Zinc Oxide Nanoparticles Boosts Phenolic Compounds and Antioxidant Activity of Capsicum annuum L. during Germination
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Characterization of ZnO-NPs
2.3. Treatments
2.4. Seed Germination and Seedling Growth
2.5. Extraction of Free Phenolic Compounds
2.6. Total Phenols Content
2.7. Total Flavonoid Content
2.8. Condensed Tannins Content
2.9. Determination of DPPH Antioxidant Capacity
2.10. Statistical Analysis
3. Results
3.1. Characterization of ZnO-NPs
3.2. Effect of ZnO-NPs on Germination and Vigor
3.3. Effect of ZnO-NPs on Seedling Growth
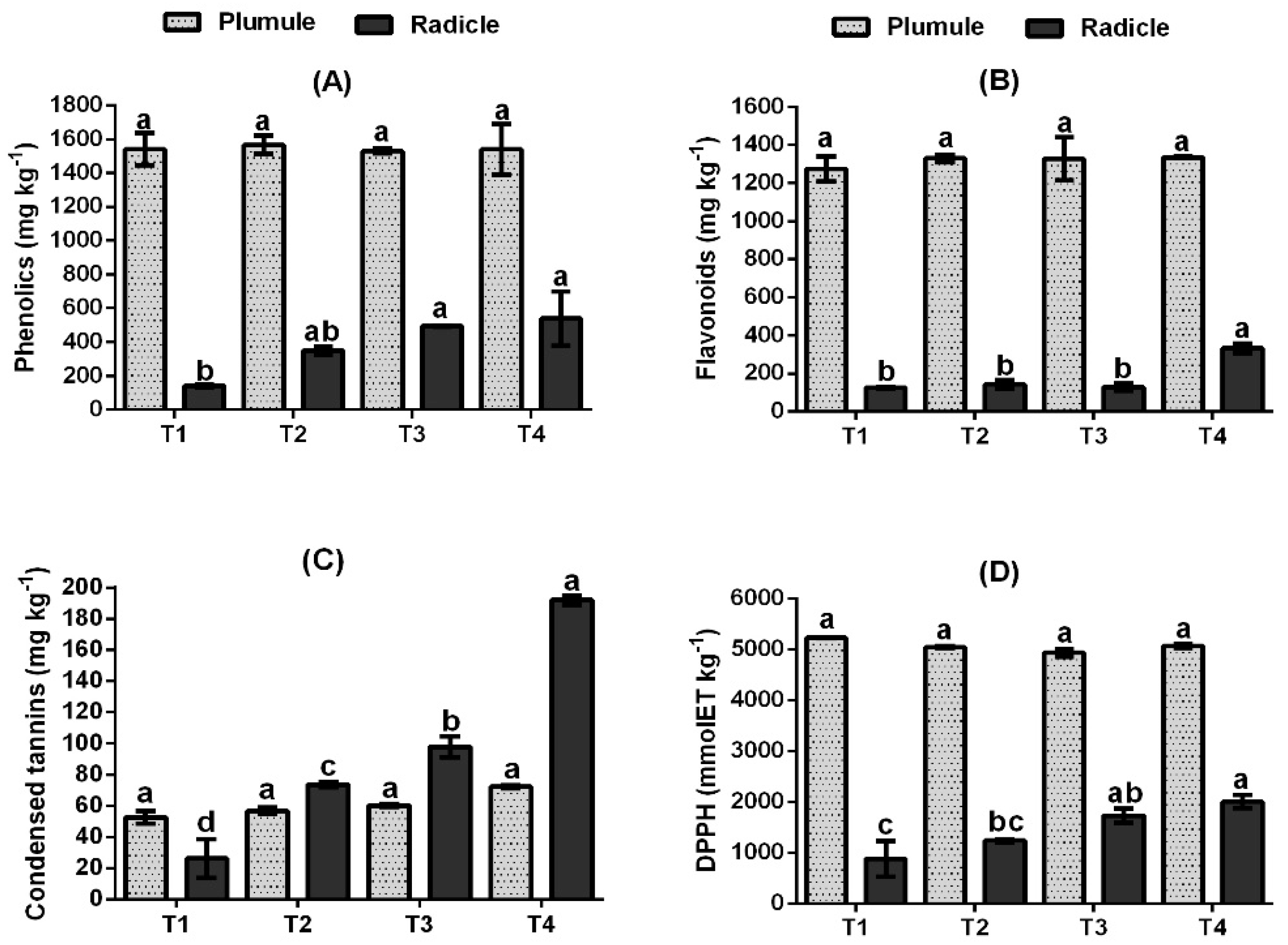
3.4. Effect of ZnO-NPs on Phenolic Compounds Content and DPPH Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hajra, A.; Mondal, N.K. Effects of ZnO and TiO2 nanoparticles on germination, biochemical and morphoanatomical attributes of Cicer arietinum L. Energy Ecol. Environ. 2017, 2, 277–288. [Google Scholar] [CrossRef]
- Anusuya, S.; Banu, K.N. Silver-chitosan nanoparticles induced biochemical variations of chickpea (Cicer arietinum L.). Biocatal. Agric. Biotechnol. 2016, 8, 39–44. [Google Scholar] [CrossRef]
- Lira-Saldívar, R.H.; Méndez-Argüello, B.; Vera Reyes, I.; de los Santos-Villarreal, G. Agronanotecnología: Una nueva herramienta para la agricultura moderna. [Agronanotechnology: A new tool for modern agriculture]. Rev. Fac. Cienc. Agrar. 2018, in press. [Google Scholar]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Kumar, S.V.; Rajesh kumar, S. A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour. Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Narendhran, S.; Rajiv, P.; Sivaraj, R. Toxicity of ZnO nanoparticles on germinating Sesamum indicum (Co-1) and their antibacterial activity. Bull. Mater. Sci. 2016, 39, 415–421. [Google Scholar] [CrossRef]
- Raskar, S.V.; Laware, S.L. Effect of zinc oxide nanoparticles on cytology and seed germination in onion. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 467–473. [Google Scholar]
- Singh, A.; Singh, N.B.; Hussain, I.; Singh, H.; Yadav, V.; Singh, S.C. Green synthesis of nano zinc oxide and evaluation of its impact on germination and metabolic activity of Solanum lycopersicum. J. Biotechnol. 2016, 233, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Alli, A.; Ali, J.S.; Haq, I.U.; Zia, M. Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: Growth dynamics and antioxidative response. Front. Plant Sci. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Burman, U.; Saini, M.; Kumar, P. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, J.; Dou, R.; Gao, X.; Mao, C.; Wang, L. Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants, maize (Zea mays L.) and rice (Oryza sativa L.). Int. J. Environ. Res. Public Health 2015, 12, 15100–15109. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Zhao, H.M.; Li, Y.W.; Huang, X.P.; Wu, X.L.; Zhai, T.; Yuan, Y.; Cai, Q.Y.; Mo, C.H. Effects of the size and morphology of zinc oxide nanoparticles on the germination of Chinese cabbage seeds. Environ. Sci. Pollut. Res. 2015, 22, 10452–10462. [Google Scholar] [CrossRef] [PubMed]
- Večeřová, K.; Večeř, Z.; Dočekal, B.; Oravec, M.; Pompeiano, A.; Tríska, J.; Urban, O. Changes of primary and secondary metabolites in barley plants exposed to CdO nanoparticles. Environ. Pollut. 2016, 218, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Franklin, G.; Conceição, L.F.; Kombrink, E.; Dias, A.C. Xanthone biosynthesis in Hypericum perforatum cells provides antioxidant and antimicrobial protection upon biotic stress. Phytochemistry 2009, 70, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Bansal, S.; Jangir, L.K.; Awasthi, G.; Awasthi, K.K.; Awasthi, K. Effect of ZnO nanoparticles on germination of Triticum aestivum seeds. Macromol. Symp. 2017, 376, 1700043. [Google Scholar] [CrossRef]
- Sheteiwy, M.; Fu, Y.; Hu, Q.; Nawaz, A.; Guan, Y.; Li, Z.; Huang, Y.; Hu, J. Seed priming with polyethylene glycol induces antioxidative defense and metabolic regulation of rice under nano-ZnO stress. Environ. Sci. Pollut. Res. Int. 2016, 19, 19989–20002. [Google Scholar] [CrossRef] [PubMed]
- Salah, S.M.; Guan, Y.J.; Cao, D.D.; Li, J.; Nawaz, A.; Hu, Q.J.; Hu, W.M.; Ning, M.Y.; Hu, J. Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Sci. Rep. 2015, 30, 14278. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.L.; Adisa, I.O.; Rawat, S.; Kalagara, S.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. ZnO nanoparticles increase photosynthetic pigments and decrease lipid peroxidation in soil grown cilantro (Coriandrum sativum). Plant Physiol. Biochem. 2018, 132, 120–127. [Google Scholar] [CrossRef] [PubMed]
- International Seed Testing Association (ISTA). International Rules for Seed Testing; International Seed Testing Association: Zurich, Switzerland, 2004; p. 243. [Google Scholar]
- Corral-Diaz, B.; Peralta-Videa, J.R.; Alvarez-Parrilla, E.; Rodrigo-García, J.; Morales, M.I.; Osuna-Avila, P.; Niu, G.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Cerium oxide nanoparticles alter the antioxidant capacity but do not impact tuber ionome in Raphanus sativus (L.). Plant Physiol. Biochem. 2014, 84, 277–285. [Google Scholar] [CrossRef] [PubMed]
- López-Contreras, J.J.; Zavala-García, F.; Urías-Orona, V.; Martínez-Ávila, G.C.G.; Rojas, R.; Nino-Medina, G. Chromatic, phenolic and antioxidant properties of Sorghum bicolor genotypes. Not. Bot. Horti Agrobot. 2015, 43, 366–370. [Google Scholar]
- Van-Dongen, J.T.; Ammerlaan, A.M.H.; Wouterlood, M.; Van Aelst, A.C.; Borstlap, A.C. Structure of the developing pea seed coat and the post-phloem transport pathway of nutrients. Ann. Bot. 2003, 91, 729–737. [Google Scholar] [CrossRef] [PubMed]
- El-Kereti, M.A.; El-Feky, S.A.; Khater, M.S.; Osman, Y.A.; El-sherbini, E.S.A. ZnO nanofertilizer and He Ne Laser irradiation for promoting growth and yield of sweet basil plant. Recent Pat. Food Nutr. Agric. 2013, 5, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Gokak, I.B.; Taranath, T.C. Seed germination and growth responses of Macrotyloma uniflorum (Lam.) Verdc. exposed to zinc and zinc nanoparticles. Int. J. Environ. Sci. 2015, 5, 840–847. [Google Scholar]
- Rameshraddy, G.J.; Pavithra, G.J.; Mahesh, S.; Geetha, K.N.; Shankar, A.G. Seed priming and foliar spray with nano zinc improves stress adaptability and seed zinc content without compromising seed yield in ragi (Finger millet). Int. J. Pure Appl. Biosci. 2017, 5, 251–258. [Google Scholar]
- Afrayeem, S.M.; Chaurasia, A.K. Effect of zinc oxide nanoparticles on seed germination and seed vigour in chilli (Capsicum annuum L.). Int. J. Pharmacol. Phytochem. 2017, 6, 1564–1566. [Google Scholar]
- Raigond, B.; Raigond, B.; Kaundal, B.; Singh, B.; Joshi, A.; Dutt, S. Effect of zinc nanoparticles on antioxidative system of potato plants. J. Environ. Biol. 2017, 38, 435–439. [Google Scholar] [CrossRef]
- Comotto, M.; Casazza, A.A.; Aliakbarian, B.; Caratto, V.; Ferretti, M.; Perego, P. Influence of TiO2 nanoparticles on growth and phenolic compounds production in photosynthetic microorganisms. Sci. World J. 2014, 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. 2006, 15, 523–530. [Google Scholar]
- Hatami, M.; Ghorbanpour, M.; Salehiarjomand, H. Nano-anatase TiO2 modulates the germination behavior and seedling vigority of some commercially important medicinal and aromatic plants. J. Biol. Environ. Sci. 2014, 8, 53–59. [Google Scholar]
- Ali, S.; Shahbaz, M.; Shahzad, A.N.; Khan, H.A.; Anees, M.; Haider, M.S.; Fatima, A. Impact of copper toxicity on stone-head cabbage (Brassica oleracea var. capitata) in hydroponics. PeerJ 2015, 3, e1119. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Kuang, L.; He, X.; Bai, W.; Ding, Y.; Zhang, Z.; Zhao, Y.; Chai, Z. Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere 2010, 78, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.; Kim, S.; Lee, I. Assessment of phytotoxicity of ZnO NPs on a medicinal plant, Fagopyrum esculentum. Environ. Sci. Pollut. Res. 2013, 20, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Mahendra, S.; Zodrow, K.; Li, D.; Tsai, Y.C.; Braam, J.; Alvarez, P.J. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ. Toxicol. Chem. 2010, 29, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, H.; Tu, C.; Hu, X.; Li, L.; Luo, Y.; Christie, P. Phytotoxicity of ZnO nanoparticles and the released Zn (II) ion to corn (Zea mays L.) and cucumber (Cucumis sativus L.) during germination. Environ. Sci. Pollut. Res. 2015, 22, 11109–11117. [Google Scholar] [CrossRef] [PubMed]
- El-Temsah, Y.S.; Joner, E.J. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 2012, 27, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lombi, E.; Zhao, F.J.; Kopittke, P.M. Nanotechnology: A new opportunity in plant sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global plant stress signaling: Reactive oxygen species at the cross-road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Ebbs, S.D.; Bradfield, S.J.; Kumar, P.; White, J.C.; Musante, C.; Ma, X. Accumulation of zinc, copper, or cerium in carrot (Daucus carota) exposed to metal oxide nanoparticles and metal ions. Environ. Sci. Nano 2016, 3, 114–126. [Google Scholar] [CrossRef]
- Wang, B.; Feng, W.Y.; Wang, T.C.; Jia, G.; Wang, M.; Shi, J.W.; Zhang, F.; Zhao, Y.L.; Chai, Z.F. Acute toxicity of nano- and micro-scale zinc powder in healthy adult mice. Toxicol. Lett. 2006, 161, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Van Breusegem, F.; Dat, J.F. Reactive oxygen species in plant cell death. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwari, A.; Suresh, S.; Chandrasekaran, N.; Mukherjee, A. Toxicity evaluation of gold nanoparticles using an Allium cepa bioassay. RSC Adv. 2016, 6, 24000–24009. [Google Scholar] [CrossRef]
- Sosan, A.; Svistunenko, D.; Straltsova, D.; Tsiurkina, K.; Smolich, I.; Lawson, T.; Subramaniam, S.; Golovko, V.; Anderson, D.; Sokolik, A.; et al. Engineered silver nanoparticles are sensed at the plasma membrane and dramatically modify the physiology of Arabidopsis thaliana plants. Plant J. 2016, 8, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, S.; Zhu, H.; Colvin, V.L.; Alvarez, P.J. Quantum dot weathering results in microbial toxicity. Environ. Sci. Technol. 2008, 42, 9424–9430. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Bhumi, G.; Ratna, N.; Savithramma, N. Screening of zinc oxide nanoparticles for cell proliferation synthesized. Int. J. Drug Dev. Res. 2014, 6, 0975–9344. [Google Scholar]
- Doroteo, V.H.; Díaz, C.; Terry, C.; Rojas, R. Phenolic compounds and antioxidant activity in vitro of 6 Peruvian plants. Rev. Soc. Quím. Perú 2013, 79, 13–20. [Google Scholar]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Oniki, T. Flavonoid and some other phenolics as substrates of peroxidase: Physiological significance of the redox reactions. J. Plant Res. 2000, 113, 301–309. [Google Scholar] [CrossRef]
- Parry, A.D.; Tiller, S.A.; Edwards, R. The Effects of heavy metals and root immersion on isoflavonoid metabolism in alfalfa (Medicago sativa L.). Plant Physiol. 1994, 106, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.F.; Klucas, R.V.; Grayer, R.J.; Abian, J.; Becana, M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: Prooxidant and antioxidant properties. Free Radic. Biol. Med. 1997, 22, 861–870. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatt, D.; Zaidi, M.G.; Saradhi, P.P.; Khanna, P.K.; Arora, S. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 2012, 167, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Marichali, A.; Dallali, S.; Ouerghemmi, S.; Sebei, H.; Hosni, K. Germination, morpho-physiological and biochemical responses of coriander (Coriandrum sativum L.) to zinc excess. Ind. Crop. Prod. 2014, 55, 248–257. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [PubMed]
- GhiassiTarzi, B.; Gharachorloo, M.; Baharinia, M.; Mortazavi, S.A. The effect of germination on phenolic content and antioxidant activity of chickpea. Iran J. Pharm. Res. 2012, 11, 1137–1143. [Google Scholar]
- Javed, R.; Mohamed, A.; Yücesan, B.; Gürel, E.; Kausar, R.; Zia, M. CuO nanoparticles significantly influence in vitro culture, steviol glycosides, and antioxidant activities of Stevia rebaudiana Bertoni. Plant Cell Tissue Organ Cult. 2017, 131, 611–620. [Google Scholar] [CrossRef]
- Ghorbanpour, M. Major essential oil constituents, total phenolics and flavonoids content and antioxidant activity of Salvia officinalis plant in response to nano-titanium dioxide. Ind. J. Plant Physiol. 2015, 20, 249–256. [Google Scholar] [CrossRef]
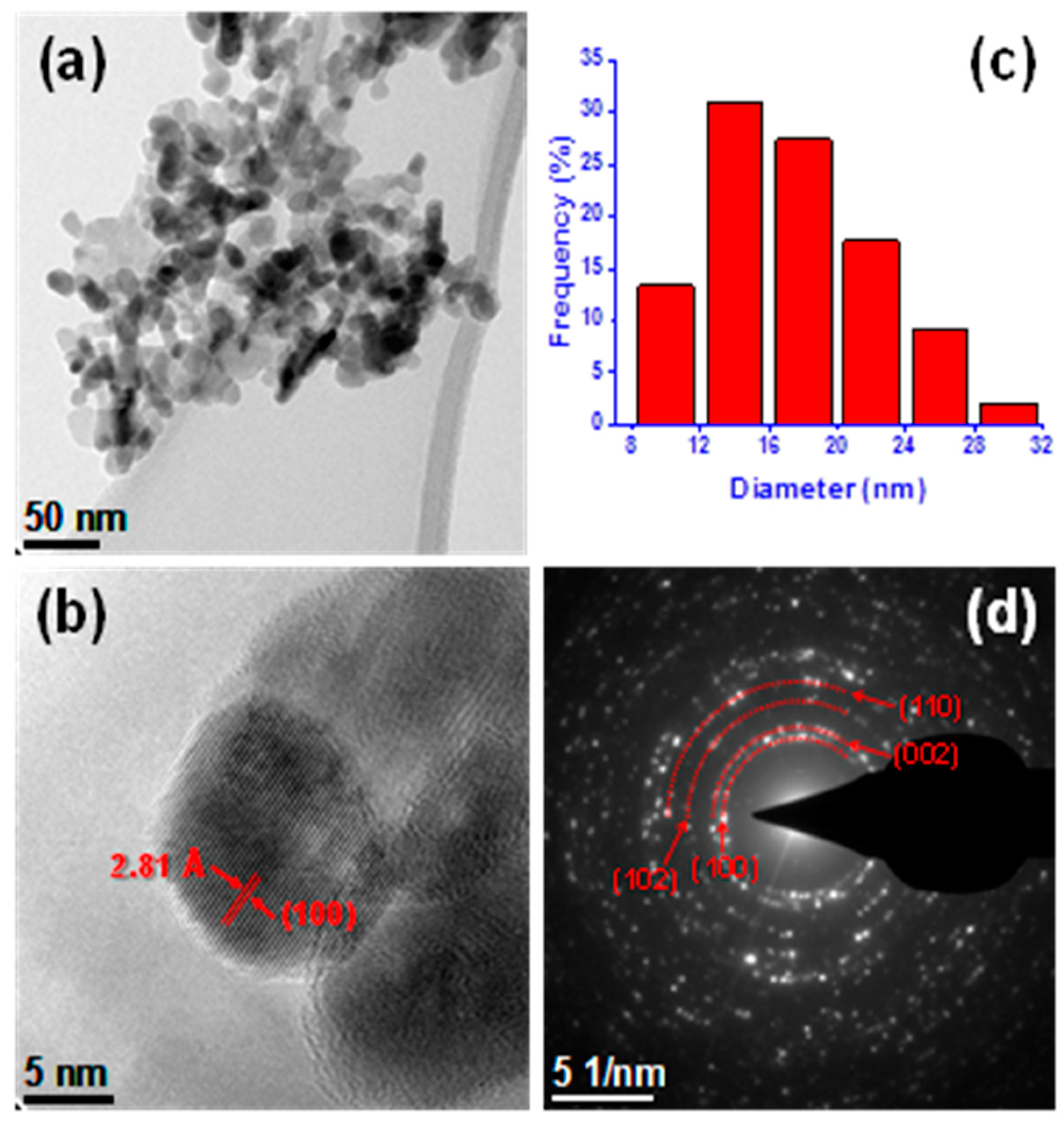

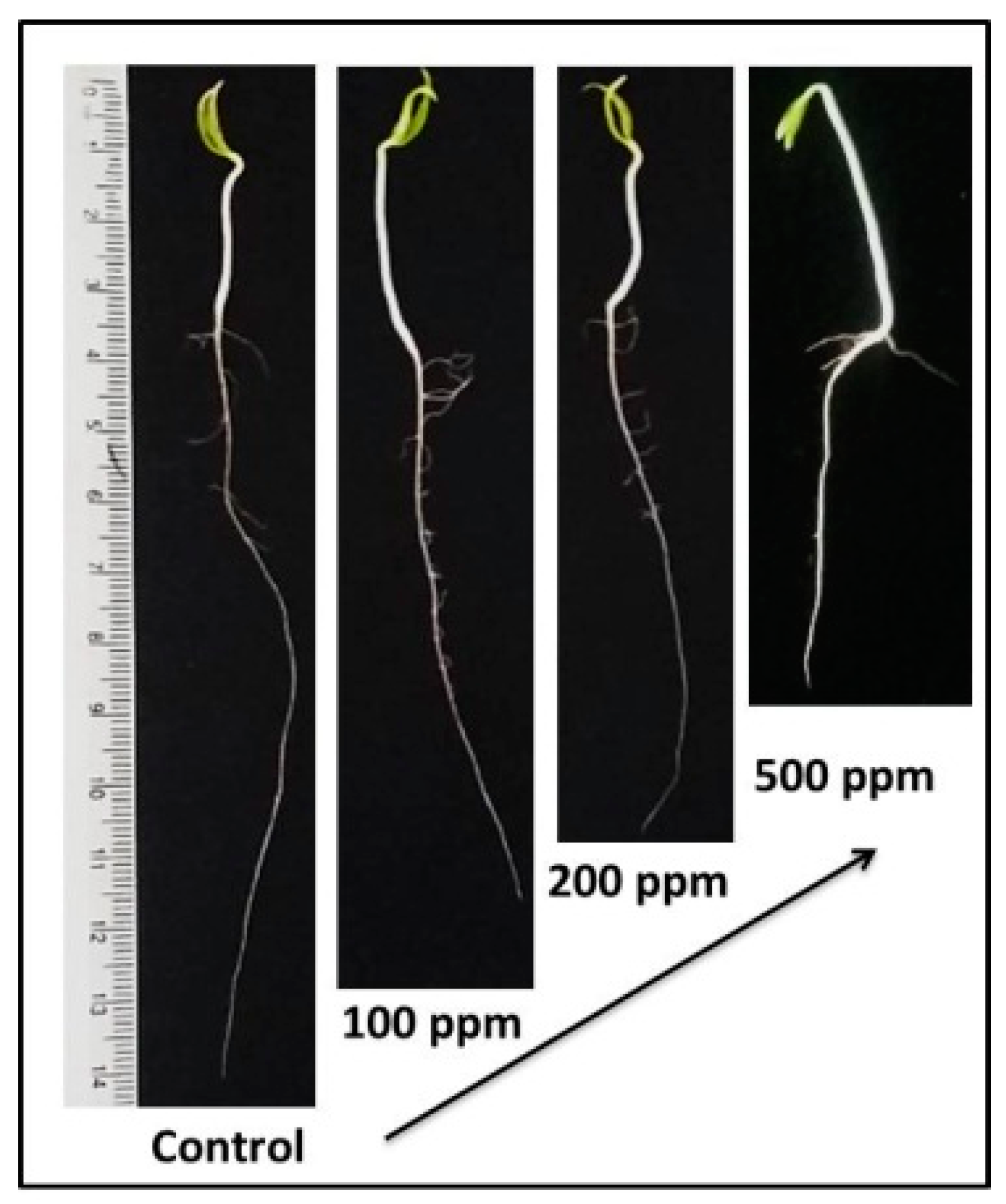
| Treatments (ppm) | Plumule Length (cm) | Radicle Length (cm) | Dry Seedling Biomass (mg Seedling−1) |
|---|---|---|---|
| 0 | 3.29 ± 0.37 a | 11.32 ± 2.48 a | 45.73 ± 3.79 a |
| 100 | 3.65 ± 0.30 a | 5.66 ± 1.40 b | 44.13 ± 0.88 a |
| 200 | 3.46 ± 0.39 a | 5.22 ± 1.65 b | 42.87 ± 4.57 a |
| 500 | 3.41 ± 0.30 a | 5.15 ± 1.47 b | 41.84 ± 5.02 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-López, J.I.; Zavala-García, F.; Olivares-Sáenz, E.; Lira-Saldívar, R.H.; Díaz Barriga-Castro, E.; Ruiz-Torres, N.A.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Niño-Medina, G. Zinc Oxide Nanoparticles Boosts Phenolic Compounds and Antioxidant Activity of Capsicum annuum L. during Germination. Agronomy 2018, 8, 215. https://doi.org/10.3390/agronomy8100215
García-López JI, Zavala-García F, Olivares-Sáenz E, Lira-Saldívar RH, Díaz Barriga-Castro E, Ruiz-Torres NA, Ramos-Cortez E, Vázquez-Alvarado R, Niño-Medina G. Zinc Oxide Nanoparticles Boosts Phenolic Compounds and Antioxidant Activity of Capsicum annuum L. during Germination. Agronomy. 2018; 8(10):215. https://doi.org/10.3390/agronomy8100215
Chicago/Turabian StyleGarcía-López, Josué I., Francisco Zavala-García, Emilio Olivares-Sáenz, Ricardo H. Lira-Saldívar, Enrique Díaz Barriga-Castro, Norma A. Ruiz-Torres, Edith Ramos-Cortez, Rigoberto Vázquez-Alvarado, and Guillermo Niño-Medina. 2018. "Zinc Oxide Nanoparticles Boosts Phenolic Compounds and Antioxidant Activity of Capsicum annuum L. during Germination" Agronomy 8, no. 10: 215. https://doi.org/10.3390/agronomy8100215
APA StyleGarcía-López, J. I., Zavala-García, F., Olivares-Sáenz, E., Lira-Saldívar, R. H., Díaz Barriga-Castro, E., Ruiz-Torres, N. A., Ramos-Cortez, E., Vázquez-Alvarado, R., & Niño-Medina, G. (2018). Zinc Oxide Nanoparticles Boosts Phenolic Compounds and Antioxidant Activity of Capsicum annuum L. during Germination. Agronomy, 8(10), 215. https://doi.org/10.3390/agronomy8100215





