Combination of Lactic Acid-Based Deep Eutectic Solvents (DES) with β-Cyclodextrin: Performance Screening Using Ultrasound-Assisted Extraction of Polyphenols from Selected Native Greek Medicinal Plants
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Preparation of DES
2.3. Plant Material
2.4. Batch Ultrasound-Assisted Extraction (UAE)
2.5. Sample Preparation and Determinations
2.6. Statistical Analysis
3. Results and Discussion
3.1. DES Synthesis
3.2. Extraction Efficiency
3.3. The Effect of β-Cyclodextrin (β-CD) Addition
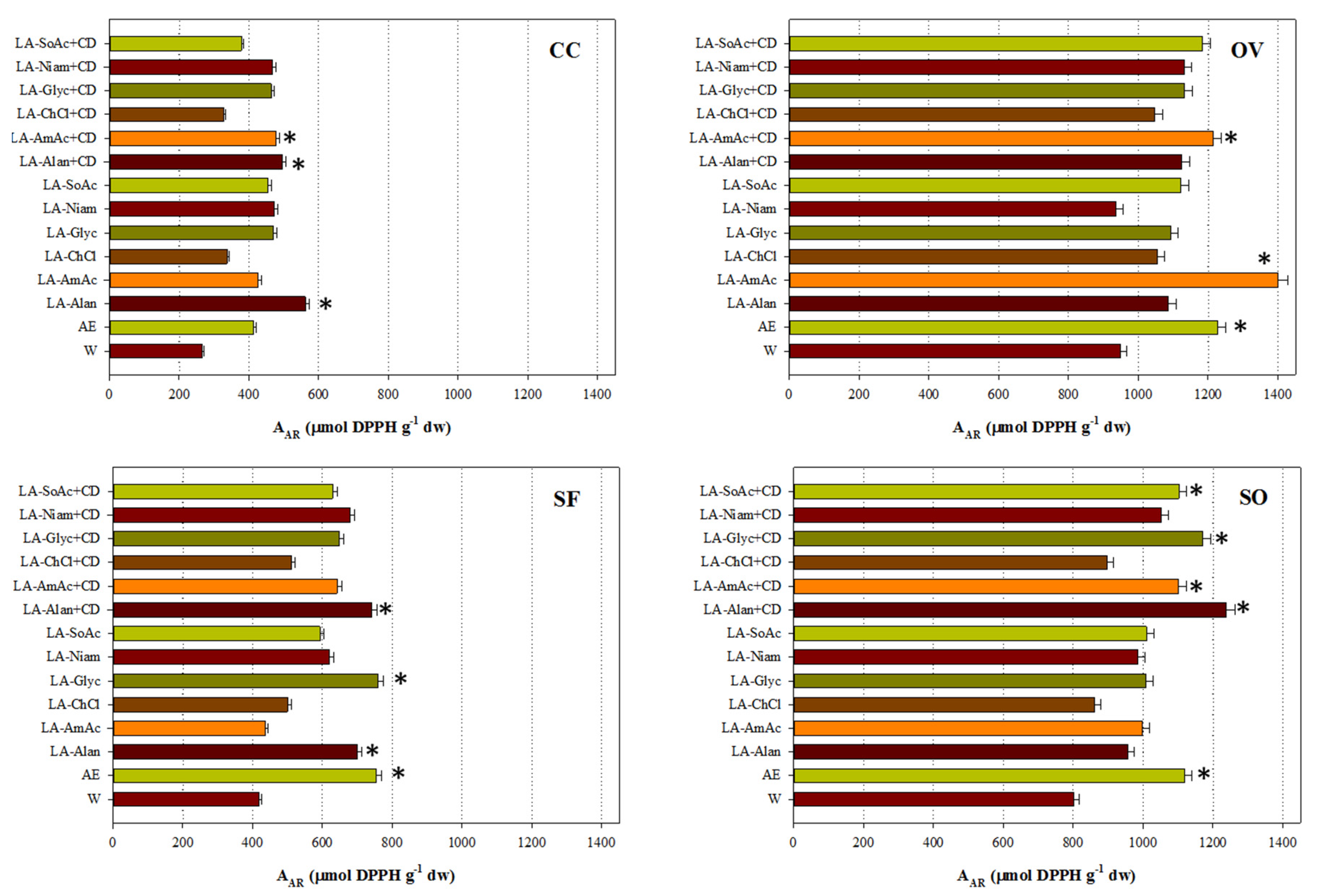
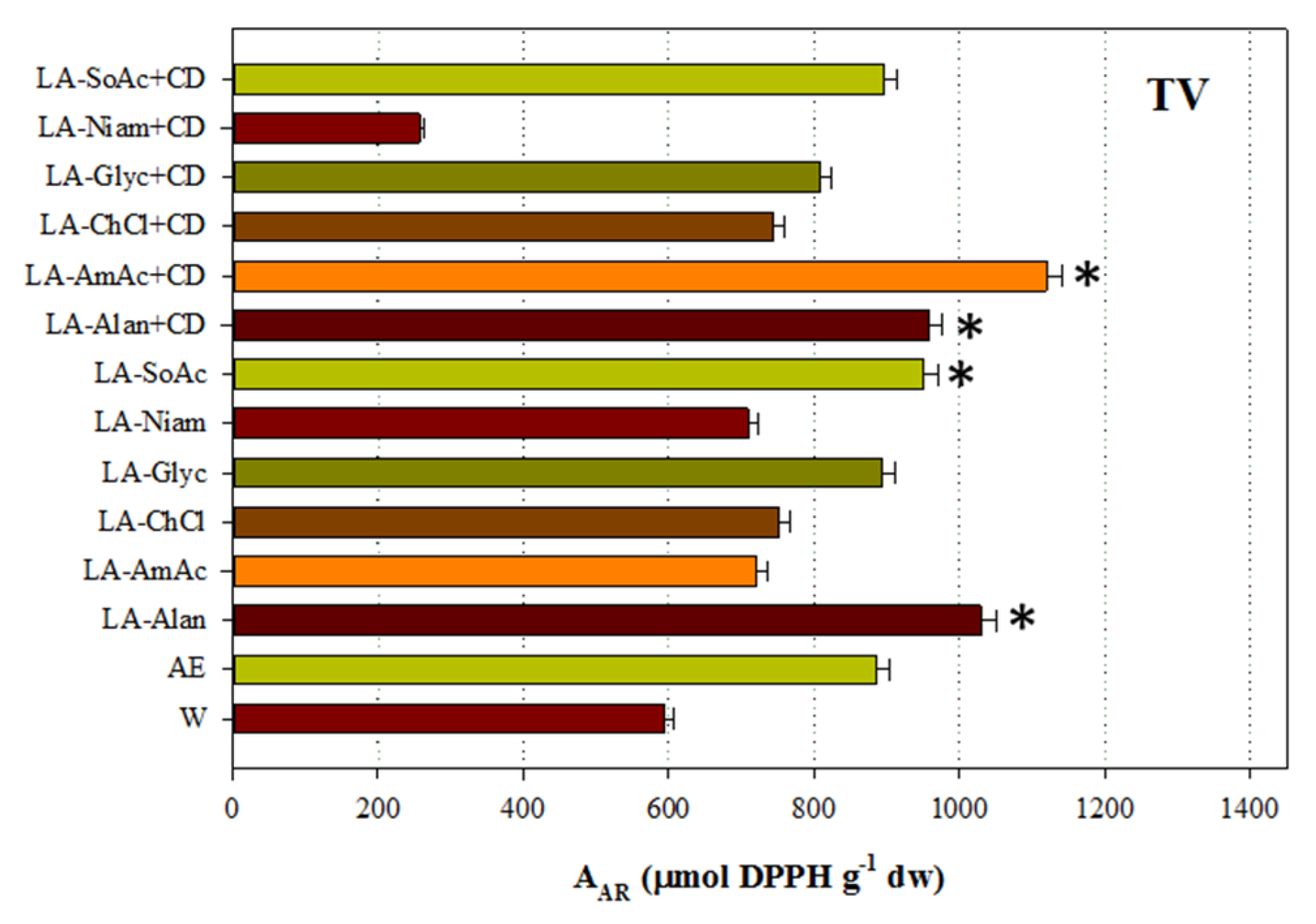
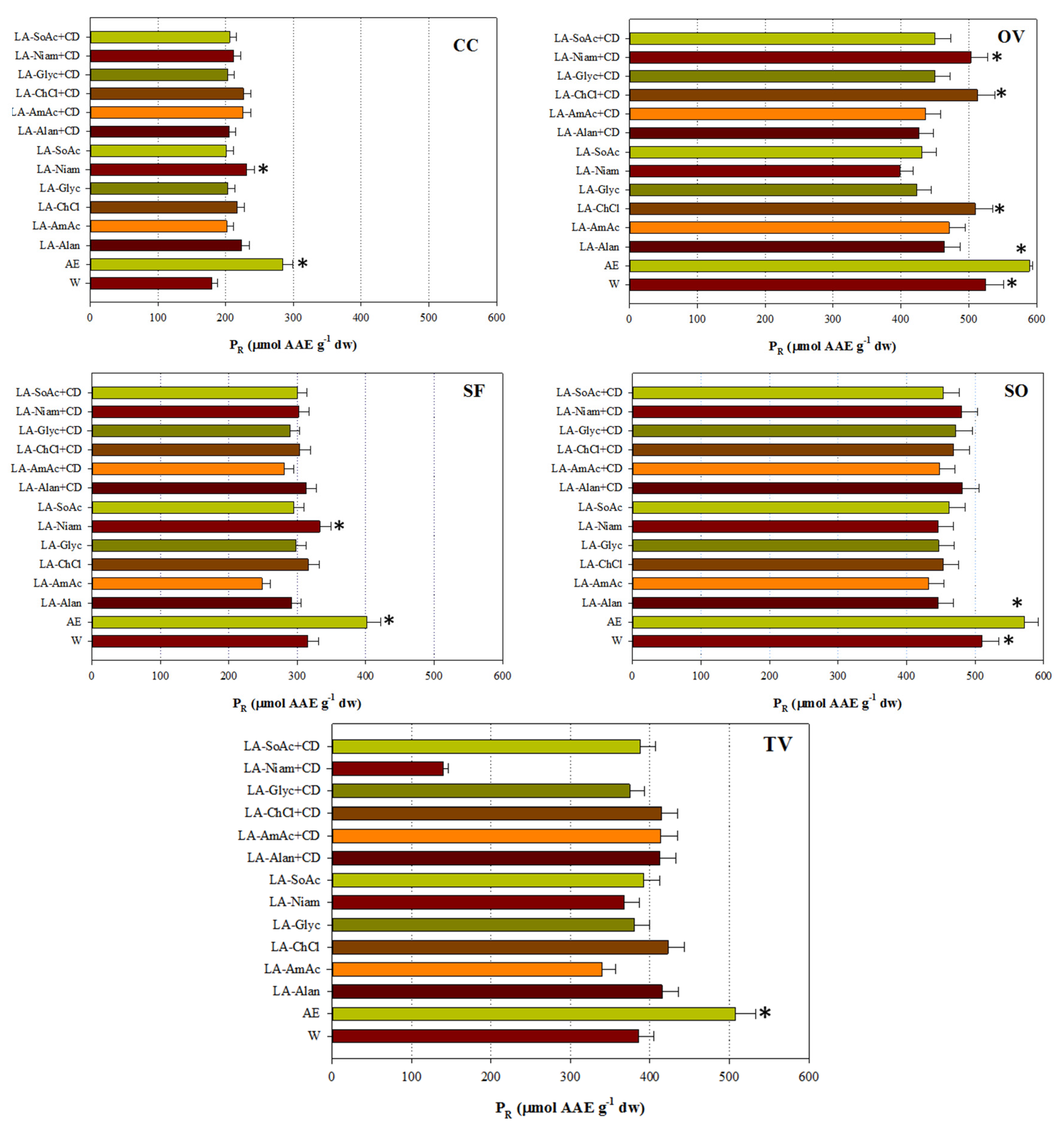
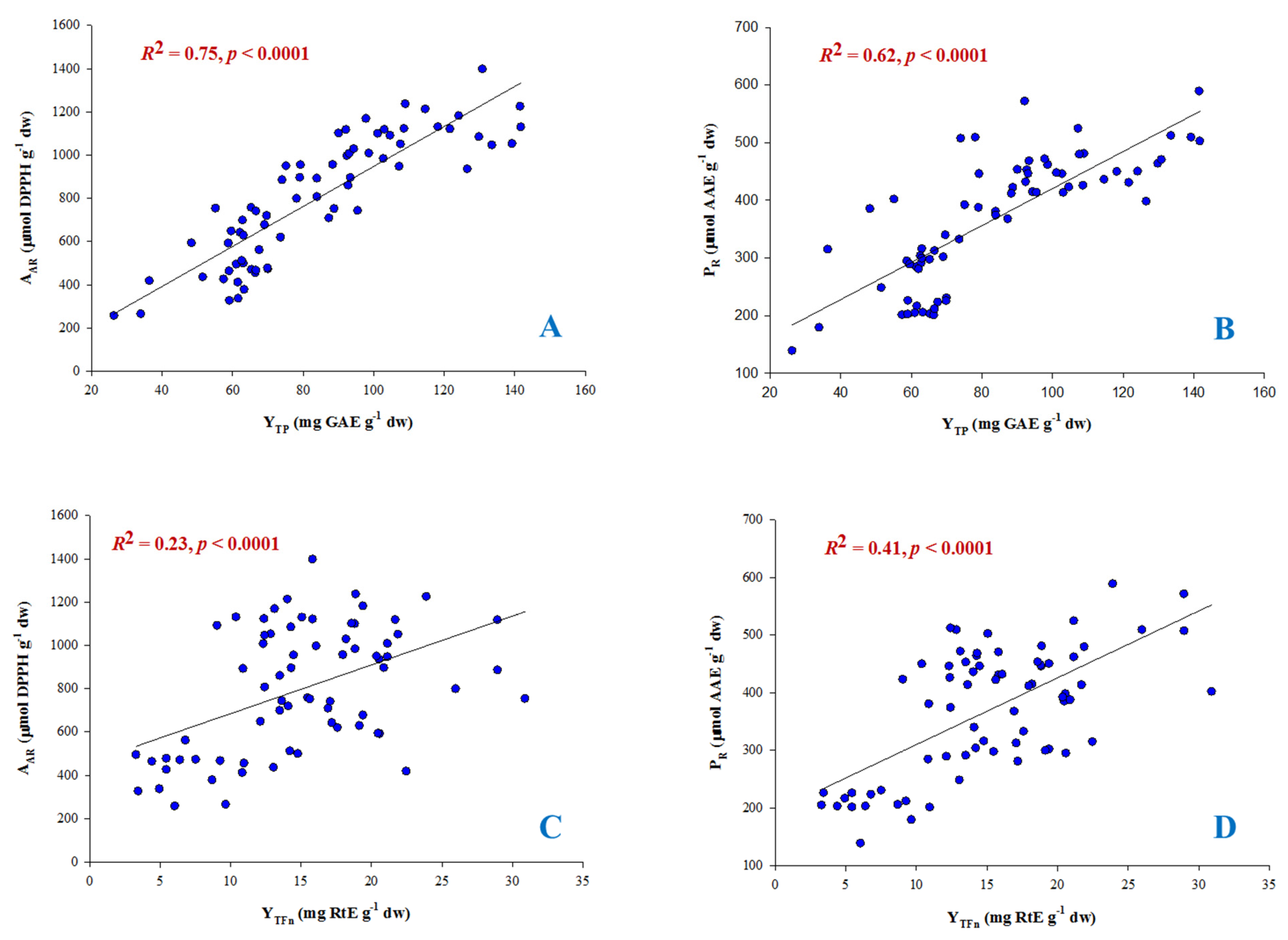
3.4. Antioxidant Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| AAR | antiradical activity (μmol DPPH g−1) |
| PR | reducing power (μmol AAE g−1) |
| RL/S | liquid-to-solid ratio (mL g−1) |
| molar HBD:HBA ratio (dimensionless) | |
| T | temperature (°C) |
| YTFn | yield in total flavonoids (mg RtE g−1) |
| YTP | yield in total polyphenols (mg CAE g−1) |
Abbreviations
| AAE | ascorbic acid equivalents |
| DES | deep eutectic solvent |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| CAE | caffeic acid equivalents |
| HBA | hydrogen bond acceptor |
| HBD | hydrogen bond donor |
| TPTZ | 2,4,6-tripyridyl-s-triazine |
References
- David, B.; Wolfender, J.-L.; Dias, D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015, 14, 299–315. [Google Scholar] [CrossRef]
- Kumar, A.P.; Graham, H.; Robson, C.; Garapati, K.; Ghosh, R. An overview of anticancer herbal medicines. In Evidence-Based Anticancer Materia Medica; Springer: New York, NY, USA, 2011; pp. 1–36. [Google Scholar]
- Farzaneh, V.; Carvalho, I.S. A review of the health benefit potentials of herbal plant infusions and their mechanism of actions. Ind. Crops Prod. 2015, 65, 247–258. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Bergez-Lacoste, M.; Thiebaud-Roux, S.; De Caro, P.; Fabre, J.F.; Gerbaud, V.; Mouloungui, Z. From chemical platform molecules to new biosolvents: Design engineering as a substitution methodology. Biofuels Bioprod. Biorefin. 2014, 8, 438–451. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S.; D’Agostino, C.; Gladden, L.F.; Mantle, M.D. Glycerol eutectics as sustainable solvent systems. Green Chem. 2011, 13, 82–90. [Google Scholar] [CrossRef]
- Wagle, D.V.; Zhao, H.; Baker, G.A. Deep eutectic solvents: Sustainable media for nanoscale and functional materials. Acc. Chem. Res. 2014, 47, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, E.; Vikmon, M.; Szente, L. Cyclodextrins in food technology and human nutrition: Benefits and limitations in 2012. Crit. Rev. Food Sci. Nutr. 2013, 56, 1981–2004. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidou, K.; Mourtzinos, I.; Biliaderis, C.G.; Makris, D.P. Optimization of a green extraction/inclusion complex formation process to recover antioxidant polyphenols from oak acorn husks (Quercus robur) using aqueous 2-hydroxypropyl-β-cyclodextrin/glycerol mixtures. Environments 2016, 3, 3. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Anastasopoulou, E.; Petrou, A.; Grigorakis, S.; Makris, D.; Biliaderis, C.G. Optimization of a green extraction method for the recovery of polyphenols from olive leaf using cyclodextrins and glycerin as co-solvents. J. Food Sci. Technol. 2016, 53, 3939–3947. [Google Scholar] [CrossRef] [PubMed]
- Parmar, I.; Sharma, S.; Rupasinghe, H.V. Optimization of β-cyclodextrin-based flavonol extraction from apple pomace using response surface methodology. J. Food Sci. Technol. 2015, 52, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Michail, A.; Sigala, P.; Grigorakis, S.; Makris, D.P. Kinetics of ultrasound-assisted polyphenol extraction from spent filter coffee using aqueous glycerol. Chem. Eng. Commun. 2016, 203, 407–413. [Google Scholar] [CrossRef]
- Manousaki, A.; Jancheva, M.; Grigorakis, S.; Makris, D.P. Extraction of antioxidant phenolics from agri-food waste biomass using a newly designed glycerol-based natural low-transition temperature mixture: A comparison with conventional eco-friendly solvents. Recycling 2016, 1, 194. [Google Scholar] [CrossRef]
- Paleologou, I.; Vasiliou, A.; Grigorakis, S.; Makris, D.P. Optimisation of a green ultrasound-assisted extraction process for potato peel (Solanum tuberosum) polyphenols using bio-solvents and response surface methodology. Biomass Convers. Biorefin. 2016, 6, 289–299. [Google Scholar] [CrossRef]
- Patsea, M.; Stefou, I.; Grigorakis, S.; Makris, D.P. Screening of natural sodium acetate-based low-transition temperature mixtures (LTTMs) for enhanced extraction of antioxidants and pigments from red vinification solid wastes. Environ. Proc. 2017, 4, 123–135. [Google Scholar] [CrossRef]
- Kottaras, P.; Koulianos, M.; Makris, D.P. Low-Transition temperature mixtures (LTTMs) made of bioorganic molecules: Enhanced extraction of antioxidant phenolics from industrial cereal solid wastes. Recycling 2017, 2, 3. [Google Scholar] [CrossRef]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel glycerol-based natural eutectic mixtures and their efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from agri-food waste biomass. Waste Biomass Valoriz. 2016, 7, 1377–1387. [Google Scholar] [CrossRef]
- Jancheva, M.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimised extraction of antioxidant polyphenols from Satureja thymbra using newly designed glycerol-based natural low-transition temperature mixtures (LTTMs). J. Appl. Res. Med. Aromat. Plants 2017. [Google Scholar] [CrossRef]
- Karageorgou, I.; Grigorakis, S.; Lalas, S.; Makris, D.P. Enhanced extraction of antioxidant polyphenols from Moringa oleifera Lam. leaves using a biomolecule-based low-transition temperature mixture. Eur. Food Res. Technol. 2017. [Google Scholar] [CrossRef]
- Philippi, K.; Tsamandouras, N.; Grigorakis, S.; Makris, D.P. Ultrasound-assisted green extraction of eggplant peel (Solanum melongena) polyphenols using aqueous mixtures of glycerol and ethanol: Optimisation and kinetics. Environ. Proc. 2016, 3, 369–386. [Google Scholar] [CrossRef]
- Jeong, K.M.; Lee, M.S.; Nam, M.W.; Zhao, J.; Jin, Y.; Lee, D.-K.; Kwon, S.W.; Jeong, J.H.; Lee, J. Tailoring and recycling of deep eutectic solvents as sustainable and efficient extraction media. J. Chromatogr. A 2015, 1424, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Exarchou, V.; Nenadis, N.; Tsimidou, M.; Gerothanassis, I.; Troganis, A.; Boskou, D. Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J. Agric. Food Chem. 2002, 50, 5294–5299. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Żegarska, Z.; Rafałowski, R.; Pegg, R.B.; Karamać, M.; Kosińska, A. Antioxidant activity and free radical-scavenging capacity of ethanolic extracts of thyme, oregano, and marjoram. Eur. J. Lipid Sci. Technol. 2009, 111, 1111–1117. [Google Scholar] [CrossRef]
- Generalić, I.; Skroza, D.; Šurjak, J.; Možina, S.S.; Ljubenkov, I.; Katalinić, A.; Šimat, V.; Katalinić, V. Seasonal variations of phenolic compounds and biological properties in sage (Salvia officinalis L.). Chem. Biodivers. 2012, 9, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Miliauskas, G.; Venskutonis, P.; Van Beek, T. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- López-Miranda, S.; Serrano-Martínez, A.; Hernández-Sánchez, P.; Guardiola, L.; Pérez-Sánchez, H.; Fortea, I.; Gabaldón, J.A.; Núñez-Delicado, E. Use of cyclodextrins to recover catechin and epicatechin from red grape pomace. Food Chem. 2016, 203, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, H.; Xu, Z.; Wu, M.; Xia, W.; Zhang, W. Chimonanthus praecox extract/cyclodextrin inclusion complexes: Selective inclusion, enhancement of antioxidant activity and thermal stability. Ind. Crops Prod. 2017, 95, 60–65. [Google Scholar] [CrossRef]
- Diamanti, A.C.; Igoumenidis, P.E.; Mourtzinos, I.; Yannakopoulou, K.; Karathanos, V.T. Green extraction of polyphenols from whole pomegranate fruit using cyclodextrins. Food Chem. 2017, 214, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Liu, Y.; Liu, T.; Yuan, Y.; Yue, T.; Cai, R.; Wang, Z. Extraction of epigallocatechin gallate and epicatechin gallate from tea leaves using β-cyclodextrin. J. Food Sci. 2017, 82, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; Chacar, S.; Afif, C.; Vorobiev, E.; Louka, N.; Maroun, R.G. β-Cyclodextrin-assisted extraction of polyphenols from vine shoot cultivars. J. Agric. Food Chem. 2015, 63, 3387–3393. [Google Scholar] [CrossRef] [PubMed]
- Mantegna, S.; Binello, A.; Boffa, L.; Giorgis, M.; Cena, C.; Cravotto, G. A one-pot ultrasound-assisted water extraction/cyclodextrin encapsulation of resveratrol from Polygonum cuspidatum. Food Chem. 2012, 130, 746–750. [Google Scholar] [CrossRef]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Antioxidant capacity of quercetin and its glycosides in the presence of β-cyclodextrins: Influence of glycosylation on inclusion complexation. J. Incl. Phenom. Macrocycl. Chem. 2015, 83, 309–319. [Google Scholar] [CrossRef]
- Medronho, B.; Valente, A.J.; Costa, P.; Romano, A. Inclusion complexes of rosmarinic acid and cyclodextrins: stoichiometry, association constants, and antioxidant potential. Colloid Polym. Sci. 2014, 292, 885–894. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; Rosa, L.A.D.L.; Torres-Rivas, F.; Rodrigo-Garcia, J.; González-Aguilar, G.A. Complexation of apple antioxidants: Chlorogenic acid, quercetin and rutin by β-cyclodextrin (β-CD). J. Incl. Phenom. Macrocycl. Chem. 2005, 53, 121–129. [Google Scholar] [CrossRef]
- Shao, P.; Zhang, J.; Fang, Z.; Sun, P. Complexing of chlorogenic acid with β-cyclodextrins: Inclusion effects, antioxidative properties and potential application in grape juice. Food Hydrocoll. 2014, 41, 132–139. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Pałecz, B.; Rachwał-Rosiak, D.; Hodurek, P.; Miśkiewicz, K.; Oracz, J.; Żyżelewicz, D. Inclusion complexes of β-cyclodextrin with chlorogenic acids (CHAs) from crude and purified aqueous extracts of green Robusta coffee beans (Coffea canephora L.). Food Res. Int. 2014, 61, 202–213. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Salta, F.; Yannakopoulou, K.; Chiou, A.; Karathanos, V.T. Encapsulation of olive leaf extract in β-cyclodextrin. J. Agric. Food Chem. 2007, 55, 8088–8094. [Google Scholar] [CrossRef] [PubMed]
- Aoun, M.; Makris, D. Binary mixtures of natural polyphenolic antioxidants with ascorbic acid: Impact of interactions on the antiradical activity. Int. Food Res. J. 2012, 19, 603–606. [Google Scholar]
- Karvela, E.; Makris, D.P.; Karathanos, V.T. Implementation of response surface methodology to assess the antiradical behaviour in mixtures of ascorbic acid and α-tocopherol with grape (Vitis vinifera) stem extracts. Food Chem. 2012, 132, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Karvela, E.; Makris, D.P. Assessment of the reducing effects in mixtures of grape (Vitis vinifera) seed extracts with α-tocopherol using response surface methodology. J. Microbiol. Biotechnol. Food Sci. 2012, 2, 771. [Google Scholar]
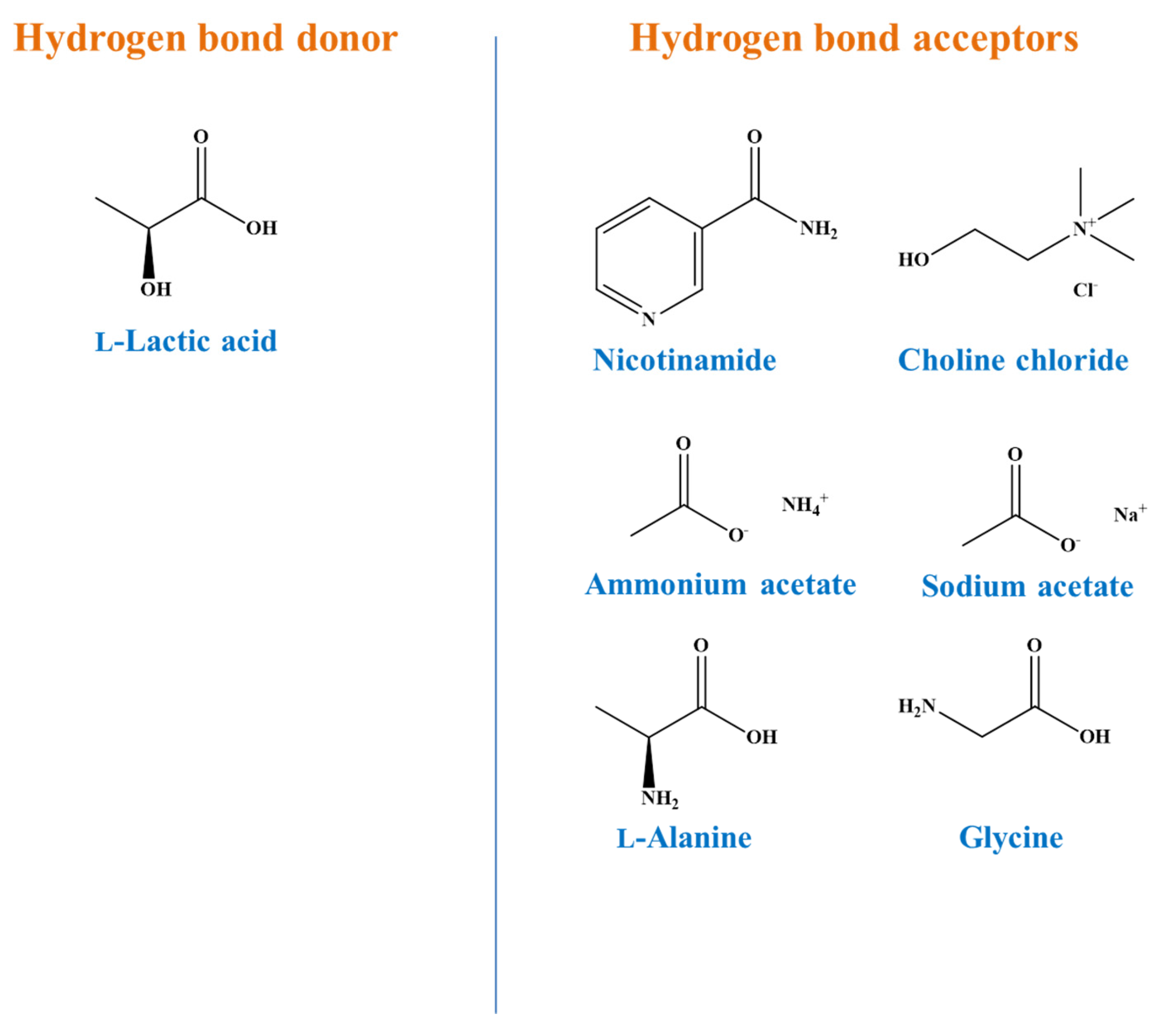
| Code | Common Name | Systematic Name |
|---|---|---|
| CC | Thyme | Coridothymus capitatus |
| OV | Oregano | Origanum vulgare hirtum |
| SF | Greek sage | Salvia fruticosa (triloba) |
| SO | Sage | Salvia officinalis |
| TV | Thyme | Thymus vulgaris |
| Code | HBA | Appearance |
|---|---|---|
| LA-Nic | Nicotinamide | Colourless |
| LA-Ccl | Choline chloride | Colourless |
| LA-Sac | Sodium acetate | Colourless |
| LA-Aac | Ammonium acetate | Colourless |
| LA-Gly | Glycine | Colourless |
| LA-Ala | l-Alanine | Colourless |
| Solvent | YTP (mg CAE g−1 dw) | ||||
|---|---|---|---|---|---|
| CC | OV | SF | SO | TV | |
| Water | 33.91 ± 3.05 | 107.18 ± 9.65 | 34.87 ± 3.27 | 78.10 ± 7.03 | 50.02 ± 4.34 |
| 60% EtOH | 63.17 ± 5.69 | 124.61 ± 11.21 | 55.09 ± 4.96 | 96.77 ± 8.71 | 73.98 ± 6.66 |
| LA-Alan | 69.92 ± 6.29 * | 129.82 ± 9.69 | 65.51 ± 5.90 | 75.84 ± 6.83 | 97.29 ± 8.67 * |
| LA-AmAc | 57.37 ± 5.16 | 134.80 ± 12.13 * | 51.46 ± 4.63 | 92.38 ± 8.31 | 69.60 ± 6.26 |
| LA-ChCl | 61.51 ± 5.54 | 140.72 ± 12.66 * | 62.97 ± 5.67 | 92.73 ± 8.35 | 88.73 ± 7.99 |
| LA-Glyc | 60.48 ± 5.44 | 102.62 ± 7.20 | 65.17 ± 5.86 | 90.72 ± 7.16 | 83.83 ± 7.54 |
| LA-Niam | 72.45 ± 6.52 * | 130.60 ± 9.75 | 73.54 ± 6.62 * | 102.70 ± 7.98 * | 87.28 ± 7.86 |
| LA-SoAc | 66.32 ± 5.97 | 121.57 ± 8.94 | 58.72 ± 5.28 | 98.60 ± 7.88 | 81.53 ± 5.34 |
| with 1.5% (w/v) β-CD | |||||
| LA-Alan | 60.99 ± 5.49 | 104.37 ± 9.39 | 66.51 ± 4.99 * | 108.94 ± 7.80 * | 88.32 ± 7.95 |
| LA-AmAc | 72.48 ± 6.52 * | 117.50 ± 5.57 | 62.03 ± 5.58 | 101.12 ± 9.10 * | 102.99 ± 9.27 * |
| LA-ChCl | 59.03 ± 5.31 | 131.60 ± 11.84 | 62.55 ± 5.63 | 93.35 ± 8.40 | 95.42 ± 8.59 * |
| LA-Glyc | 58.92 ± 5.30 | 113.58 ± 10.22 | 59.55 ± 3.36 | 97.81 ± 6.80 | 83.89 ± 6.45 |
| LA-Niam | 66.54 ± 3.98 | 144.92 ± 10.04 * | 69.02 ± 3.21 * | 110.88 ± 5.88 * | 27.66 ± 2.49 |
| LA-SoAc | 63.18 ± 5.69 | 124.08 ± 11.17 | 62.97 ± 5.35 | 87.59 ± 7.88 | 79.00 ± 6.11 |
| Solvent | YTFn (mg RtE g−1 dw) | ||||
|---|---|---|---|---|---|
| CC | OV | SF | SO | TV | |
| Water | 9.64 ± 0.39 * | 21.13 ± 0.85 * | 22.46 ± 0.90 * | 25.96 ± 1.04 * | 20.46 ± 0.98 * |
| 60% EtOH | 10.81 ± 0.43 * | 23.89 ± 0.96 * | 30.89 ± 1.24 * | 28.93 ± 1.16 * | 28.94 ± 0.87 * |
| LA-Alan | 6.78 ± 0.37 | 14.26 ± 0.57 | 13.48 ± 0.54 | 14.46 ± 0.58 | 18.19 ± 0.95 |
| LA-AmAc | 5.43 ± 0.32 | 15.80 ± 0.33 | 13.03 ± 0.52 | 16.07 ± 0.84 | 14.07 ± 0.43 |
| LA-ChCl | 4.93 ± 0.20 | 12.83 ± 0.61 | 14.76 ± 0.87 | 13.48 ± 0.54 | 15.61 ± 0.62 |
| LA-Glyc | 6.39 ± 0.46 | 9.03 ± 0.38 | 15.45 ± 0.62 | 12.30 ± 0.59 | 10.87 ± 0.76 |
| LA-Niam | 7.51 ± 0.32 | 20.54 ± 0.90 * | 17.58 ± 0.70 | 18.83 ± 0.75 | 16.91 ± 0.68 |
| LA-SoAc | 10.94 ± 0.44 * | 15.80 ± 0.97 | 20.57 ± 1.02 * | 21.13 ± 1.02 | 20.35 ± 0.81 * |
| with 1.5% (w/v) β-CD | |||||
| LA-Alan | 3.27 ± 0.13 | 12.36 ± 0.49 | 17.05 ± 0.76 | 18.86 ± 0.75 | 17.95 ± 0.71 |
| LA-AmAc | 5.43 ± 0.21 | 14.02 ± 0.56 | 17.17 ± 0.99 | 18.80 ± 0.62 | 21.68 ± 0.77 * |
| LA-ChCl | 3.43 ± 0.14 | 12.41 ± 0.50 | 14.19 ± 0.57 | 14.29 ± 0.57 | 13.62 ± 0.76 |
| LA-Glyc | 4.40 ± 0.18 | 10.37 ± 0.76 | 12.11 ± 0.68 | 13.11 ± 0.52 | 12.41 ± 0.50 |
| LA-Niam | 9.25 ± 0.37 * | 15.05 ± 0.60 | 19.38 ± 0.78 | 21.87 ± 0.99 * | 6.03 ± 0.24 |
| LA-SoAc | 8.68 ± 0.45 * | 19.38 ± 0.78 * | 19.13 ± 1.00 | 18.58 ± 0.74 | 20.87 ± 0.98 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgantzi, C.; Lioliou, A.-E.; Paterakis, N.; Makris, D.P. Combination of Lactic Acid-Based Deep Eutectic Solvents (DES) with β-Cyclodextrin: Performance Screening Using Ultrasound-Assisted Extraction of Polyphenols from Selected Native Greek Medicinal Plants. Agronomy 2017, 7, 54. https://doi.org/10.3390/agronomy7030054
Georgantzi C, Lioliou A-E, Paterakis N, Makris DP. Combination of Lactic Acid-Based Deep Eutectic Solvents (DES) with β-Cyclodextrin: Performance Screening Using Ultrasound-Assisted Extraction of Polyphenols from Selected Native Greek Medicinal Plants. Agronomy. 2017; 7(3):54. https://doi.org/10.3390/agronomy7030054
Chicago/Turabian StyleGeorgantzi, Chrysa, Antonia-Eleni Lioliou, Nikos Paterakis, and Dimitris P. Makris. 2017. "Combination of Lactic Acid-Based Deep Eutectic Solvents (DES) with β-Cyclodextrin: Performance Screening Using Ultrasound-Assisted Extraction of Polyphenols from Selected Native Greek Medicinal Plants" Agronomy 7, no. 3: 54. https://doi.org/10.3390/agronomy7030054
APA StyleGeorgantzi, C., Lioliou, A.-E., Paterakis, N., & Makris, D. P. (2017). Combination of Lactic Acid-Based Deep Eutectic Solvents (DES) with β-Cyclodextrin: Performance Screening Using Ultrasound-Assisted Extraction of Polyphenols from Selected Native Greek Medicinal Plants. Agronomy, 7(3), 54. https://doi.org/10.3390/agronomy7030054







