Impacts of Fertilization Type on Soil Microbial Biomass and Nutrient Availability in Two Agroecological Zones of Ghana
Abstract
:1. Introduction
2. Results
2.1. Basic Soil Chemical Properties
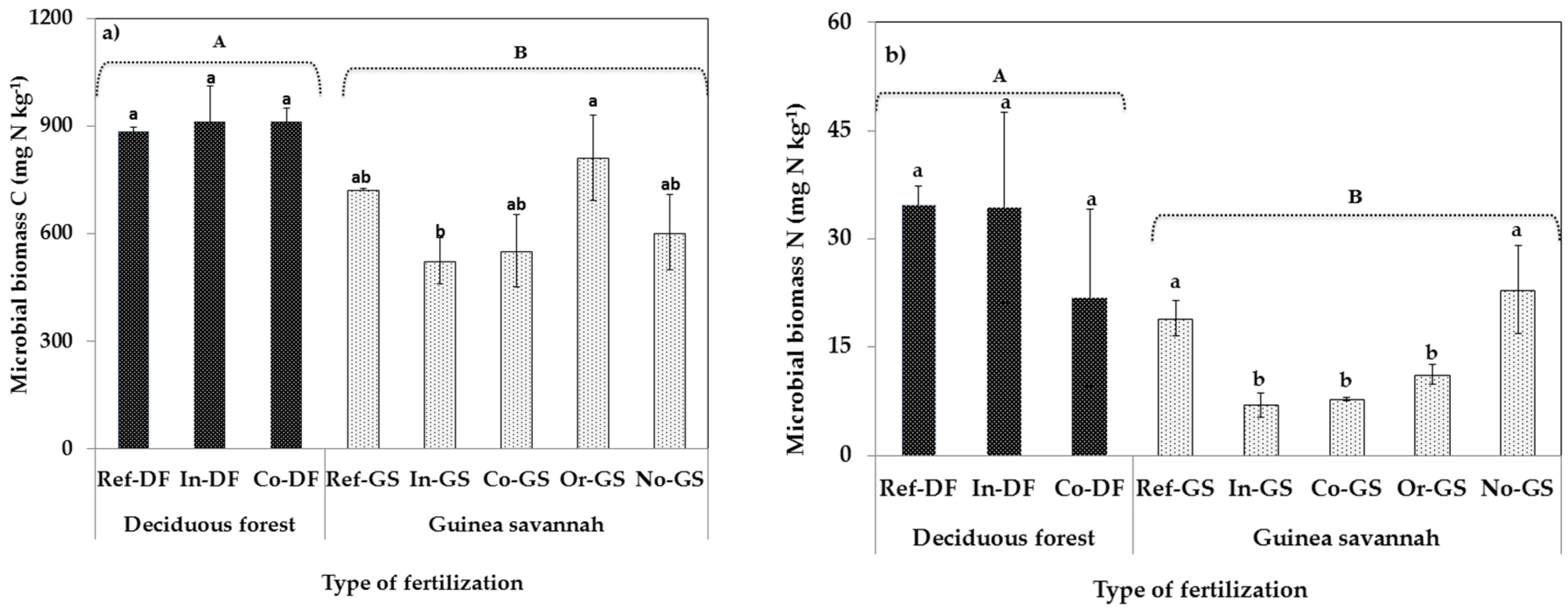
2.2. Microbial Biomass Content-Differences Among Sites
3. Discussion
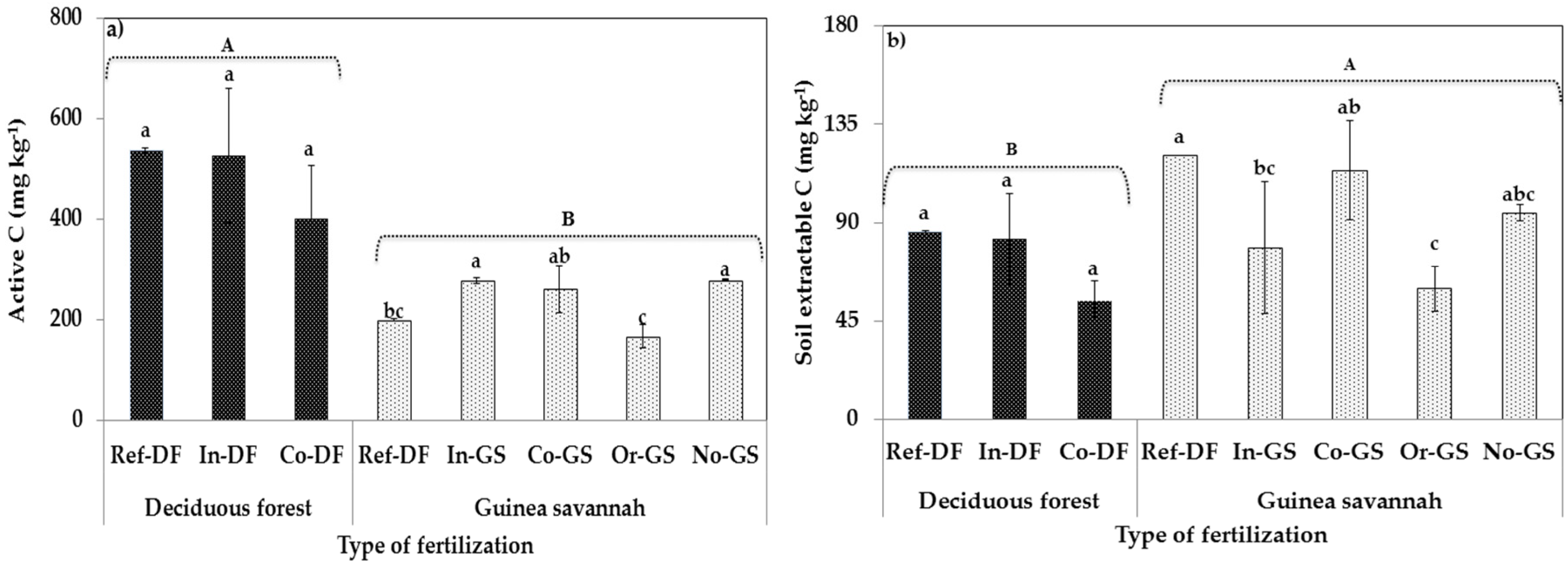
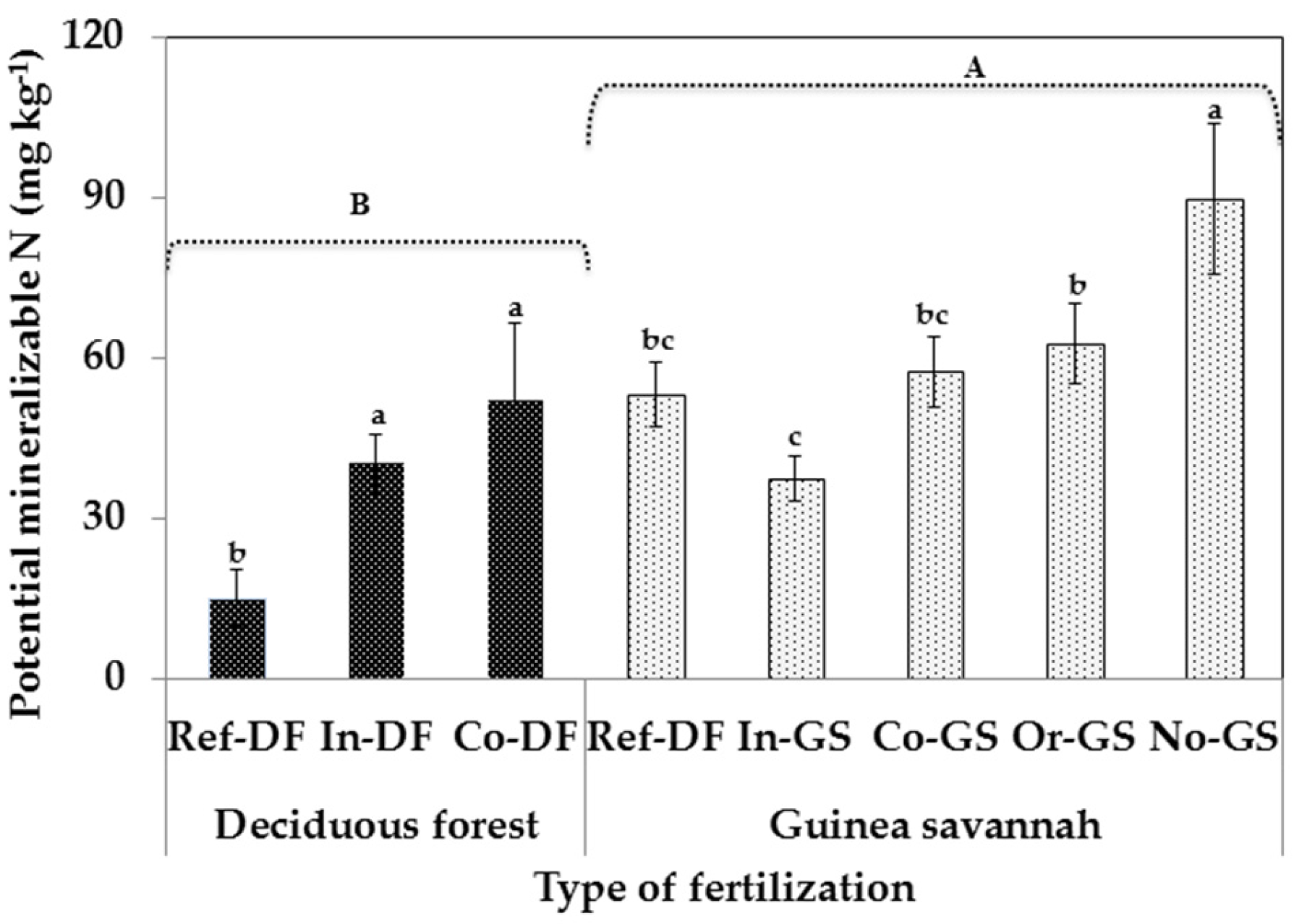
3.1. Fertilization Type and Climate Effects on Soil Chemical Properties
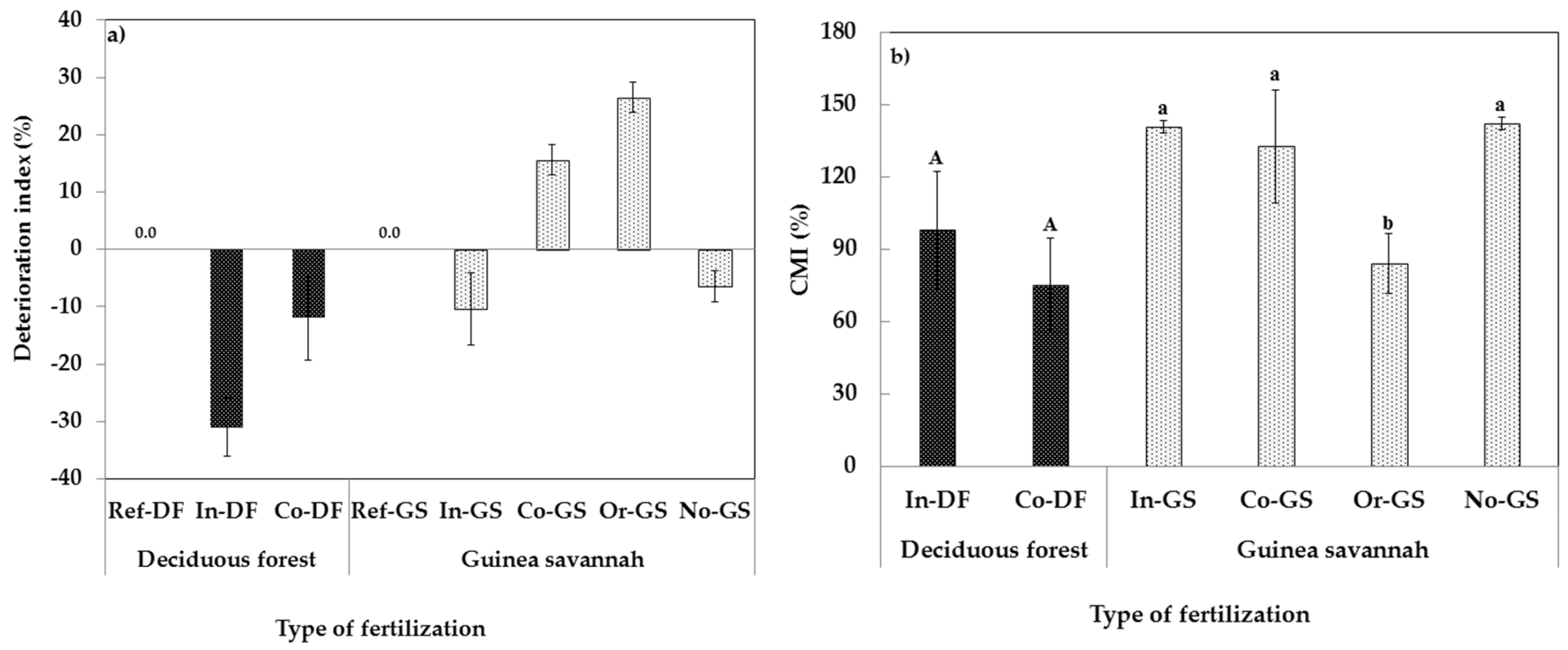
3.2. Fertilization Type on Soil Microbial Biomass Indicators
4. Materials and Methods
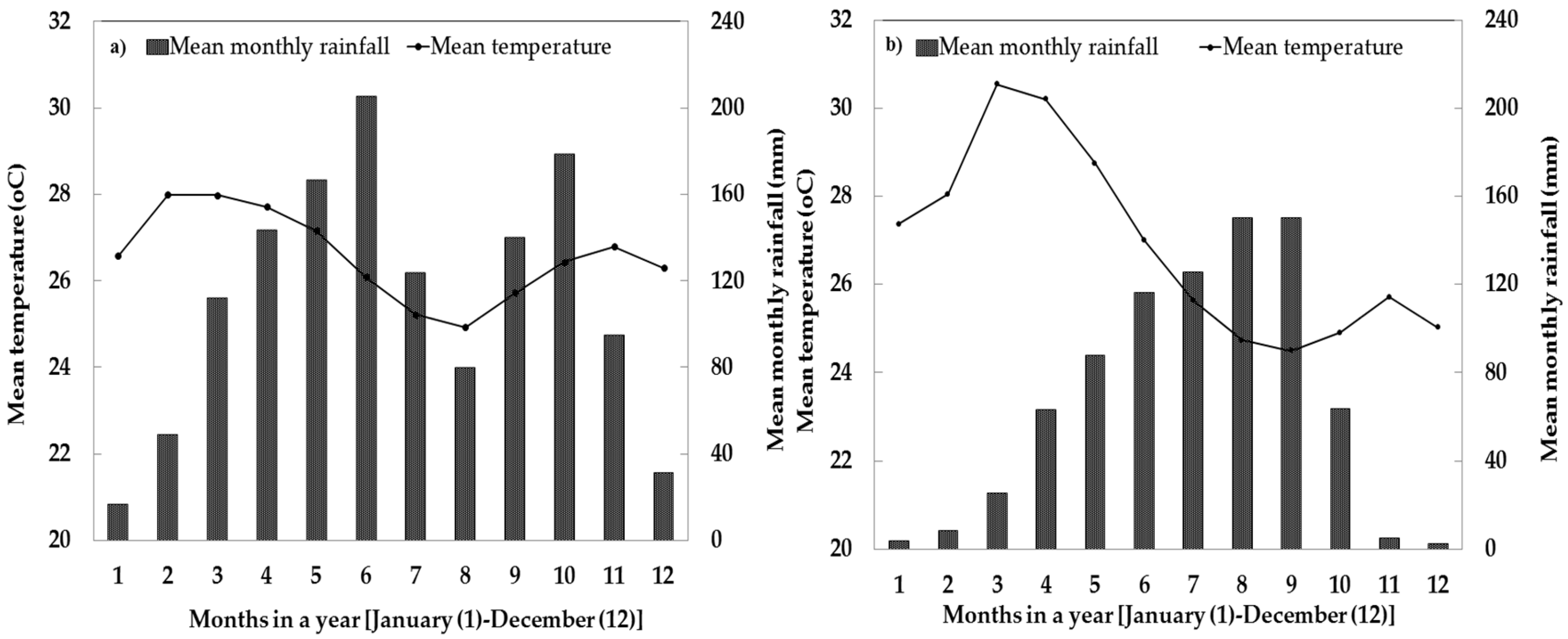
4.1. Site Description and Soil Samplings
4.2. Soil Physicochemical Analyses
4.3. Soil Microbial Analyses
4.4. Calculation of Deterioration Index (DI) and Carbon Management Index (CMI)
- (1)
- C pool index (CPI) was expressed as
- (2)
- Lability index (LI) was calculated as where,
- (3)
- (4)
- And CMI is given as
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Deininger, K.; Byerlee, D.; Lindsay, J.; Norton, A.; Selod, H.; Stickler, M. Rising Global Interest in Farmland. Can It Yield Sustainable and Equitable Benefits; World Bank Publisher: Washington, DC, USA, 2011; pp. 9–44. [Google Scholar]
- OECD-FAO. Agriculture in Sub-Saharan Africa: Prospects and challenges for the next decade. In OECD-FAO Agricultural Outlook 2016–2025; OECD Publishing: Paris, France, 2016; pp. 1–39. [Google Scholar]
- Giller, K.E.; Witter, E.; Corbeels, M.; Tittonell, P. Conservation agriculture and smallholder farming in Africa: The heretics’ view. F. Crop. Res. 2009, 114, 23–34. [Google Scholar] [CrossRef]
- MoFA. Agriculture in Ghana. Facts and Figures (2010); MoFA: Accra, Ghana, 2011.
- Vanlauwe, B.; Bationo, A.; Chianu, J.; Giller, K.E.; Merckx, R.; Mokwunye, U.; Ohiokpehai, O.; Pypers, P.; Tabo, R.; Shepherd, K.D.; et al. Integrated soil fertility management. Operational definition and consequences for implementation and dissemination. Outlook Agric. 2010, 39, 17–24. [Google Scholar] [CrossRef]
- González-Estrada, E.; Rodriguez, L.C.; Walen, V.K.; Naab, J.B.; Koo, J.; Jones, J.W.; Herrero, M.; Thornton, P.K. Carbon sequestration and farm income in West Africa: Identifying best management practices for smallholder agricultural systems in northern Ghana. Ecol. Econ. 2008, 67, 492–502. [Google Scholar] [CrossRef]
- Houssou, N.; Johnson, M.; Kolavalli, S.; Asante-Addo, C. Changes in Ghanaian Farming Systems: Stagnation or a Quiet Transformation? In IFPRI Discussion Paper 01504; International Food Policy Research Institute: Washington, DC, USA, 2016. [Google Scholar]
- Úbeda, X.; Outeiro, L. Physical and Chemical Effects of Fire on Soil. In Fire Effects on Soils and Restoration Strategies; Cerdà Artemi, R.R.P., Ed.; Science Publishers: Enfield, NH, USA, 2009; pp. 105–132. [Google Scholar]
- Mataix-Solera, J.; Cerdà, A.; Arcenegui, V.; Jordàn, A.; Zavala, L.M. Fire effects on soil aggregation: A review. Earth-Sci. Rev. 2011, 109, 44–60. [Google Scholar] [CrossRef]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient Use Efficiency in Plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Spiertz, J.H.J. Nitrogen, sustainable agriculture and food security. A review. Agron. Sustain. Dev. 2010, 30, 43–55. [Google Scholar] [CrossRef]
- McBride, A.C.; Dale, V.H.; Baskaran, L.M.; Downing, M.E.; Eaton, L.M.; Efroymson, R.A.; Garten, C.T.; Kline, K.L.; Jager, H.I.; Mulholland, P.J.; et al. Indicators to support environmental sustainability of bioenergy systems. Ecol. Indic. 2011, 11, 1277–1289. [Google Scholar] [CrossRef]
- Sanchez, P.A. ECOLOGY: Soil Fertility and Hunger in Africa. Science 2002, 295, 2019–2020. [Google Scholar] [CrossRef] [PubMed]
- Issaka, N.R.; Mohammed, M.; Tobita, S.; Nakamura, S.; Owusu-Adjei, E. Indigenous fertilizing materials to enhance soil productivity in Ghana. In Soil Fertility Improvement and Integrated Nutrient Management—A Global Perspective; Whalen, K.J., Ed.; InTech: Rijeka, Croatia, 2012; pp. 119–134. [Google Scholar]
- Marx, E.S.; Hart, J.; Stevens, R.G. Soil Test Interpretation Guide. Ec 1478; Oregon State University: Corvallis, OR, USA, 1999. [Google Scholar]
- Šimon, T. The influence of long-term organic and mineral fertilization on soil organic matter. Soil Water Res. 2008, 3, 41–51. [Google Scholar]
- Benin, S.; Johnson, M.; Abokyi, E.; Ahorbo, G.; Jimah, K.; Nasser, G.; Owusu, V.; Taabazuing, J.T.A. Revisiting agricultural input and farm support subsidies in Africa: The case of Ghana’s mechanization, fertilizer, block farms, and marketing programs. In IFPRI Discussion Paper 01300; International Food Policy Research Institute: Washington, DC, USA, 2013. [Google Scholar]
- Adjei-Gyapong, T.; Asiamah, R. The interim Ghana soil classification system and its relation with the World Reference Base for Soil Resources. In FAO Report on Soil Resources; Food and Agriculture Organization: Rome, Italy, 2002; pp. 9–13. [Google Scholar]
- Heve, W.K.; Olesen, J.E.; Chirinda, N.; Adiku, S.G. K. Targeted management of organic resources for sustainably increasing soil organic carbon: Observations and perspectives for resource use and climate adaptations in northern Ghana. Acta Agric. Scand. Sect. B 2016, 66, 178–190. [Google Scholar] [CrossRef]
- Fuentes, P.A.; Bumb, B.; Johnson, M. Improving Fertilizer Markets in West Africa: The Fertilizer Supply Chain in Ghana; International Fertilizer Development Center (IFDC) and International Food Policy Research Institute (IFPRI): Muscle Shoals, AL, USA; Washington, DC, USA, 2012. [Google Scholar]
- Vanlauwe, B.; Giller, K.E. Popular myths around soil fertility management in sub-Saharan Africa. Agric. Ecosyst. Environ. 2006, 116, 34–46. [Google Scholar] [CrossRef]
- Chirinda, N.; Olesen, J.E.; Porter, J.R.; Schjønning, P. Soil properties, crop production and greenhouse gas emissions from organic and inorganic fertilizer-based arable cropping systems. Agric. Ecosyst. Environ. 2010, 139, 584–594. [Google Scholar] [CrossRef]
- Smith, J.; Abegaz, A.; Matthews, R.B.; Subedi, M.; Orskov, E.R.; Tumwesige, V.; Smith, P. What is the potential for biogas digesters to improve soil carbon sequestration in Sub-Saharan Africa? Comparison with other uses of organic residues. Biomass Bioenergy 2014, 70, 73–86. [Google Scholar] [CrossRef]
- Omari, R.A.; Aung, H.P.; Hou, M.; Yokoyama, T.; Onwona-Agyeman, S.; Oikawa, Y.; Fujii, Y.; Bellingrath-Kimura, S.D. Influence of Different Plant Materials in Combination with Chicken Manure on Soil Carbon and Nitrogen Contents and Vegetable Yield. Pedosphere 2016, 26, 510–521. [Google Scholar] [CrossRef]
- Apusigah, A.A. Promoting sustainable wildfire management in Northern Ghana: Learning from history. Eur. J. Soc. Sci. 2007, 5, 61–76. [Google Scholar]
- Liang, B.; Yang, X.; He, X.; Zhou, J. Effects of 17-year fertilization on soil microbial biomass C and N and soluble organic C and N in loessial soil during maize growth. Biol. Fertil. Soils 2011, 47, 121–128. [Google Scholar] [CrossRef]
- Lovell, R.D.; Jarvis, S.C.; Bardgett, R.D. Soil microbial biomass and activity in long-term grassland: Effects of management changes. Soil Biol. Biochem. 1995, 27, 969–975. [Google Scholar] [CrossRef]
- Allison, S.D.; Treseder, K.K. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob. Chang. Biol. 2008, 14, 2898–2909. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Ajwa, H.A.; Dell, C.J.; Rice, C.W. Changes in enzyme activities and microbial biomass of tallgrass prairie soil as related to burning and nitrogen fertilization. Soil Biol. Biochem. 1999, 31, 769–777. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.Y.; Wang, J.Y.; Hu, T.X.; Gong, Y.B. Long-term manuring and fertilization effects on soil organic carbon pools under a wheat-maize cropping system in North China Plain. Plant Soil 2009, 314, 67–76. [Google Scholar] [CrossRef]
- Ghani, A.; Dexter, M.; Perrott, K.W. Hot-water extractable carbon in soils: A sensitive measurement for determining impacts of fertilisation, grazing and cultivation. Soil Biol. Biochem. 2003, 35, 1231–1243. [Google Scholar] [CrossRef]
- Grego, S.; Marinari, S.; Moscatelli, M.; Badalucco, L. Effect of ammonium nitrate and stabilized farmyard manure on microbial biomass and metabolic quotient of soil under Zea mays. Agric. Mediterr. 1998, 128, 132–137. [Google Scholar]
- Adejuwon, J.O.; Ekanade, O. A comparison of soil properties under different land use types in a part of the Nigerian cocoa belt. Catena 1988, 15, 319–331. [Google Scholar] [CrossRef]
- Islam, K.R.; Weil, R.R. Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agric. Ecosyst. Environ. 2000, 79, 9–16. [Google Scholar] [CrossRef]
- Blair, N.; Faulkner, R.D.; Till, A.R.; Poulton, P.R. Long-term management impacts on soil C, N and physical fertility. Part I: Broadbalk experiment. Soil Tillage Res. 2006, 91, 30–38. [Google Scholar] [CrossRef]
- Yang, X.; Ren, W.; Sun, B.; Zhang, S. Effects of contrasting soil management regimes on total and labile soil organic carbon fractions in a loess soil in China. Geoderma 2012, 177–178, 49–56. [Google Scholar] [CrossRef]
- FAO/ISRIC/ISSS. World Reference Base for Soil Resources (WRB). World Soil Resources Report 84; Food and Agriculture Organization: Rome, Italy, 1998. [Google Scholar]
- Hu, S.; Coleman, D.C.; Carroll, C.R.; Hendrix, P.F.; Beare, M.H. Labile soil carbon pools in subtropical forest and agricultural ecosystems as influenced by management practices and vegetation types. Agric. Ecosyst. Environ. 1997, 65, 69–78. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis, 1st ed.; Pergamon Press: Oxford, UK, 1984; p. 173. [Google Scholar]
- US Environmental Protection Agency. Methods for Chemical Analysis of Water and Wastes. In Environmental Protection Agency; US Environmental Protection Agency: Washington, DC, USA, 1983; p. 491. [Google Scholar]
- Truog, E. The Determination of the Readily Available Phosphorus of Soils. Agron. J. 1930, 22, 874–882. [Google Scholar] [CrossRef]
- Schollenberger, C.J.; Simon, R.H. Determination of exchange capacity and exchangeable bases in soil-ammonium acetate method. Soil Sci. 1945, 59, 13–24. [Google Scholar] [CrossRef]
- Hobbie, S.E. Chloroform Fumigation Direct Extraction (CFDE) Protocol for Microbial Biomass Carbon and Nitrogen. Available online: https://web.stanford.edu/group/Vitousek/cfde.htm (accessed on 29 June 2017).
- Beck, T.; Joergensen, R.G.; Kandeler, E.; Makeshin, E.; Nuss, E.; Oberholzer, H.R.; Scheu, S. An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol. Biochem. 1997, 29, 1023–1032. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Gugino, B.K.; Idowu, O.J.; Schindelbeck, R.R.; Van Es, H.M.; Wolfe, D.W.; Thies, J.E.; Abawi, G.S. Cornell Soil Health Assessment Training Manual, edition 2.0; Cornell University: Geneva, NY, USA, 2009. [Google Scholar]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis Part 3—Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; Soil Science Society of America, American Society of Agronomy: Fitchburg, WI, USA; Madison, WI, USA, 1996; Volume sssabookse, pp. 961–1010. [Google Scholar]
- Blair, N.; Daniel, H. The impact of cropping on soil aggregate stability and carbon content. In ASSSI and NZSSS National Soils Conference, Oral Papers’; Australian Soil Science Society Inc.: Melbourne, Vic., Australia, 1996; pp. 17–18. [Google Scholar]
| Treatment Code | TC (g kg−1) | TN (g kg−1) | CN Ratio | NO3 (mg kg−1) | NH4 (mg kg−1) | Phosphorus (mg kg−1) | SOM (%) | CEC | pH |
|---|---|---|---|---|---|---|---|---|---|
| Ref-DF | 16.85 ± 0.14a | 2.38 ± 0.08a | 7.09 ± 0.30a | 176.17 ± 1.17b | 76.0 ± 0.48ab | 5.82 ± 0.00b | 2.63 ± 0.00a | 9.60 ± 0.01a | 6.12 |
| In-DF | 15.97 ± 4.95a | 2.64 ± 0.62a | 6.00 ± 0.61a | 362.32 ± 42.25a | 78.2 ± 0.58a | 19.77 ± 4.26a | 3.22 ± 0.58a | 10.66 ± 1.49a | 5.94 |
| Co-DF | 10.04 ± 4.42a | 1.91 ± 0.22a | 5.14 ± 1.65a | 307.88 ± 21.39a | 68.3 ± 1.18abc | 16.53 ± 8.24ab | 2.13 ± 0.81a | 11.07 ± 3.09a | 5.85 |
| Mean-DF | 14.29 ± 4.62A | 2.31 ± 0.46A | 6.07 ± 1.23A | 282.12 ± 86.2A | 77.62 ± 8.19A | 14.04 ± 7.84A | 2.66 ± 0.69A | 10.44 ± 0.76B | 5.97 |
| Ref-GS | 6.05 ± 0.43ab | 1.30 ± 0.04a | 4.68 ± 0.47b | 38.60 ± 0.06b | 49.4 ± 0.74bc | 16.89 ± 0.01a | 2.48 ± 0.01a | 16.82 ± 4.46ab | ND |
| In-GS | 6.84 ± 0.62a | 1.19 ± 0.08a | 5.73 ± 0.16a | 107.89 ± 5.36a | 65.0 ± 2.14a | 14.55 ± 3.12b | 1.15 ± 0.13c | 19.72 ± 5.88a | 5.80 |
| Co-GS | 5.71 ± 1.12ab | 1.26 ± 0.170a | 4.51 ± 0.33b | 24.44 ± 0.45b | 44.0 ± 0.48bc | 11.05 ± 1.36bc | 0.89 ± 0.12cd | ND | 5.90 |
| Or-GS | 4.32 ± 0.68b | 1.05 ± 0.09a | 4.11 ± 0.51b | 39.19 ± 1.27b | 31.4 ± 1.16c | 13.68 ± 1.19bc | 0.46 ± 0.08d | 10.53 ± 1.86b | 6.40 |
| No-GS | 6.24 ± 1.88ab | 1.28 ± 0.22a | 4.79 ± 0.63b | 33.44 ± 0.73b | 43.9 ± 0.81bc | 17.25 ± 8.44a | 1.82 ± 0.76b | 19.20 ± 3.26a | 5.45 |
| Mean-GS | 5.83 ± 1.26B | 1.22 ± 0.15B | 4.76 ± 0.67B | 62.45 ± 44.38B | 46.72 ± 15.21B | 13.35 ± 2.21A | 1.36 ± 0.79B | 16.57 ± 4.21A | 5.89 |
| Rate | TN | TC | CN | NO3 | Extr C | MBC | MBN | ACC | P | PMN | SOM | AM | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate | |||||||||||||
| TN | 0.57 ** | ||||||||||||
| TC | 0.60 ** | 0.96 ** | |||||||||||
| CN | 0.54 ** | 0.52 ** | 0.73 ** | ||||||||||
| NO3 | 0.73 ** | 0.82 ** | 0.76 ** | 0.43 ** | |||||||||
| Extr C | −0.38 * | −0.14 NS | −0.09 NS | −0.03 NS | −0.54 ** | ||||||||
| MBC | 0.43 ** | 0.83 ** | 0.72 ** | 0.24NS | 0.84 ** | −0.42 ** | |||||||
| MBN | 0.17 NS | 0.65 ** | 0.66 ** | 0.45 ** | 0.60 ** | −0.16 NS | 0.62 ** | ||||||
| ACC | 0.57 ** | 0.95 ** | 0.96 ** | 0.62 ** | 0.80 ** | −0.16 NS | 0.73 ** | 0.66 ** | |||||
| P | 0.21NS | 0.44 ** | 0.40 * | 0.15 NS | 0.48 ** | −0.19 NS | 0.47 ** | 0.38 * | 0.50 ** | ||||
| PMN | −0.83 ** | −0.30 * | −0.35 * | −0.42 ** | −0.50 ** | 0.27 NS | 0.14 NS | 0.03 NS | −0.34 * | −0.11 NS | |||
| SOM | 0.15 NS | 0.52 ** | 0.60 ** | 0.50 ** | 0.24 NS | −0.12 NS | 0.38 * | 0.24 NS | 0.55 ** | 0.26 NS | −0.02 NS | ||
| AM | 0.71 ** | 0.60 ** | 0.56 ** | 0.38 * | 0.79 ** | −0.41 ** | 0.61 ** | 0.35 * | 0.62 ** | 0.45 ** | 0.63 ** | 0.13 NS | |
| CEC | −0.55 ** | −0.56 ** | −0.50 ** | −0.19 NS | −0.62 ** | 0.25 NS | −0.76 | −0.32 * | −0.46 * | 0.01 | 0.32 * | −0.16 NS | −0.28 NS |
| Variable | Microbial Properties | |||||||
|---|---|---|---|---|---|---|---|---|
| MBN | MBC | BIO CN | ACC | PMN | EXTR C | MBC/TC | MBN/TN | |
| Rate | ||||||||
| R2 | 0.03 | 0.18 | 0.02 | 0.33 | 0.69 | 0.14 | 0.23 | 0.10 |
| Sig. | 0.3 NS | 0.004 * | 0.4 NS | 0.001 ** | 0.001 ** | 0.01 * | 0.001 ** | 0.04 * |
| Soil TN | ||||||||
| R2 | 0.42 | 0.69 | 0.10 | 0.91 | 0.09 | 0.02 | 0.25 | 0.04 |
| Sig. | 0.001 ** | 0.001 ** | 0.03 * | 0.001 ** | 0.04 * | 0.4 NS | 0.001 ** | 0.2 NS |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omari, R.A.; Sarkodee-Addo, E.; Fujii, Y.; Oikawa, Y.; Bellingrath-Kimura, S.D. Impacts of Fertilization Type on Soil Microbial Biomass and Nutrient Availability in Two Agroecological Zones of Ghana. Agronomy 2017, 7, 55. https://doi.org/10.3390/agronomy7030055
Omari RA, Sarkodee-Addo E, Fujii Y, Oikawa Y, Bellingrath-Kimura SD. Impacts of Fertilization Type on Soil Microbial Biomass and Nutrient Availability in Two Agroecological Zones of Ghana. Agronomy. 2017; 7(3):55. https://doi.org/10.3390/agronomy7030055
Chicago/Turabian StyleOmari, Richard Ansong, Elsie Sarkodee-Addo, Yoshiharu Fujii, Yosei Oikawa, and Sonoko Dorothea Bellingrath-Kimura. 2017. "Impacts of Fertilization Type on Soil Microbial Biomass and Nutrient Availability in Two Agroecological Zones of Ghana" Agronomy 7, no. 3: 55. https://doi.org/10.3390/agronomy7030055
APA StyleOmari, R. A., Sarkodee-Addo, E., Fujii, Y., Oikawa, Y., & Bellingrath-Kimura, S. D. (2017). Impacts of Fertilization Type on Soil Microbial Biomass and Nutrient Availability in Two Agroecological Zones of Ghana. Agronomy, 7(3), 55. https://doi.org/10.3390/agronomy7030055







