Remote Sensing Meets Agronomy: A Three-Year Field Study of Tritordeum’s Response to Enhanced Efficiency Fertilisers
Abstract
1. Introduction
2. Materials and Methods
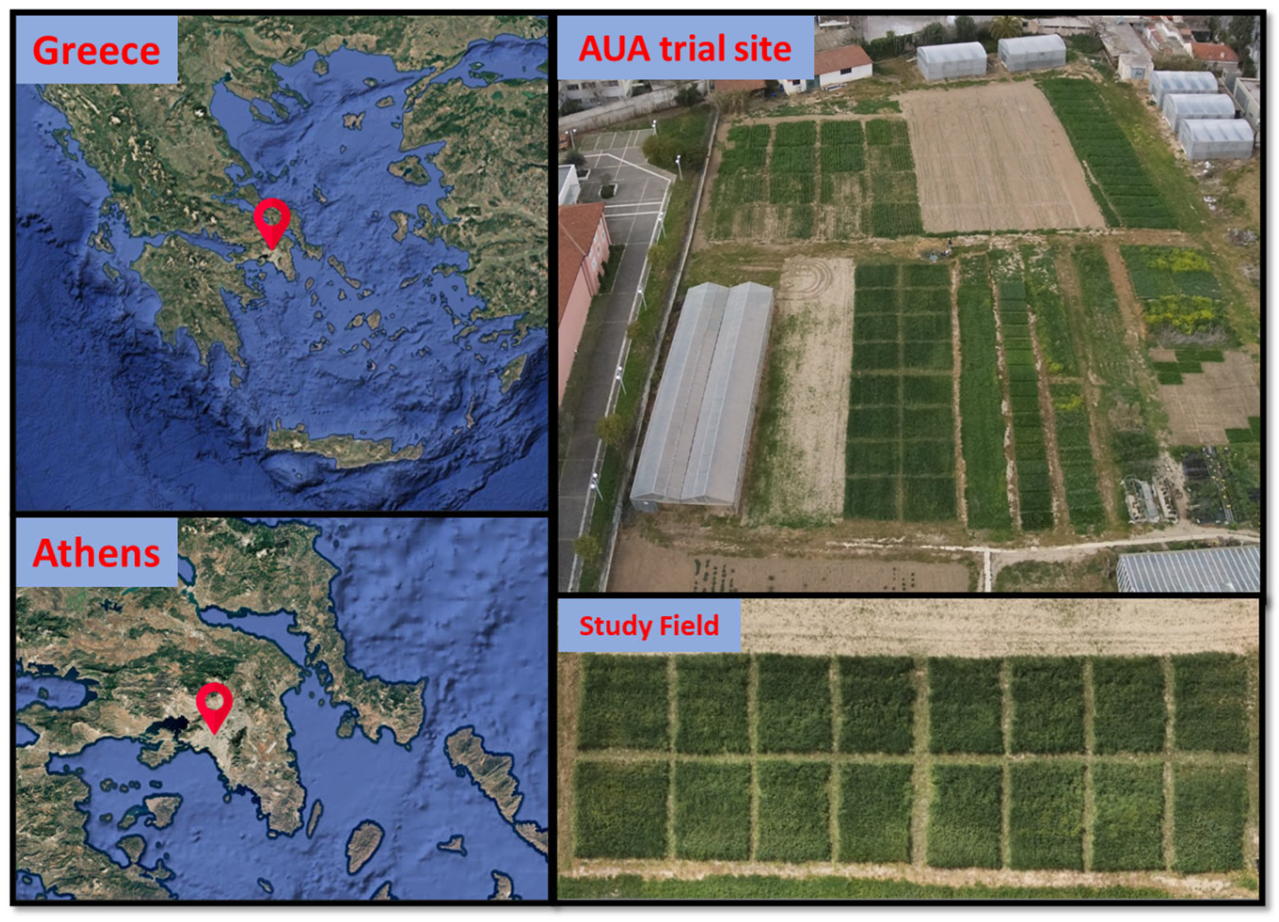
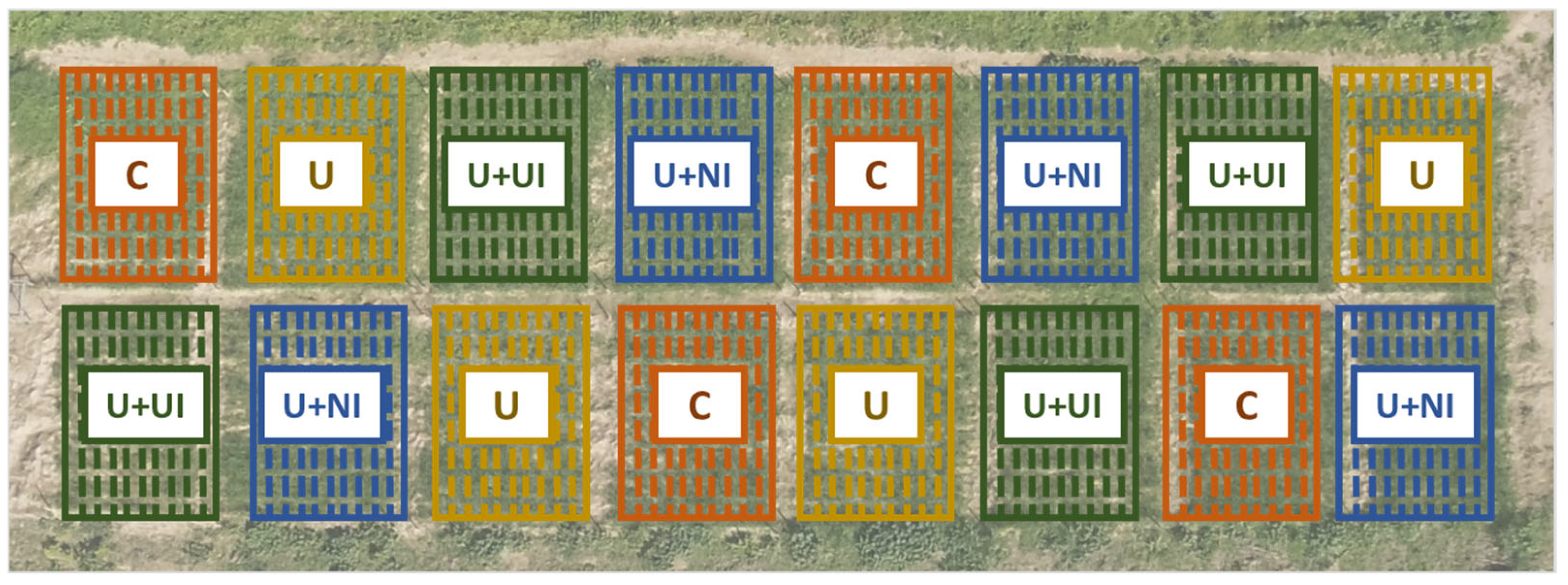
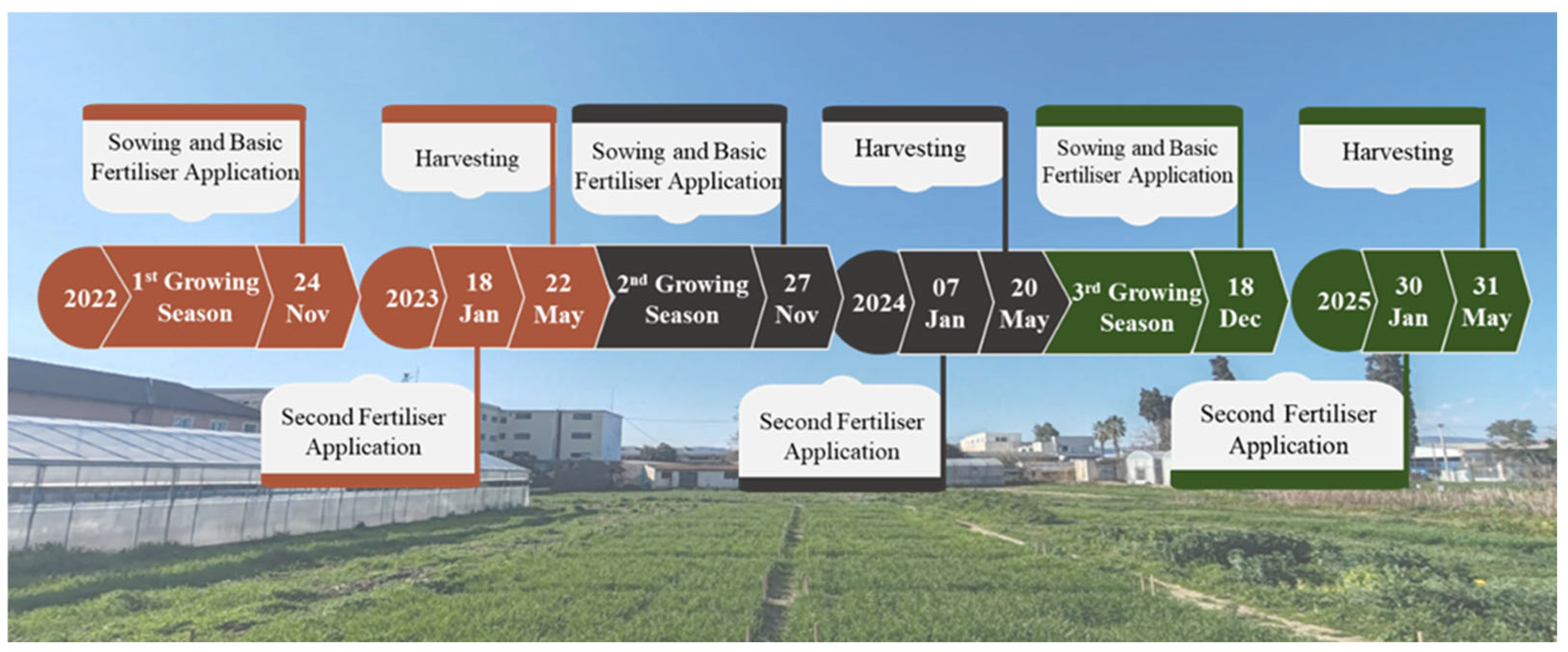
2.1. Experimental Site and Design
2.2. Measured Parameters
2.2.1. Soil Sampling and Analysis
2.2.2. Phenological Staging Based on Growing Degree Days
2.2.3. Agronomic Traits and Qualitative Characteristics
2.2.4. Multispectral Indices—UAV Flights
2.3. Statistical Analysis
3. Results
3.1. Soil Properties of the Studied Soil
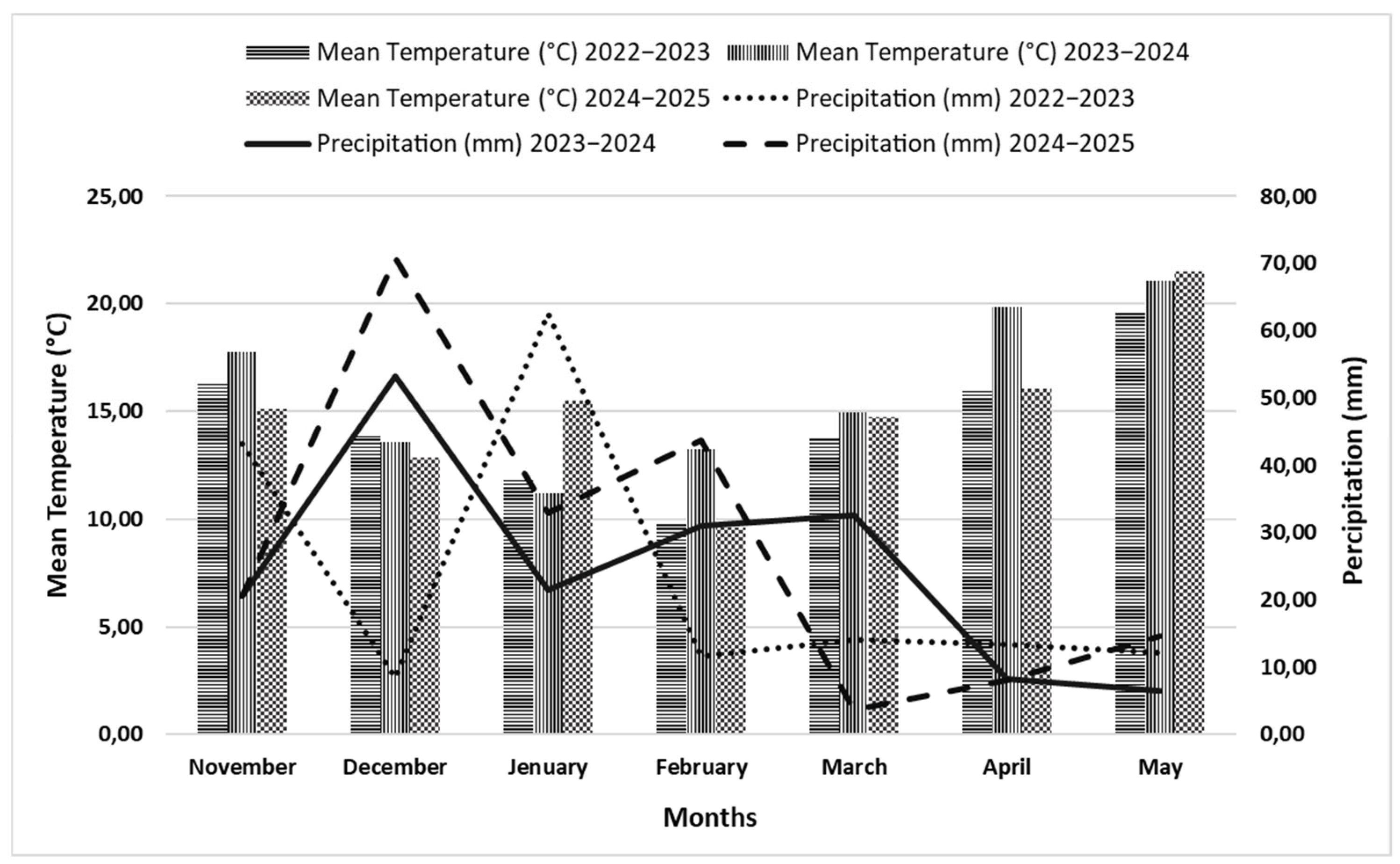
3.2. Growing Degree Days (GDDs)
3.3. Effect of Different Types of N Fertilization on Agronomic Characteristics
3.4. Effect of Different Types of N Fertilization on Multispectral Indices
3.5. Effect of Different Types of N Fertilization on Qualitative Characteristics
4. Discussion
4.1. Nitrogen Strategy Performance
4.2. Temporal Response to EEFs
4.3. Remote Sensing Insight
4.4. Sustainability Implications
4.5. Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ν | Nitrogen |
| NUE | Nitrogen Use Efficiency |
| EC | Electrical Conductivity |
| NBPT | N-(n-butyl) thiophosphoric triamide |
| EEFs | Enhanced-Efficiency Fertilisers |
| DCD | Dicyandiamide |
| NIs | Nitrification Inhibitors |
| SOM | Soil Organic Matter |
| DTPA | Diethylenetriaminepentaacetic Acid |
| VIs | Vegetation Indices |
| UAV | Unmanned Aerial Vehicles |
| GDS | Ground Sampling Distance |
References
- Ali, A.; Martelli, R.; Lupia, F.; Barbanti, L. Assessing Multiple Years’ Spatial Variability of Crop Yields Using Satellite Vegetation Indices. Remote Sens. 2019, 11, 2384. [Google Scholar] [CrossRef]
- Miller, J.O.; Shober, A.L.; Taraila, J. Assessing Relationships of Cover Crop Biomass and Nitrogen Content to Multispectral Imagery. Agron. J. 2024, 116, 1417–1427. [Google Scholar] [CrossRef]
- Tilly, N.; Aasen, H.; Bareth, G. Fusion of Plant Height and Vegetation Indices for the Estimation of Barley Biomass. Remote Sens. 2015, 7, 11449–11480. [Google Scholar] [CrossRef]
- Vidican, R.; Mălinaș, A.; Ranta, O.; Moldovan, C.; Marian, O.; Ghețe, A.; Ghișe, C.R.; Popovici, F.; Cătunescu, G.M. Using Remote Sensing Vegetation Indices for the Discrimination and Monitoring of Agricultural Crops: A Critical Review. Agronomy 2023, 13, 3040. [Google Scholar] [CrossRef]
- Walsh, O.S.; Shafian, S.; Marshall, J.M.; Jackson, C.; McClintick-Chess, J.R.; Blanscet, S.M.; Swoboda, K.; Thompson, C.; Belmont, K.M.; Walsh, W.L. Assessment of UAV Based Vegetation Indices for Nitrogen Concentration Estimation in Spring Wheat. Adv. Remote Sens. 2018, 07, 71–90. [Google Scholar] [CrossRef]
- Barnes, M.C.; Addai-Mensah, J.; Gerson, A.R. A Methodology for Quantifying Sodalite and Cancrinite Phase Mixtures and the Kinetics of the Sodalite to Cancrinite Phase Transformation. Microporous Mesoporous Mater. 1999, 31, 303–319. [Google Scholar] [CrossRef]
- Daughtry, C. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a Green Channel in Remote Sensing of Global Vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Samborski, S.M.; Tremblay, N.; Fallon, E. Strategies to Make Use of Plant Sensors-Based Diagnostic Information for Nitrogen Recommendations. Agron. J. 2009, 101, 800–816. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Lemaire, G.; Gastal, F. Nitrogen, Plant Growth and Crop Yield. In Plant Nitrogen; Lea, P.J., Morot-Gaudry, J.-F., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 343–367. ISBN 978-3-642-08731-8. [Google Scholar]
- Rehman, H.U.; Alharby, H.F.; Al-Zahrani, H.S.; Bamagoos, A.A.; Alsulami, N.B.; Alabdallah, N.M.; Iqbal, T.; Wakeel, A. Enriching Urea with Nitrogen Inhibitors Improves Growth, N Uptake and Seed Yield in Quinoa (Chenopodium Quinoa Willd) Affecting Photochemical Efficiency and Nitrate Reductase Activity. Plants 2022, 11, 371. [Google Scholar] [CrossRef]
- Bagheri, N. Development of a High-Resolution Aerial Remote-Sensing System for Precision Agriculture. Int. J. Remote Sens. 2017, 38, 2053–2065. [Google Scholar] [CrossRef]
- Lu, N.; Wang, W.; Zhang, Q.; Li, D.; Yao, X.; Tian, Y.; Zhu, Y.; Cao, W.; Baret, F.; Liu, S.; et al. Estimation of Nitrogen Nutrition Status in Winter Wheat from Unmanned Aerial Vehicle Based Multi-Angular Multispectral Imagery. Front. Plant Sci. 2019, 10, 1601. [Google Scholar] [CrossRef] [PubMed]
- Gallegos-Cedillo, V.M.; Diánez, F.; Nájera, C.; Santos, M. Plant Agronomic Features Can Predict Quality and Field Performance: A Bibliometric Analysis. Agronomy 2021, 11, 2305. [Google Scholar] [CrossRef]
- Tshikunde, N.M.; Mashilo, J.; Shimelis, H.; Odindo, A. Agronomic and Physiological Traits, and Associated Quantitative Trait Loci (QTL) Affecting Yield Response in Wheat (Triticum aestivum L.): A Review. Front. Plant Sci. 2019, 10, 1428. [Google Scholar] [CrossRef]
- Kakabouki, I.; Beslemes, D.F.; Tigka, E.L.; Folina, A.; Karydogianni, S.; Zisi, C.; Papastylianou, P. Performance of Six Genotypes of Tritordeum Compare to Bread Wheat under East Mediterranean Condition. Sustainability 2020, 12, 9700. [Google Scholar] [CrossRef]
- Olesen, J.E.; Børgesen, C.D.; Elsgaard, L.; Palosuo, T.; Rötter, R.P.; Skjelvåg, A.O.; Peltonen-Sainio, P.; Börjesson, T.; Trnka, M.; Ewert, F.; et al. Changes in Time of Sowing, Flowering and Maturity of Cereals in Europe under Climate Change. Food Addit. Contam. Part A 2012, 29, 1527–1542. [Google Scholar] [CrossRef]
- Cabo, S.; Carvalho, A.; Rocha, L.; Martin, A.; Lima-Brito, J. IRAP, REMAP and ISSR Fingerprinting in Newly Formed Hexaploid Tritordeum (X Tritordeum Ascherson et Graebner) and Respective Parental Species. Plant Mol. Biol. Report. 2014, 32, 761–770. [Google Scholar] [CrossRef]
- Martín, A.; Alvarez, J.B.; Martín, L.M.; Barro, F.; Ballesteros, J. The Development of Tritordeum: A Novel Cereal for Food Processing. J. Cereal Sci. 1999, 30, 85–95. [Google Scholar] [CrossRef]
- Villegas, D.; Casadesús, J.; Atienza, S.G.; Martos, V.; Maalouf, F.; Karam, F.; Aranjuelo, I.; Nogués, S. Tritordeum, Wheat and Triticale Yield Components under Multi-Local Mediterranean Drought Conditions. Field Crops Res. 2010, 116, 68–74. [Google Scholar] [CrossRef]
- Visioli, G.; Lauro, M.; Vamerali, T.; Dal Cortivo, C.; Panozzo, A.; Folloni, S.; Piazza, C.; Ranieri, R. A Comparative Study of Organic and Conventional Management on the Rhizosphere Microbiome, Growth and Grain Quality Traits of Tritordeum. Agronomy 2020, 10, 1717. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Cabrera-Bosquet, L.; Araus, J.L.; Nogués, S. Carbon and Nitrogen Partitioning during the Post-anthesis Period Is Conditioned by N Fertilisation and Sink Strength in Three Cereals. Plant Biol. 2013, 15, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, H.; Otto, R.; Soares, J.R.; Silva, A.G.D.B. Agronomic Efficiency of NBPT as a Urease Inhibitor: A Review. J. Adv. Res. 2018, 13, 19–27. [Google Scholar] [CrossRef]
- Cantarella, H.; Mattos, D.; Quaggio, J.A.; Rigolin, A.T. Fruit Yield of Valencia Sweet Orange Fertilized with Different N Sources and the Loss of Applied N. Nutr. Cycl. Agroecosyst. 2003, 67, 215–223. [Google Scholar] [CrossRef]
- Dawar, K.; Rahman, U.; Alam, S.S.; Tariq, M.; Khan, A.; Fahad, S.; Datta, R.; Danish, S.; Saud, S.; Noor, M. Nitrification Inhibitor and Plant Growth Regulators Improve Wheat Yield and Nitrogen Use Efficiency. J. Plant Growth Regul. 2022, 41, 216–226. [Google Scholar] [CrossRef]
- Meena, A.K.; Singh, D.K.; Pandey, P.C.; Nanda, G. Dynamics of Dry Matter and Nitrogen Distribution in Transplanted Rice on Mollisols. J. Plant Nutr. 2019, 42, 749–758. [Google Scholar] [CrossRef]
- Silva, A.G.B.; Sequeira, C.H.; Sermarini, R.A.; Otto, R. Urease Inhibitor NBPT on Ammonia Volatilization and Crop Productivity: A Meta-Analysis. Agron. J. 2017, 109, 1–13. [Google Scholar] [CrossRef]
- Cui, M.; Sun, X.; Hu, C.; Di, H.J.; Tan, Q.; Zhao, C. Effective Mitigation of Nitrate Leaching and Nitrous Oxide Emissions in Intensive Vegetable Production Systems Using a Nitrification Inhibitor, Dicyandiamide. J. Soils Sediments 2011, 11, 722–730. [Google Scholar] [CrossRef]
- Soares, J.R.; Souza, B.R.; Mazzetto, A.M.; Galdos, M.V.; Chadwick, D.R.; Campbell, E.E.; Jaiswal, D.; Oliveira, J.C.; Monteiro, L.A.; Vianna, M.S.; et al. Mitigation of Nitrous Oxide Emissions in Grazing Systems through Nitrification Inhibitors: A Meta-Analysis. Nutr. Cycl. Agroecosyst. 2023, 125, 359–377. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. A Recalibration of the Hydrometer Method for Making Mechanical Analysis of Soils. Agron. J. 1951, 43, 434–438. [Google Scholar] [CrossRef]
- Klute, A.; Page, A.L. (Eds.) Methods of Soil Analysis, 2nd ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; ISBN 978-0-89118-088-3. [Google Scholar]
- Nelson, D.W.; Sommers, E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; Wiley: Hoboken, NJ, USA, 1996. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Government Printing Office: Washington, DC, USA, 1954.
- Bremner, J.M. Determination of Nitrogen in Soil by the Kjeldahl Method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Thomas, G.W. Exchangeable Cations. In Agronomy Monographs; Page, A.L., Ed.; Wiley: Hoboken, NJ, USA, 1982; Volume 9, pp. 159–165. ISBN 978-0-89118-072-2. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Mcmaster, G. Growing Degree-Days: One Equation, Two Interpretations. Agric. For. Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Open Agrar Repositorium: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Nitrogen Analysis of Soil and Plant Tissues. J. AOAC Int. 1980, 63, 770–778. [Google Scholar] [CrossRef]
- Shewry, P.R.; Brouns, F.; Dunn, J.; Hood, J.; Burridge, A.J.; America, A.H.P.; Gilissen, L.; Proos-Huijsmans, Z.A.M.; Van Straaten, J.P.; Jonkers, D.; et al. Comparative Compositions of Grain of Tritordeum, Durum Wheat and Bread Wheat Grown in Multi-Environment Trials. Food Chem. 2023, 423, 136312. [Google Scholar] [CrossRef] [PubMed]
- Rouse, W.; Haas, R.H. Monitoring Vegetation Systems in the Great Plains with Erts; NASA: Washington, DC, USA, 1974. [Google Scholar]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote Estimation of Canopy Chlorophyll Content in Crops. Geophys. Res. Lett. 2005, 32, 2005GL022688. [Google Scholar] [CrossRef]
- Bailey, S.J. Using Growing Degree Days to Predict Plant Stages; MSU Extension Service: Bozeman, MT, USA, 2001. [Google Scholar]
- Litke, L.; Gaile, Z.; Ruža, A. Effect of Nitrogen Fertilization on Winter Wheat Yield and Yield Quality. Agron. Res. 2018, 16, 500–509. [Google Scholar] [CrossRef]
- Arduini, I.; Masoni, A.; Ercoli, L.; Mariotti, M. Grain Yield, and Dry Matter and Nitrogen Accumulation and Remobilization in Durum Wheat as Affected by Variety and Seeding Rate. Eur. J. Agron. 2006, 25, 309–318. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. Grain Nitrogen Source Changes over Time in Maize: A Review. Crop Sci. 2013, 53, 366–377. [Google Scholar] [CrossRef]
- Lan, T.; He, X.; Wang, Q.; Deng, O.; Zhou, W.; Luo, L.; Chen, G.; Zeng, J.; Yuan, S.; Zeng, M.; et al. Synergistic Effects of Biological Nitrification Inhibitor, Urease Inhibitor, and Biochar on NH3 Volatilization, N Leaching, and Nitrogen Use Efficiency in a Calcareous Soil–Wheat System. Appl. Soil Ecol. 2022, 174, 104412. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, J.J.; Wei, Z.; Dodla, S.K.; Fultz, L.M.; Gaston, L.A.; Xiao, R.; Park, J.; Scaglia, G. Nitrification Inhibitors Reduce Nitrogen Losses and Improve Soil Health in a Subtropical Pastureland. Geoderma 2021, 388, 114947. [Google Scholar] [CrossRef]
- Guzman-Bustamante, I.; Schulz, R.; Müller, T.; Ruser, R. Split N Application and DMP Based Nitrification Inhibitors Mitigate N2O Losses in a Soil Cropped with Winter Wheat. Nutr. Cycl. Agroecosystems 2022, 123, 119–135. [Google Scholar] [CrossRef]
- Olšovská, K.; Rybarova, Z.; Sytar, O. Effectiveness of N Fertilizers with Nitrification Inhibitors on Winter Barley Nutrition and Yield. Sustainability 2025, 17, 2610. [Google Scholar] [CrossRef]
- Rawal, N.; Pande, K.R.; Shrestha, R.; Vista, S.P. Nutrient Use Efficiency (NUE) of Wheat (Triticum aestivum L.) as Affected by NPK Fertilization. PLoS ONE 2022, 17, e0262771. [Google Scholar] [CrossRef]
- Magney, T.S.; Eitel, J.U.H.; Huggins, D.R.; Vierling, L.A. Proximal NDVI Derived Phenology Improves In-Season Predictions of Wheat Quantity and Quality. Agric. For. Meteorol. 2016, 217, 46–60. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Wen, T.; Xu, J.; Huang, B.; Yan, S.; Gao, G.; Zhao, Y.; Li, H.; Qiao, J.; et al. On Correlation between Canopy Vegetation and Growth Indexes of Maize Varieties with Different Nitrogen Efficiencies. Open Life Sci. 2023, 18, 20220566. [Google Scholar] [CrossRef] [PubMed]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated Narrow-Band Vegetation Indices for Prediction of Crop Chlorophyll Content for Application to Precision Agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Sharifi, A. Remotely Sensed Vegetation Indices for Crop Nutrition Mapping. J. Sci. Food Agric. 2020, 100, 5191–5196. [Google Scholar] [CrossRef]
- Hao, T.; Zhu, Q.; Zeng, M.; Shen, J.; Shi, X.; Liu, X.; Zhang, F.; De Vries, W. Impacts of Nitrogen Fertilizer Type and Application Rate on Soil Acidification Rate under a Wheat-Maize Double Cropping System. J. Environ. Manag. 2020, 270, 110888. [Google Scholar] [CrossRef]

| Multispectral Indices | Equation | Reference |
|---|---|---|
| NDVI | [41] (Rouse & Haas, 1974) | |
| GNDVI | [42] (Gitelson et al., 2005) | |
| NDRE | [6] (Barnes et al., 1999) | |
| MCARI | ) | [7] (Daughtry, 2000) |
| Soil Properties | pH | EC | SOM | N | P | K | Fe | Cu | Mn | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 7.96 | 219 | 2.54 | 0.19 | 30.65 | 285.00 | 4.56 | 8.94 | 12.77 | 8.41 | |
| Season 2024–2025 | C | 7.82 | 474 | 2.50 | 0.17 | 27.20 | 207.80 | 4.12 | 8.45 | 11.56 | 8.45 |
| U | 7.73 | 485 | 2.51 | 0.19 | 22.85 | 211.10 | 3.96 | 8.26 | 12.36 | 8.22 | |
| U+NI | 7.78 | 522 | 2.48 | 0.20 | 23.07 | 216.40 | 4.05 | 8.32 | 12.04 | 8.17 | |
| U+UI | 7.76 | 553 | 2.46 | 0.21 | 22.13 | 225.00 | 4.10 | 8.56 | 11.78 | 8.34 | |
| Growing Season | 2022–2023 | 2023–2024 | 2024–2025 |
|---|---|---|---|
| Emergence [BBCH 00–19] | 127.60 | 129.65 | 130.45 |
| Tillering [BBCH 20–29] | 373.15 | 369.45 | 370.80 |
| Stem Elongation [BBCH 30–39] | 597.80 | 597.00 | 598.30 |
| Flowering [BBCH 60–69] | 811.15 | 807.20 | 810.50 |
| Seed Filling [BBCH 70–79] | 1077.05 | 1072.40 | 1078.05 |
| Ripening [BBCH 80–89] | 1430.40 | 1479.05 | 1449.15 |
| Senescence [BBCH 90–99] | 1617.20 | 1795.35 | 1606.45 |
| Tillering [BBCH 20–29] | |||||
|---|---|---|---|---|---|
| Treatment | C | U | U+NI | U+UI | |
| Mean ± SD 2023 | Height | 23.433 ± 1.159 | 23.673 ± 1.780 | 25.268 ± 1.464 | 25.000 ± 1.284 |
| F | 1.646 ns | ||||
| Fresh Weight | 1.192 ± 0.220 | 1.155 ± 0.132 | 1.367 ± 0.280 | 1.234 ± 0.082 | |
| F | 0.907 ns | ||||
| Mean ± SD 2024 | Height | 21.017 ± 1.394 a | 23.775 ± 1.220 b | 24.500 ± 0.850 b | 23.683 ± 1.101 b |
| F | 6.973 * | ||||
| Fresh Weight | 1.390 ± 0.383 | 1.314 ± 0.394 | 1.457 ± 0.225 | 1.665 ± 0.458 | |
| F | 0.646 ns | ||||
| Mean ± SD 2025 | Height | 15.025 ± 0.791 | 16.050 ± 0.775 | 15.050 ± 0.795 | 15.150 ± 2.293 |
| F | 0.541 ns | ||||
| Fresh Weight | 0.628 ± 0.061 | 0.656 ± 0.165 | 0.665 ± 0.167 | 0.665 ± 0.035 | |
| F | 0.079 ns | ||||
| Stem Elongation [BBCH 30–39] | |||||
|---|---|---|---|---|---|
| Treatment | C | U | U+NI | U+UI | |
| Mean ± SD 2023 | Height | 28.158 ± 1.977 a | 31.942 ± 1.947 b | 32.692 ± 0.394 b | 36.208 ± 1.213 c |
| F | 12.463 * | ||||
| Fresh Weight | 2.506 ± 0.197 | 2.550 ± 0.202 | 2.632 ± 0.000 | 2.526 ± 0.426 | |
| F | 0.102 ns | ||||
| Mean ± SD 2024 | Height | 33.475 ± 1.229 a | 43.018 ± 1.068 b | 47.108 ± 1.014 c | 43.700 ± 1.909 b |
| F | 74.699 *** | ||||
| Fresh Weight | 4.874 ± 0.411 | 5.123 ± 1.067 | 5.130 ± 1.164 | 4.488 ± 0.501 | |
| F | 0.500 ns | ||||
| Mean ± SD 2025 | Height | 27.983 ± 1.601 a | 32.742 ± 2.113 b | 30.950 ± 0.923 ab | 33.050 ± 1.801 b |
| F | 7.771 * | ||||
| Fresh Weight | 3.168 ± 0.135 a | 3.500 ± 0.098 b | 4.041 ± 0.174 c | 3.782 ± 0.216 bc | |
| F | 21.490 ** | ||||
| Flowering [BBCH 60–69] | |||||
|---|---|---|---|---|---|
| Treatment | C | U | U+NI | U+UI | |
| Mean ± SD 2023 | Height | 55.208 ± 2.878 a | 60.925 ± 1.820 b | 60.574 ± 2.661 b | 62.883 ± 2.400 b |
| F | 7.075 * | ||||
| Fresh Weight | 8.895 ± 0.762 | 9.904 ± 0.767 | 9.649 ± 0.723 | 9.510 ± 1.140 | |
| F | 0.983 ns | ||||
| Mean ± SD 2024 | Height | 65.205 ± 1.606 a | 74.905 ± 1.701 b | 78.151 ± 1.949 b | 77.232 ± 1.516 b |
| F | 48.761 *** | ||||
| Fresh Weight | 11.721 ± 1.770 | 13.271 ± 1.222 | 12.599 ± 1.475 | 13.985 ± 0.956 | |
| F | 1.932 ns | ||||
| Mean ± SD 2025 | Height | 66.508 ± 1.425 a | 73.125 ± 1.243 b | 78.067 ± 1.156 c | 73.658 ± 1.658 b |
| F | 47.456 *** | ||||
| Fresh Weight | 8.356 ± 1.899 a | 10.263 ± 2.008 ab | 11.217 ± 2.330 ab | 12.386 ± 1.252 b | |
| F | 3.175 * | ||||
| Seed Filling [BBCH 70–79] | |||||
|---|---|---|---|---|---|
| Treatment | C | U | U+NI | U+UI | |
| Mean ± SD 2023 | Height | 64.833 ± 2.704 a | 74.317 ± 2.745 b | 79.230 ± 1.725 c | 84.292 ± 2.279 d |
| F | 47.808 ** | ||||
| Fresh Weight | 9.118 ± 0.669 a | 15.173 ± 2.227 c | 12.633 ± 0.531 b | 14.456 ± 0.812 bc | |
| F | 18.450 ** | ||||
| Mean ± SD 2024 | Height | 72.483 ± 1.569 a | 83.418 ± 2.131 b | 87.633 ± 1.666 c | 92.833 ± 2.285 d |
| F | 79.701 *** | ||||
| Fresh Weight | 10.925 ± 1.103 a | 15.225 ± 0.497 b | 13.598 ± 1.729 b | 15.493 ± 1.494 b | |
| F | 10.531 * | ||||
| Mean ± SD 2025 | Height | 73.942 ± 3.163 a | 79.815 ± 2.628 b | 85.117 ± 1.168 c | 82.542 ± 1.980 bc |
| F | 16.548 *** | ||||
| Fresh Weight | 10.498 ± 1.081 | 12.658 ± 1.375 | 13.963 ± 2.503 | 13.545 ± 1.271 | |
| F | 3.489 ns | ||||
| Ripening [BBCH 80–89]—Senescence [BBCH 90–99] | |||||
|---|---|---|---|---|---|
| Treatment | C | U | U+NI | U+UI | |
| Mean ± SD 2023 | Height | 64.833 ± 2.704 a | 71.534 ± 2.703 b | 72.115 ± 1.799 b | 73.705 ± 1.770 b |
| F | 27.728 ** | ||||
| Fresh Weight | 9.118 ± 0.669 a | 11.627 ± 0.884 b | 10.174 ± 0.687 b | 11.354 ± 1.076 b | |
| F | 25.937 ** | ||||
| Spike Length | 7.068 ± 0.302 a | 8.500 ± 0.782 b | 8.858 ± 0.637 b | 8.792 ± 0.629 b | |
| F | 7.490 * | ||||
| Mean ± SD 2024 | Height | 68.417 ± 2.217 a | 77.875 ± 1.259 b | 82.000 ± 1.639 c | 81.658 ± 1.948 c |
| F | 49.383 ** | ||||
| Fresh Weight | 8.298 ± 0.408 a | 13.358 ± 1.519 bc | 11.388 ± 1.182 b | 14.733 ± 1.390 c | |
| F | 21.489 ** | ||||
| Spike Length | 8.333 ± 0.471 | 9.125 ± 0.599 | 9.167 ± 0.430 | 9.250 ± 0.167 | |
| F | 3.671 ns | ||||
| Mean ± SD 2025 | Height | 66.989 ± 1.932 a | 73.067 ± 3.113 b | 75.900 ± 2.156 b | 73.525 ± 0.856 b |
| F | 12.262 * | ||||
| Fresh Weight | 9.555 ± 1.721 | 11.003 ± 2.374 | 12.185 ± 2.337 | 11.783 ± 3.076 | |
| F | 0.915 ns | ||||
| Spike Length | 8.875 ± 0.831 | 8.917 ± 0.399 | 8.232 ± 0.136 | 8.217 ± 0.382 | |
| F | 2.376 ns | ||||
| Tillering [BBCH 20–29] | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | GNDVI | MCARI | NDRE | NDVI | ||||||||||||
| Treatment | C | U | U+NI | U+UI | C | U | U+NI | U+UI | C | U | U+NI | U+UI | C | U | U+NI | U+UI |
| Mean ± SD 2023 | 0.548 ± 0.011 b | 0.502 ± 0.010 a | 0.543 ± 0.012 ab | 0.505 ± 0.013 a | 0.540 ± 0.016 | 0.479 ± 0.016 | 0.531 ± 0.018 | 0.491 ± 0.020 | 0.178 ± 0.006 ab | 0.157 ± 0.006 a | 0.181 ± 0.007 b | 0.165 ± 0.007 ab | 0.683 ± 0.015 b | 0.617 ± 0.013 a | 0.665 ± 0.016 ab | 0.613 ± 0.018 a |
| F | 4.268 * | 2.859 ns | 3.078 * | 5.013 ** | ||||||||||||
| Mean ± SD 2024 | 0.626 ± 0.006 | 0.635 ± 0.007 | 0.627 ± 0.007 | 0.617 ± 0.008 | 0.526 ± 0.012 | 0.479 ± 0.013 | 0.489 ± 0.014 | 0.485 ± 0.013 | 0.217 ± 0.004 ab | 0.231 ± 0.005 c | 0.224 ± 0.005 ab | 0.214 ± 0.005 a | 0.809 ± 0.006 | 0.815 ± 0.008 | 0.814 ± 0.008 | 0.798 ± 0.009 |
| F | 1.116 ns | 2.657 ns | 3.137 * | 0.965 ns | ||||||||||||
| Mean ± SD 2025 | 0.367 ± 0.009 | 0.367 ± 0.009 | 0.367 ± 0.011 | 0.383 ± 0.010 | 0.340 ± 0.014 | 0.357 ± 0.013 | 0.329 ± 0.015 | 0.351 ± 0.014 | 0.078 ± 0.004 | 0.076 ± 0.004 | 0.078 ± 0.005 | 0.086 ± 0.005 | 0.451 ± 0.015 | 0.469 ± 0.014 | 0.466 ± 0.016 | 0.489 ± 0.015 |
| F | 0.593 ns | 0.783 ns | 0.960 ns | 1.061 ns | ||||||||||||
| Stem Elongation [BBCH 30–39] | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | GNDVI | MCARI | NDRE | NDVI | ||||||||||||
| Treatment | C | U | U+NI | U+UI | C | U | U+NI | U+UI | C | U | U+NI | U+UI | C | U | U+NI | U+UI |
| Mean ± SD 2023 | 0.733 ± 0.004 | 0.738 ± 0.005 | 0.723 ± 0.005 | 0.722 ± 0.006 | 0.613 ± 0.014 | 0.651 ± 0.014 | 0.611 ± 0.013 | 0.614 ± 0.013 | 0.288 ± 0.004 | 0.294 ± 0.005 | 0.291 ± 0.004 | 0.285 ± 0.005 | 0.879 ± 0.003 | 0.887 ± 0.003 | 0.874 ± 0.003 | 0.874 ± 0.005 |
| F | 2.517 ns | 1.993 ns | 0.780 ns | 2.850 ns | ||||||||||||
| Mean ± SD 2024 | 0.764 ± 0.003 | 0.768 ± 0.003 | 0.763 ± 0.003 | 0.759 ± 0.004 | 0.980 ± 0.010 | 0.986 ± 0.011 | 1.005 ± 0.013 | 0.989 ± 0.013 | 0.367 ± 0.003 | 0.374 ± 0.004 | 0.365 ± 0.004 | 0.361 ± 0.004 | 0.900 ± 0.002 ab | 0.903 ± 0.002 b | 0.902 ± 0.002 ab | 0.895 ± 0.003 a |
| F | 1.176 ns | 0.997 ns | 2.234 ns | 2.824 * | ||||||||||||
| Mean ± SD 2025 | 0.746 ± 0.007 | 0.751 ± 0.005 | 0.758 ± 0.006 | 0.746 ± 0.005 | 0.878 ± 0.000 | 0.877 ± 0.012 | 0.875 ± 0.013 | 0.862 ± 0.011 | 0.339 ± 0.007 | 0.348 ± 0.005 | 0.353 ± 0.005 | 0.346 ± 0.005 | 0.891 ± 0.015 | 0.893 ± 0.005 | 0.899 ± 0.005 | 0.890 ± 0.004 |
| F | 0.999 ns | 0.401 ns | 1.321 ns | 0.802 ns | ||||||||||||
| Flowering [BBCH 60–69] | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | GNDVI | MCARI | NDRE | NDVI | ||||||||||||
| Treatment | C | U | U+NI | U+UI | C | U | U+NI | U+UI | C | U | U+NI | U+UI | C | U | U+NI | U+UI |
| Mean ± SD 2023 | 0.744 ± 0.005 | 0.751 ± 0.005 | 0.741 ± 0.005 | 0.745 ± 0.006 | 0.841 ± 0.014 | 0.871 ± 0.015 | 0.828 ± 0.014 | 0.841 ± 0.014 | 0.355 ± 0.005 | 0.356 ± 0.005 | 0.355 ± 0.005 | 0.349 ± 0.008 | 0.875 ± 0.003 | 0.875 ± 0.003 | 0.873 ± 0.003 | 0.869 ± 0.004 |
| F | 0.596 ns | 1.635 ns | 0.248 ns | 0.805 ns | ||||||||||||
| Mean ± SD 2024 | 0.799 ± 0.004 bc | 0.802 ± 0.004 c | 0.761 ± 0.006 a | 0.785 ± 0.005 b | 0.923 ± 0.012 | 0.936 ± 0.011 | 0.947 ± 0.015 | 0.924 ± 0.012 | 0.417 ± 0.005 bc | 0.422 ± 0.005 c | 0.370 ± 0.007 a | 0.400 ± 0.006 b | 0.912 ± 0.002 b | 0.914 ± 0.002 b | 0.892 ± 0.003 a | 0.906 ± 0.002 b |
| F | 16.077 *** | 0.777 ns | 17.296 *** | 14.871 *** | ||||||||||||
| Mean ± SD 2025 | 0.766 ± 0.006 ab | 0.767 ± 0.007 ab | 0.777 ± 0.007 b | 0.749 ± 0.007 a | 0.786 ± 0.014 | 0.778 ± 0.015 | 0.799 ± 0.016 | 0.770 ± 0.017 | 0.399 ± 0.006 | 0.403 ± 0.006 | 0.411 ± 0.006 | 0.388 ± 0.006 | 0.871 ± 0.005 ab | 0.868 ± 0.006 ab | 0.882 ± 0.006 b | 0.852 ± 0.007 a |
| F | 3.109 * | 0.613 ns | 2.529 ns | 4.267 ** | ||||||||||||
| Seed Filling [BBCH 70–79] | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | GNDVI | MCARI | NDRE | NDVI | ||||||||||||
| Treatment | C | U | U+NI | U+UI | C | U | U+NI | U+UI | C | U | U+NI | U+UI | C | U | U+NI | U+UI |
| Mean ± SD 2023 | 0.554 ± 0.008 | 0.560 ± 0.007 | 0.563 ± 0.007 | 0.546 ± 0.008 | 0.444 ± 0.016 | 0.406 ± 0.015 | 0.441 ± 0.015 | 0.435 ± 0.016 | 0.197 ± 0.005 | 0.201 ± 0.005 | 0.212 ± 0.004 | 0.196 ± 0.005 | 0.559 ± 0.010 | 0.548 ± 0.010 | 0.579 ± 0.009 | 0.551 ± 0.011 |
| F | 1.013 ns | 1.285 ns | 2.088 ns | 2.108 ns | ||||||||||||
| Mean ± SD 2024 | 0.577 ± 0.007 | 0.579 ± 0.006 | 0.590 ± 0.007 | 0.569 ± 0.007 | 0.454 ± 0.013 ab | 0.415 ± 0.013 a | 0.475 ± 0.014 b | 0.416 ± 0.012 a | 0.226 ± 0.005 | 0.219 ± 0.004 | 0.226 ± 0.005 | 0.214 ± 0.005 | 0.600 ± 0.009 ab | 0.578 ± 0.009 a | 0.620 ± 0.010 b | 0.577 ± 0.010 a |
| F | 1.747 ns | 5.189 ** | 5.889 ns | 4.735 ** | ||||||||||||
| Mean ± SD 2025 | 0.605 ± 0.009 b | 0.606 ± 0.009 b | 0.637 ± 0.008 c | 0.575 ± 0.009 a | 0.663 ± 0.017 b | 0.658 ± 0.019 b | 0.706 ± 0.019 b | 0.591 ± 0.019 a | 0.257 ± 0.007 b | 0.259 ± 0.007 b | 0.280 ± 0.007 b | 0.229 ± 0.007 a | 0.641 ± 0.012 b | 0.633 ± 0.013 ab | 0.696 ± 0.013 c | 0.593 ± 0.014 a |
| F | 8.545 *** | 6.698 *** | 8.688 *** | 10.616 *** | ||||||||||||
| Ripening [BBCH 80–89]—Senescence [BBCH 90–99] | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | GNDVI | MCARI | NDRE | NDVI | ||||||||||||
| Treatment | C | U | U+NI | U+UI | C | U | U+NI | U+UI | C | U | U+NI | U+UI | C | U | U+NI | U+UI |
| Mean ± SD 2023 | 0.321 ± 0.006 | 0.318 ± 0.006 | 0.325 ± 0.006 | 0.317 ± 0.006 | 0.054 ± 0.004 | 0.052 ± 0.004 | 0.058 ± 0.003 | 0.051 ± 0.004 | 0.060 ± 0.003 | 0.058 ± 0.003 | 0.061 ± 0.003 | 0.054 ± 0.003 | 0.190 ± 0.005 | 0.186 ± 0.005 | 0.194 ± 0.004 | 0.183 ± 0.005 |
| F | 0.453 ns | 0.739 ns | 1.076 ns | 1.125 ns | ||||||||||||
| Mean ± SD 2024 | 0.384 ± 0.066 b | 0.386 ± 0.006 b | 0.359 ± 0.008 a | 0.381 ± 0.005 b | 0.059 ± 0.038 | 0.059 ± 0.006 | 0.059 ± 0.005 | 0.054 ± 0.003 | 0.071 ± 0.029 | 0.069 ± 0.003 | 0.069 ± 0.003 | 0.067 ± 0.002 | 0.249 ± 0.082 b | 0.207 ± 0.005 a | 0.199 ± 0.006 a | 0.204 ± 0.004 a |
| F | 3.932 ** | 2.234 ns | 0.307 ns | 17.072 ** | ||||||||||||
| Mean ± SD 2025 | 0.342 ± 0.008 b | 0.343 ± 0.007 b | 0.358 ± 0.007 b | 0.315 ± 0.007 a | 0.049 ± 0.005 ab | 0.056 ± 0.006 bc | 0.070 ± 0.006 c | 0.036 ± 0.004 a | 0.047 ± 0.003 a | 0.052 ± 0.003 ab | 0.059 ± 0.003 b | 0.041 ± 0.003 a | 0.184 ± 0.006 bc | 0.088 ± 0.016 a | 0.204 ± 0.006 c | 0.165 ± 0.005 b |
| F | 6.126 *** | 7.240 *** | 5.424 ** | 30.932 *** | ||||||||||||
| Treat. | Yield (kg/ha) | Thousands Grain Weight (gr) | Protein Content (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | U | U+NI | U+UI | C | U | U+NI | U+UI | C | U | U+NI | U+UI | |
| Mean ± SD 2023 | 2031.963 ± 36.117 a | 2390.263 ± 42.828 c | 2180.450 ± 41.857 b | 2127.653 ± 41.022 b | 30.400± 0.807 a | 33.350 ± 0.854 b | 33.700 ± 1.232 b | 35.700 ± 0.830 c | 12.409± 0.190 a | 15.282 ± 0.531 b | 14.943 ± 0.322 b | 14.603 ± 0.751 b |
| F | 55.852 *** | 21.328 ** | 27.272 *** | |||||||||
| Mean ± SD 2024 | 1006.000 ± 29.998 ab | 1366.000 ± 45.598 b | 1208.998 ± 43.414 a | 1079.000 ± 26.330 ab | 33.667± 1.422 | 35.733± 1.364 | 35.867± 1.061 | 36.133± 1.422 | 12.797± 1.651 a | 16.040± 0.591 b | 15.178± 0.899 b | 15.433± 0.257 b |
| F | 71.993 *** | 3.013 ns | 8.211 ** | |||||||||
| Mean ± SD 2025 | 1839.063 ± 35.950 a | 2237.500 ± 39.114 b | 2319.050 ± 41.652 c | 2187.750 ± 40.267 b | 30.923± 1.279 | 32.880± 1.775 | 33.358± 1.877 | 33.245± 0.758 | 12.668± 0.247 a | 15.960± 0.533 b | 15.521± 0.273 b | 15.401± 0.113 b |
| F | 115.903 *** | 2.331 ns | 83.125 *** | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulos, G.; Zafeiriou, I.; Georgiou, E.; Oikonomou, A.; Mavroeidis, A.; Stavropoulos, P.; Kakabouki, I.; Fountas, S.; Bilalis, D. Remote Sensing Meets Agronomy: A Three-Year Field Study of Tritordeum’s Response to Enhanced Efficiency Fertilisers. Agronomy 2025, 15, 2244. https://doi.org/10.3390/agronomy15092244
Papadopoulos G, Zafeiriou I, Georgiou E, Oikonomou A, Mavroeidis A, Stavropoulos P, Kakabouki I, Fountas S, Bilalis D. Remote Sensing Meets Agronomy: A Three-Year Field Study of Tritordeum’s Response to Enhanced Efficiency Fertilisers. Agronomy. 2025; 15(9):2244. https://doi.org/10.3390/agronomy15092244
Chicago/Turabian StylePapadopoulos, George, Ioannis Zafeiriou, Evgenia Georgiou, Antonia Oikonomou, Antonios Mavroeidis, Panteleimon Stavropoulos, Ioanna Kakabouki, Spyros Fountas, and Dimitrios Bilalis. 2025. "Remote Sensing Meets Agronomy: A Three-Year Field Study of Tritordeum’s Response to Enhanced Efficiency Fertilisers" Agronomy 15, no. 9: 2244. https://doi.org/10.3390/agronomy15092244
APA StylePapadopoulos, G., Zafeiriou, I., Georgiou, E., Oikonomou, A., Mavroeidis, A., Stavropoulos, P., Kakabouki, I., Fountas, S., & Bilalis, D. (2025). Remote Sensing Meets Agronomy: A Three-Year Field Study of Tritordeum’s Response to Enhanced Efficiency Fertilisers. Agronomy, 15(9), 2244. https://doi.org/10.3390/agronomy15092244










