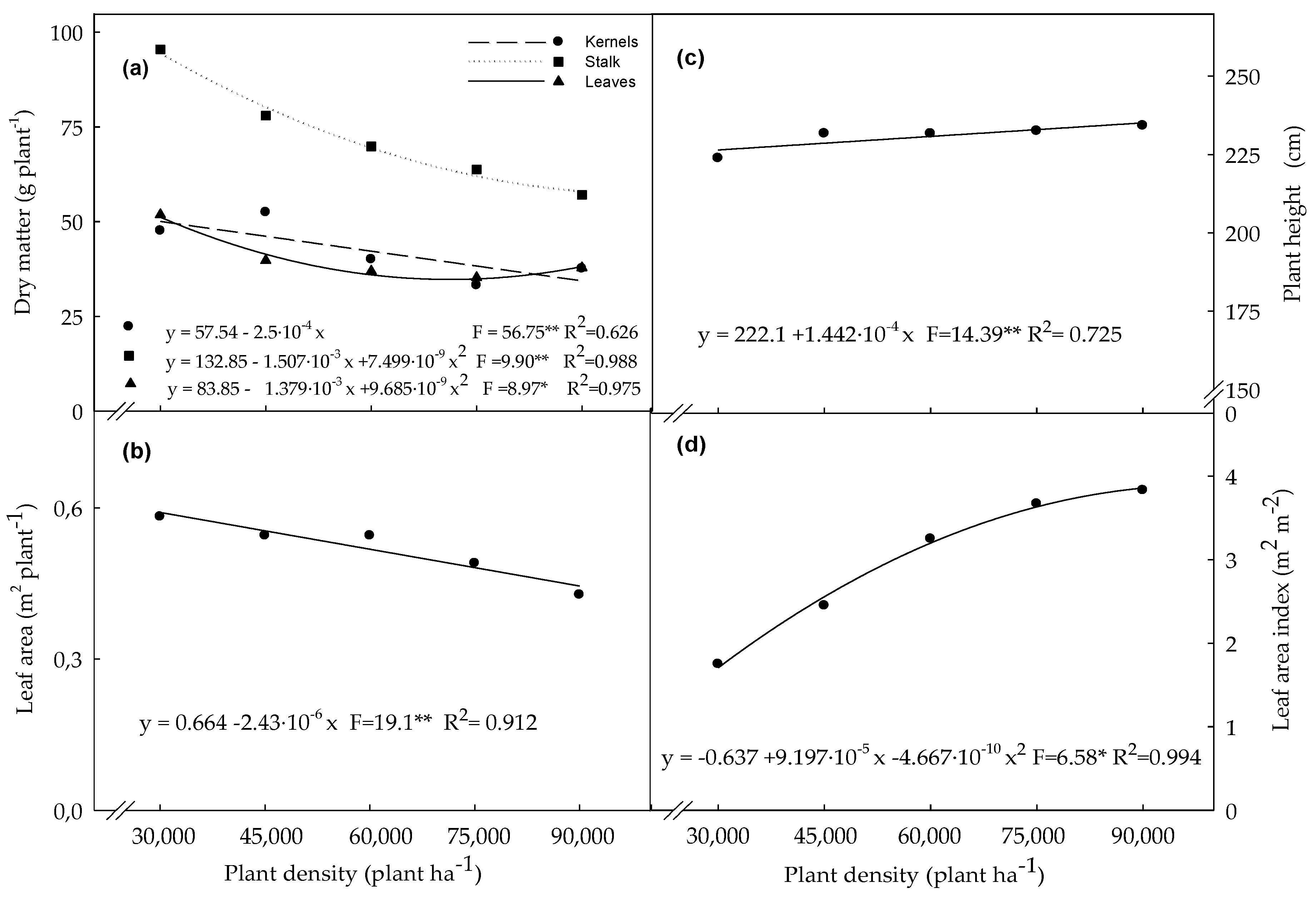
3.1. Biometric Variables Determined in Plants Evaluated at the R4 Stage
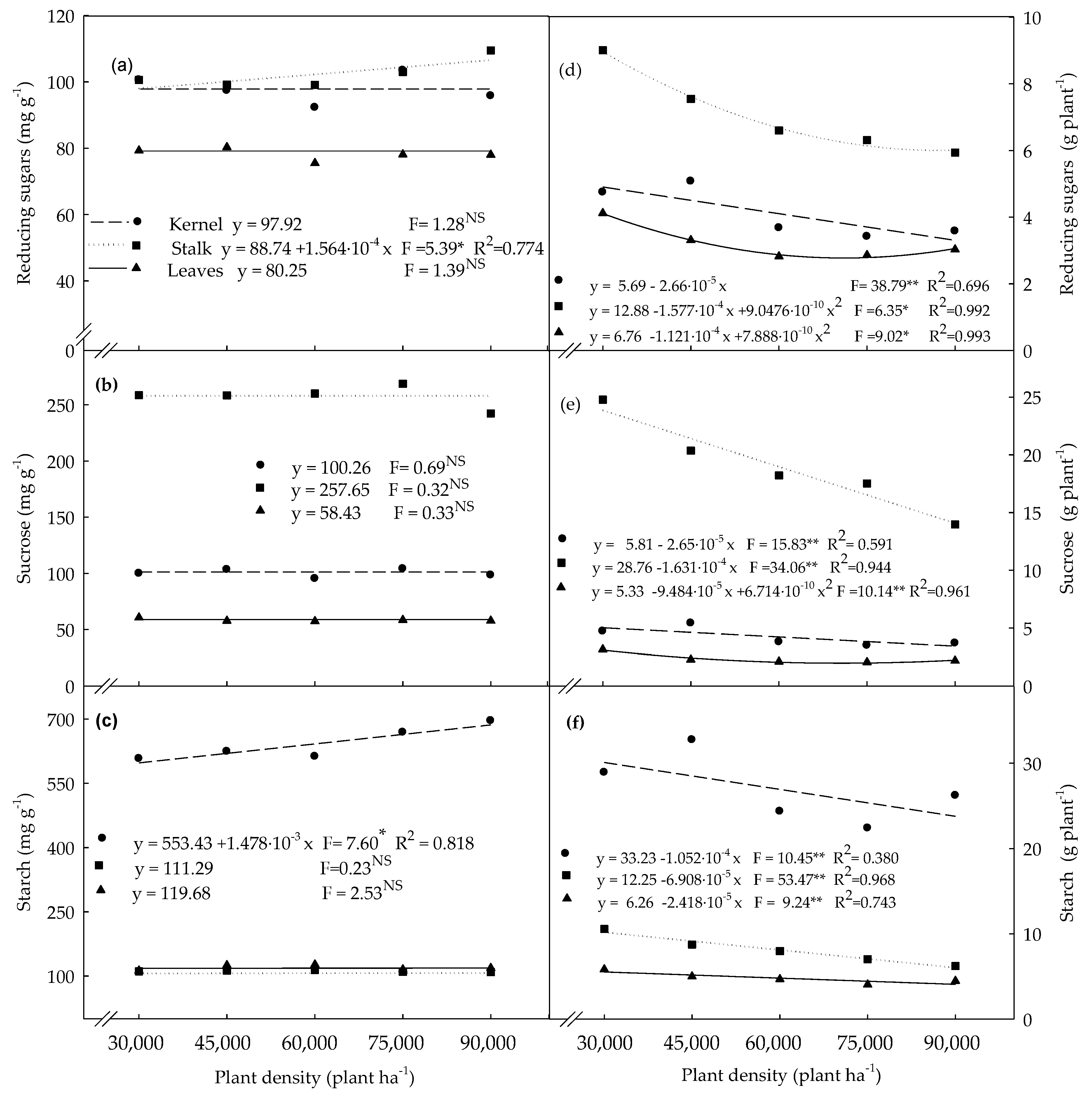
3.2. Carbohydrates Determined in Plants Evaluated at the R4 Stage
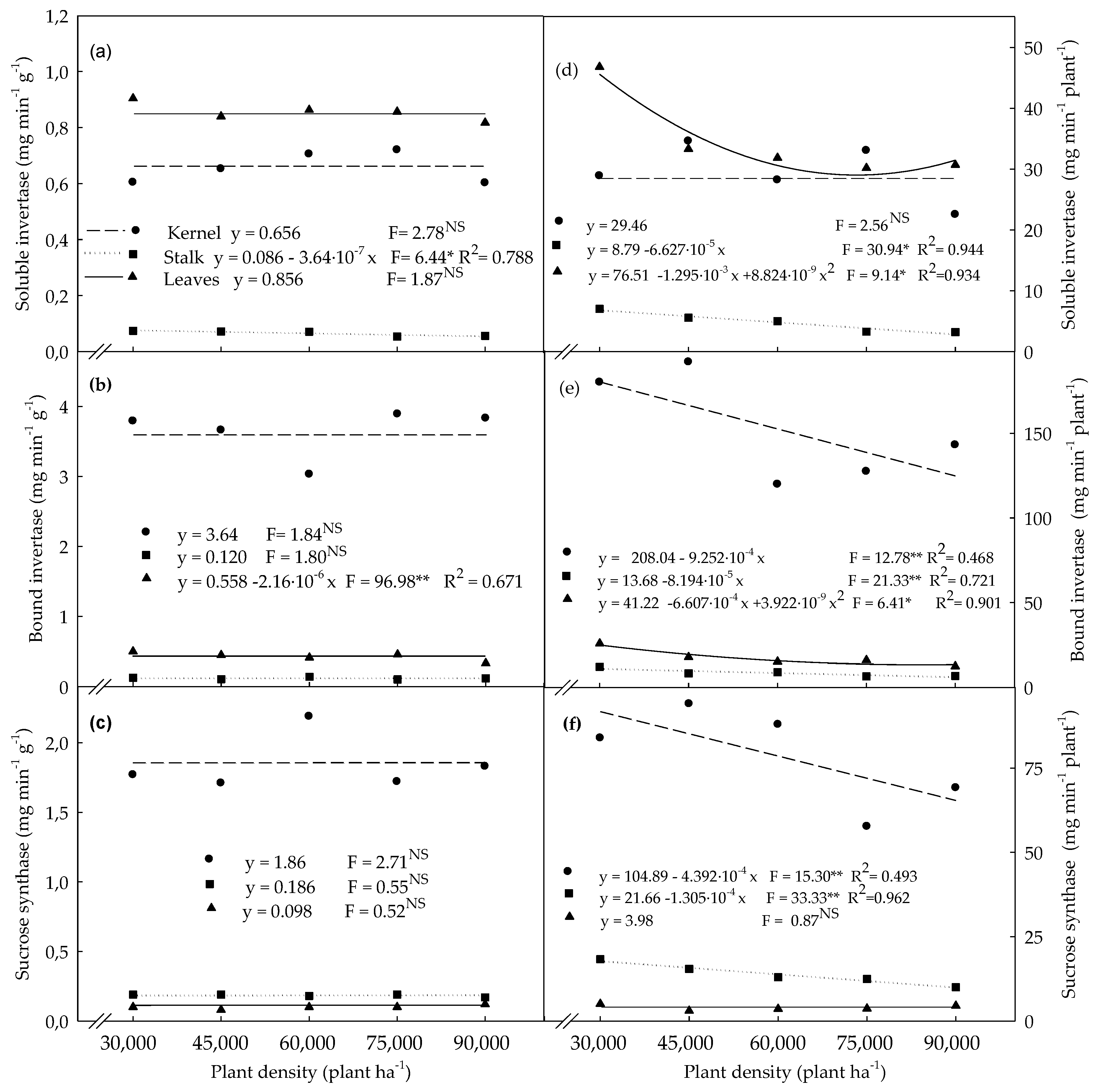
3.3. Enzymatic Activities Determined in Plants Evaluated at the R4 Stage
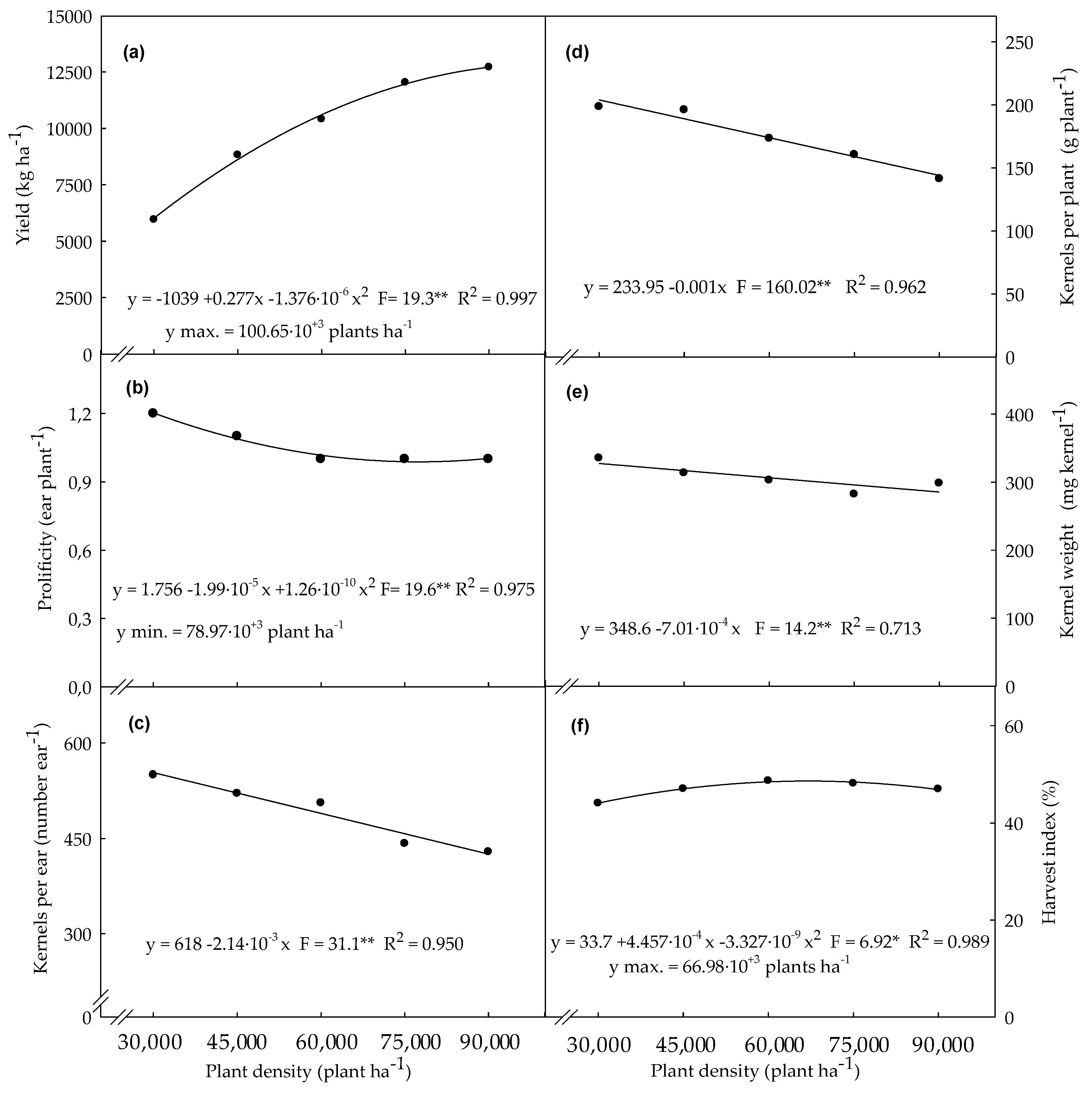
3.4. Agronomic Variables Determined in Plants Evaluated at Final Harest Time
4. Discussion
4.1. Biometric Variables Determined in Plants Evaluated at the R4 Stage
4.2. Non-Structural Carbohydrates Determined in Plants Evaluated at the R4 Stage
4.3. Enzymatic Activities Determined in Plants Evaluated at the R4 Stage
4.4. Agronomic Variables Determined in Plants Evaluated at Final Harvest Time
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gregory, P.J.; George, T.S. Feeding nine bilion: The challenge to sustainable production. J. Exp. Bot. 2011, 62, 5223–5239. [Google Scholar] [CrossRef] [PubMed]
- Slewinski, T.L. Non-structural carbohydrates partitioning in grass stems: A target to increase yield stability, stress tolerance, and biofuel production. J. Exp. Bot. 2012, 63, 4647–4670. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAOSTAT-Agriculture Database. 2018. Available online: http://faostat.fao.org/site/ (accessed on 1 August 2018).
- White, E.G.; Moose, E.P.; Weil, C.F.; McCann, M.C.; Carpita, N.C.; Below, F.E. Tropical maize: Exploiting maize genetic diversity to develop a novel annual crop for lignocellulosic biomass and sugar production. In Routs to Cellulosic Ethanol; Buckeridge, M.S., Goldman, G.G., Eds.; Springer: New York, NY, USA, 2011; pp. 167–179. [Google Scholar]
- Lobell, D.B.; Cassman, K.G.; Field, C.B. Crop yield gaps: Their importance, magnitudes, and causes. Annu. Rev. Environ. Resour. 2009, 34, 179–204. [Google Scholar] [CrossRef]
- Sangoi, L. Understanding plant density effects on maize growth and development: An important issue to maximize grain yield. Ciênc. Rural 2000, 31, 159–168. [Google Scholar] [CrossRef]
- Bisht, A.S.; Bhatnagar, A.; Sing, V. Influence of plant density and integrated nutrient management on N, P, and K contents and uptake of quality protein maize. Madras Agric. J. 2013, 100, 110–112. [Google Scholar]
- Song, Y.; Rui, Y.; Bedane, G.; Li, J. Morphological characteristics of maize canopy development as affected by increased plant density. PLoS ONE 2016, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ciampitti, I.A.; Vyn, T.J. A comprehensive study of plant density consequences on nitrogen uptake dynamics of maize plants from vegetative to reproductive stages. Field Crops Res. 2011, 121, 2–18. [Google Scholar] [CrossRef]
- Tokatlidis, I.S.; Hasb, V.; Melidisc, V.; Hasb, I.; Mylonasa, I.; Evgenidisc, G.; Copandeanb, A.; Ninouc, E.; Fasoulad, V.A. Maize hybrids less dependent on high plant densities improve resource-use efficiency in rainfed and irrigated conditions. Field Crops Res. 2011, 120, 345–351. [Google Scholar] [CrossRef]
- Testa, G.; Reyneri, A.; Blandino, M. Maize grain yield enhancement through high plant density cultivation with different inter-row and intra-row spacings. Eur. J. Agron. 2016, 72, 28–37. [Google Scholar] [CrossRef]
- Yan, P.; Zhang, Q.; Shuai, X.F.; Pan, J.X.; Zhang, W.J.; Shi, J.F.; Wang, M.; Chen, X.P.; Cui, Z.L. Interaction between plant density and nitrogen management strategy in improving maize grain yield and nitrogen use efficiency on the North China Plain. J. Agric. Sci. 2016, 154, 978–988. [Google Scholar] [CrossRef]
- Xue, J.; Zhao, Y.; Gou, L.; Shi, Z.; Yao, M.; Zhang, W. How High Plant Density of Maize Affects Basal Internode Development and Strength Formation. Crop Sci. 2016, 56, 3295–3306. [Google Scholar] [CrossRef]
- Huang, S.; Gaoa, Y.; Lia, Y.; Xub, L.; Taoa, H.; Wanga, P. Influence of plant architecture on maize physiology and yield in the Heilonggang River valley. Crop J. 2017, 5, 52–62. [Google Scholar] [CrossRef]
- Al-Naggar, A.M.M.; Shabana, R.A.; Atta, M.M.M.; Al-Khalil, T.H. Maize response to elevated plant density combined with lowered N-fertilizer rate is genotype-dependent. Crop J. 2015, 3, 96–109. [Google Scholar] [CrossRef]
- Sher, A.; Khan, A.; Cai, L.J.; Ahmad, M.I.; Asharf, U.; Jamoro, S.A. Response of maize grown under high plant density; performance, issues and management—A critical review. Adv. Crop Sci. Tech. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Xu, Z.; Lai, T.; Li, S.; Si, D.; Zhang, C.; Cui, Z.; Chen, X. Promoting potassium allocation to stalk enhances stalk bending resistance of maize (Zea mays L.). Field Crops Res. 2018, 213, 200–206. [Google Scholar] [CrossRef]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world. Past successes and future challenges to the role played by maize in global food security. Food Sec. 2011, 3, 307. [Google Scholar] [CrossRef]
- Cazetta, J.O.; Seebauer, J.R.; Below, F.E. Sucrose and nitrogen supplies regulate growth of maize kernels. Ann. Bot. 1999, 84, 747–754. [Google Scholar] [CrossRef]
- Li, B.; Liu, H.; Zhang, Y.; Kang, T.; Zhang, L.; Tong, J.; Xiao, L.; Zhang, H. Constitutive expression of cell wall invertase genes increases grain yield and starch content in maize. Plant Biotechnol. J. 2013, 11, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chi, Y.; Wang, J.; Zhou, J.; Cheng, Y.; Zhang, B.; Ma, A.; Vanitha, J.; Ramachandran, R. Sucrose metabolism gene families and their biological functions. Nature 2015, 17583. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Duan, X.; Wu, Y.; Cheng, J.; Zhang, J.; Zhang, H.; Li, B. Genetic Engineering of Maize (Zea mays L.) with Improved Grain Nutrients. J. Agric. Food Chem. 2018, 66, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Wang, Z.; Ma, B.; Zhang, C.; Zhu, L.; Ma, F.; Li, M. Structure and expression analysis of the sucrose synthase gene family in apple. J. Integr. Agric. 2018, 17, 847–856. [Google Scholar] [CrossRef]
- Van Raij, B.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, Brasil, 2001; 285p, ISBN 85-85564-05-9. [Google Scholar]
- Van Raij, B.; Cantarella, H. Cereais: Milho para grão e silagem. In Recomendações de Calagem e Adubação Para o Estado de São Paulo; van Raij, B., Cantarella, H., Quaggio, J.A., Furlani, A.M.C., Eds.; Instituto Agronômico de Campinas: Campinas, Brasil, 1997; pp. 56–59. [Google Scholar]
- Faleiros, R.R.S.; Seebauer, J.R.; Below, F.E. Nutritionally induced changes in endosperm of shrunken-1 and brittle-2 maize kernels growth in vitro. Crop Sci. 1996, 36, 947–954. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalisylic acid reagent for determination of reducing sugars. Ann. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Fieuw, S.; Willenbrink, J. Sucrose synthase and sucrose phosphate synthase in sugar beet plants (Beta vulgaris L. ssp. altissima). J. Plant Physiol. 1987, 131, 153–162. [Google Scholar] [CrossRef]
- Hendrix, D.L. Rapid extraction and analysis of nonstructural carbohydrates in plant tissues. Crop Sci. 1993, 33, 1306–1311. [Google Scholar] [CrossRef]
- Brow, C.S.; Huber, S.C. Reserve mobilization and starch formation in soybean (Glycine max) cotyledon in relation to seedling growth. Plant Physiol. 1988, 72, 518–524. [Google Scholar] [CrossRef]
- Singletary, G.W.; Doehlert, D.C.; Wilson, C.M.; Muhtich, M.J.; Below, F.E. Response of enzymes and storage proteins of maize endosperm to nitrogen supply. Plant Physiol. 1990, 94, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Doehlert, D.C.; Felker, F.C. Characterization and distribution of invertase activity in developing maize (Zea mays) kernels. Physiol. Plant. 1987, 70, 51–57. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Maldonado, W., Jr. Experimentação Agronômica & AgroEstat: Sistema Para Análise Estatística de Ensaios Agronômicos; Gráfica Multipress: Jaboticabal, Brazil, 2015; ISBN 978-85-68020-01-02. [Google Scholar]
- Balkcom, K.S.; Satterwhiteb, J.L.; Arriagaa, F.J.; Pricea, J.A.; Santenb, E.V. Conventional and glyphosate-resistant maize yields across plant densities in single- and twin-row configurations. Field Crops Res. 2011, 120, 330–337. [Google Scholar] [CrossRef]
- Schlüter, U.; Mascher, M.; Colmsee, C.; Scholz, U.; Bräutigam, A.; Fahnenstich, H.; Sonnewald, U. Maize source leaf adaptation to nitrogen deficiency affects not only nitrogen and carbon metabolism but also control of phosphate homeostasis. Plant Physiol. 2012, 160, 1384–1406. [Google Scholar] [CrossRef] [PubMed]
- Rajcan, I.; Tollenaar, M. Source: Sink ratio and leaf senescence in maize: II. Nitrogen metabolism during grain filling. Field Crops Res. 1999, 60, 255–265. [Google Scholar] [CrossRef]
- Tollenaar, M.; Aguilera, A.; Nissanka, S.P. Grain yield is reduced more by weed interference in an old than in a new maize hybrid. Agron. J. 1997, 89, 239–246. [Google Scholar] [CrossRef]
- Unkovich, M.; Baldock, J.; Forbes, M. Variability in Harvest Index of Grain Crops and Potential Significance for Carbon Accounting: Examples from Australian Agriculture. Adv. Agron. 2010, 105, 173–219. [Google Scholar] [CrossRef]
- Demetrio, C.S.; Fornasieri Filho, D.; Cazetta, J.O.; Cazetta, D.A. Desempenho de híbridos de milho submetidos a diferentes espaçamentos e densidades populacionais. Pesqui. Agropecu. Bras. 2008, 43, 1691–1697. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazetta, J.O.; Revoredo, M.D. Non-Structural Carbohydrate Metabolism, Growth, and Productivity of Maize by Increasing Plant Density. Agronomy 2018, 8, 243. https://doi.org/10.3390/agronomy8110243
Cazetta JO, Revoredo MD. Non-Structural Carbohydrate Metabolism, Growth, and Productivity of Maize by Increasing Plant Density. Agronomy. 2018; 8(11):243. https://doi.org/10.3390/agronomy8110243
Chicago/Turabian StyleCazetta, Jairo O., and Marcos D. Revoredo. 2018. "Non-Structural Carbohydrate Metabolism, Growth, and Productivity of Maize by Increasing Plant Density" Agronomy 8, no. 11: 243. https://doi.org/10.3390/agronomy8110243
APA StyleCazetta, J. O., & Revoredo, M. D. (2018). Non-Structural Carbohydrate Metabolism, Growth, and Productivity of Maize by Increasing Plant Density. Agronomy, 8(11), 243. https://doi.org/10.3390/agronomy8110243




