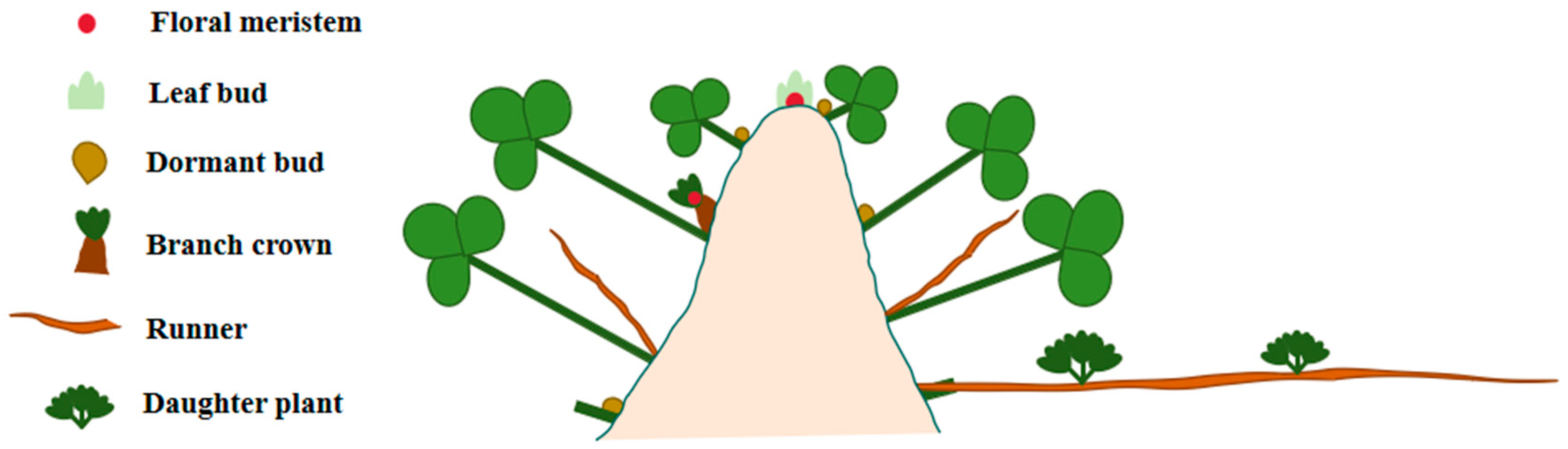
Factors Controlling Runner Formation in Strawberries
Abstract
1. Introduction
2. Environmental Control of Runner Formation
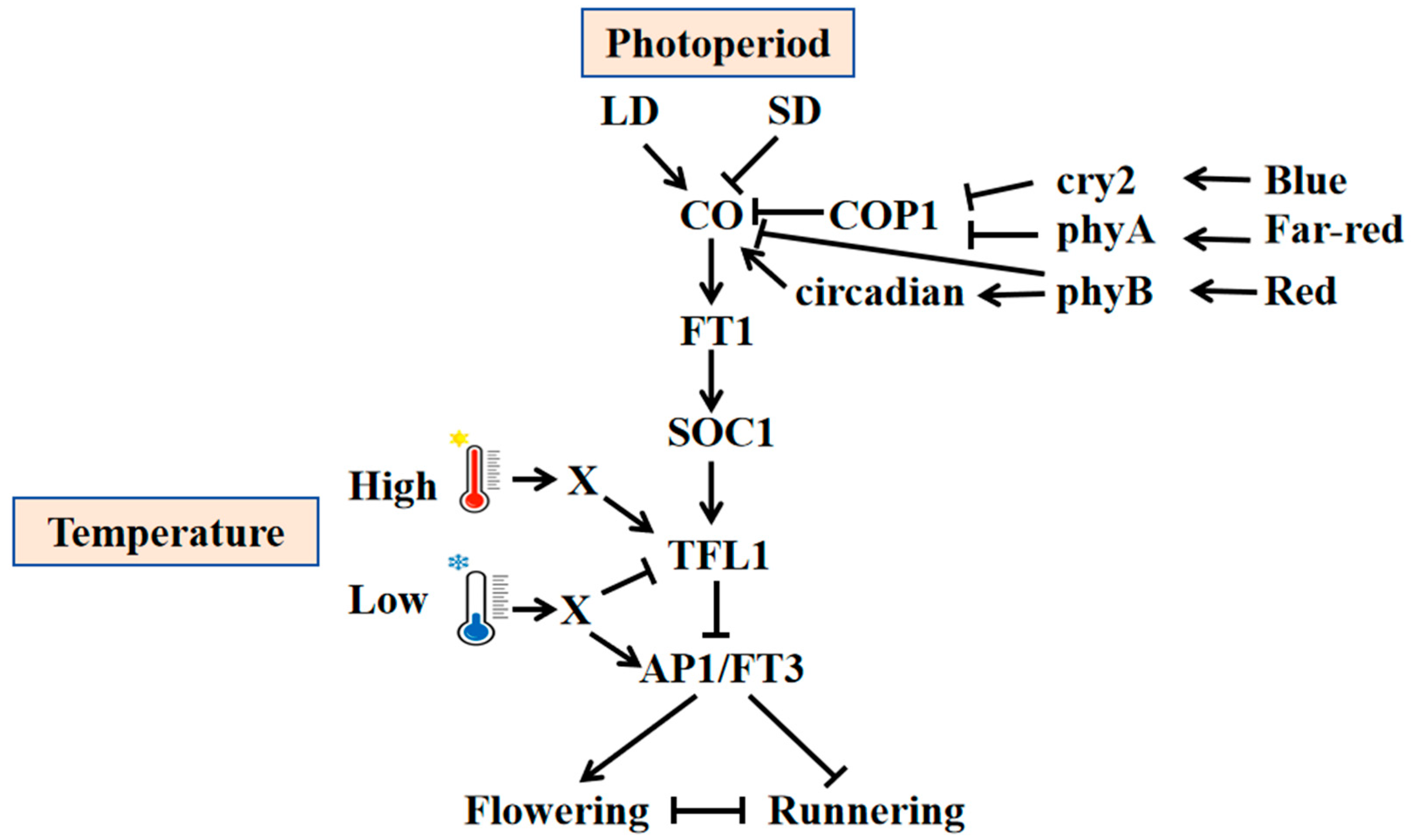
2.1. Light
2.1.1. Photoperiod
2.1.2. Light Intensity
2.1.3. Light Quality
2.2. Temperature
| No. | Variety | Cultivar | Light Condition | Temperature | Optimal Treatment | Reference |
|---|---|---|---|---|---|---|
| 1 | Fragaria × ananassa June-bearing | Seolhyang | 8, 12, or 16 h photoperiod at 300 µmol·m−2·s−1 PPFD | 27/13 °C, 25/15 °C, or 23/17 °C (day/night) | 16 h + 25/15 °C | [54] |
| 2 | Fragaria × ananassa June-bearing | Sulhyang, Maehyang | Photoperiod from 12 to 22 h with 250 µmol·m−2·s−1 PPFD; 4 h NI light provided by red, blue, green, white, or far-red LED light | 25/15 °C (day/night) | 20 h photoperiod and red LED light | [32] |
| 3 | Fragaria × ananassa Everbearing | Natsuakari, Dekoruju’ | Natural day length or 16 h photoperiod | 0, 700, 1000, 1500 and 2000 h of chilling (<5 °C) | More than 1000 h chilling | [52] |
| 4 | Fragaria × ananassa June-bearing | Tochiotome | Plants grown under 10 h SD in a glasshouse for 0, 14 or 28 d, then under 13 h LD for 49, 35 and 21 d, respectively, | 23/17 °C (day/night) | 13 h LD for 49 d | [55] |
| 5 | Fragaria × ananassa June-bearing | Toyonoka | 100% red light, 70% red light + 30% blue light, 70% red light + 20% blue light + 10% green light | 25/20 °C (day/night) | 70% red light + 30% blue light | [38] |
| 6 | Fragaria × ananassa | Saga, Sonata, Nobel, Florence, Rumba, Malwina | 10 h SD or 20 h LD | 9, 15, 21, or 27 °C | Temperature over 21 °C in SD and over 15 °C in LD | [56] |
| 7 | Fragaria × ananassa June-bearing | Akihime | White LEDs, white and red LEDs, red and blue LEDs, and red, blue and green LEDs | 25/20 °C (day/night) | White LEDs | [57] |
| 8 | F1-Hybrid, Everbearing | Delizzimo | 10 h SD or 20 h LD | 12, 19, or 26 °C | 10 h SD + 26 °C | [34,58] |
| 9 | Fragaria vesca | Original cultivars in Norwegian | 10 h SD or 24 h LD | 9, 15, or 21 °C | 24 h LD + 21 °C | [45] |
| 10 | Fragaria × ananassa | Earliglow, Seneca, Jewel, Chandler Cavendish | Preforcing 8 h SD for 0, 1, 2, 4 weeks, then transfer to 16 h LD | Preforcing at 15 °C for 0, 1, 2, 4 weeks, then transfer to 20 °C | Chandler: preforcing at 15 °C + 8 h for 4 weeks; other cultivars: no preforcing treatment | [59] |
| 11 | Fragaria × ananassa Everbearing | Albion | 10 h SD, 18 h and 24 h LD; far-red: blue (1:5, 5:1 and 1:1) | Nature temperature for photoperiod expt.; 24 °C/18 °C (day/night) for light quality expt. | Very few runners produced, and no significant difference in runner production between photoperiods and light qualities | [60] |
| 12 | Fragaria × ananassa Everbearing | Capri | 2 or 4 weeks NI light for 30 min·h−1 at 10 W·m−2 | Nature temperature | No NI treatment | [61] |
| 13 | Fragaria × ananassa | Honeoye (SD), Tribute (day-neutral), RH 30 (day-neutral) | 9 h SD or 16 h LD | 14, 17, 20, 23, 26, or 29 °C | Honeoye: 26 °C + 16 h LD; RH30: 26 and 29 °C + 16 h LD; Tribute: 23 °C + 16 h LD | [62] |
| 14 | Fragaria × ananassa Everbearing | Favori | 10 h SD or 20 h LD | 6, 16 or 26 °C for 5 and 10 weeks | 21 °C + 20 h preconditioning for 10 weeks | [34] |
2.3. Inorganic Nutrients
2.4. Regulation Network Behind Environment
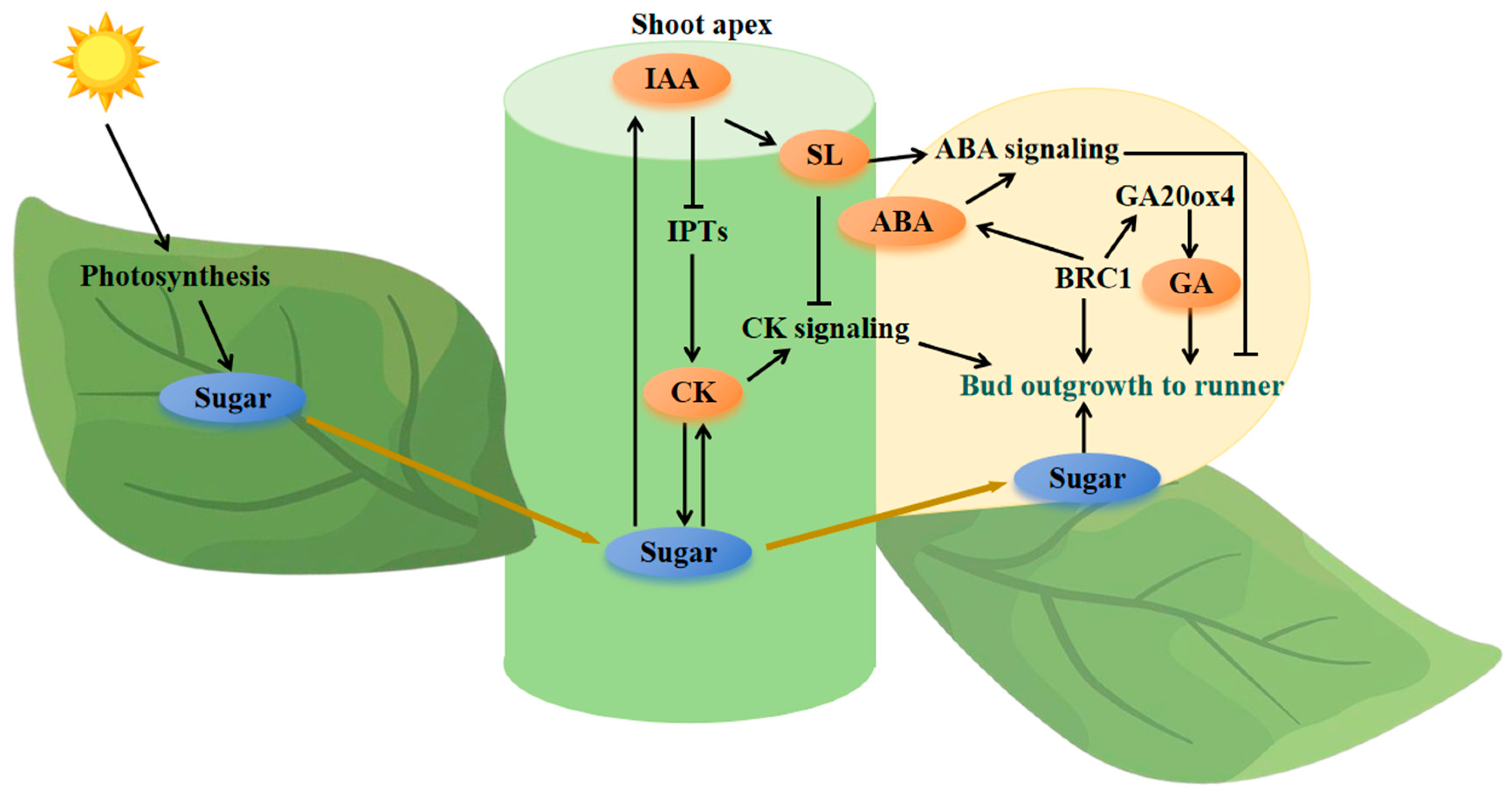
3. Hormonal Control of Runner Formation
3.1. Gibberellic Acids (GAs)
3.2. Cytokinins and Auxins
3.3. Other Plant Growth Regulators
| No. | Cultivar | Hormone | Concentration | Runner Development | Reference |
|---|---|---|---|---|---|
| 1 | Pajaro, Queen Eliza, Paros | GA and BA | 300 and 1200 ppm, respectively | Inhibited runner production | [14] |
| 2 | Seolhyang | BA and IBA | 50 mg·L−1 | Increased runner production | [87] |
| 3 | Seolhyang | TDZ | 50 mg·L−1 | Increased runner production | [87] |
| 4 | Seolhyang | TDZ and IBA | 50 mg·L−1 | Increased runner production | [87] |
| 5 | Seolhyang | TDZ and IAA | 50 mg·L−1 | Increased runner production | [87] |
| 6 | Korona | GA3 | 5000 mg·L−1 | Increased runner production | [30] |
| 7 | Ruegen | GA3 | 50, 75, 100 mg·L−1 | Increased runner production | [97] |
| 8 | Maehyang | 6-BA | 900 or 1500 mg·L−1 | Increased runner production | [98] |
| 9 | Maehyang, Sulhyang | BA | 100 mg·L−1 | Increased runner production | [86] |
| 10 | Maehyang, Sulhyang | Chlormequat chloride | 100 mg·L−1 | No effect | [86] |
| 11 | Cardinal | Paclobutrazol | 75, 150, 300, 600, or 1200 mg·L−1 | Inhibited runner production | [99] |
| 12 | Benihoppe | Strigolactones | 5, 10, or 20 µmol·L−1 | Inhibited runner production | [91] |
| 13 | Superfection | ABA | 50 ppm | Inhibited runner production | [92] |
3.4. Sugar
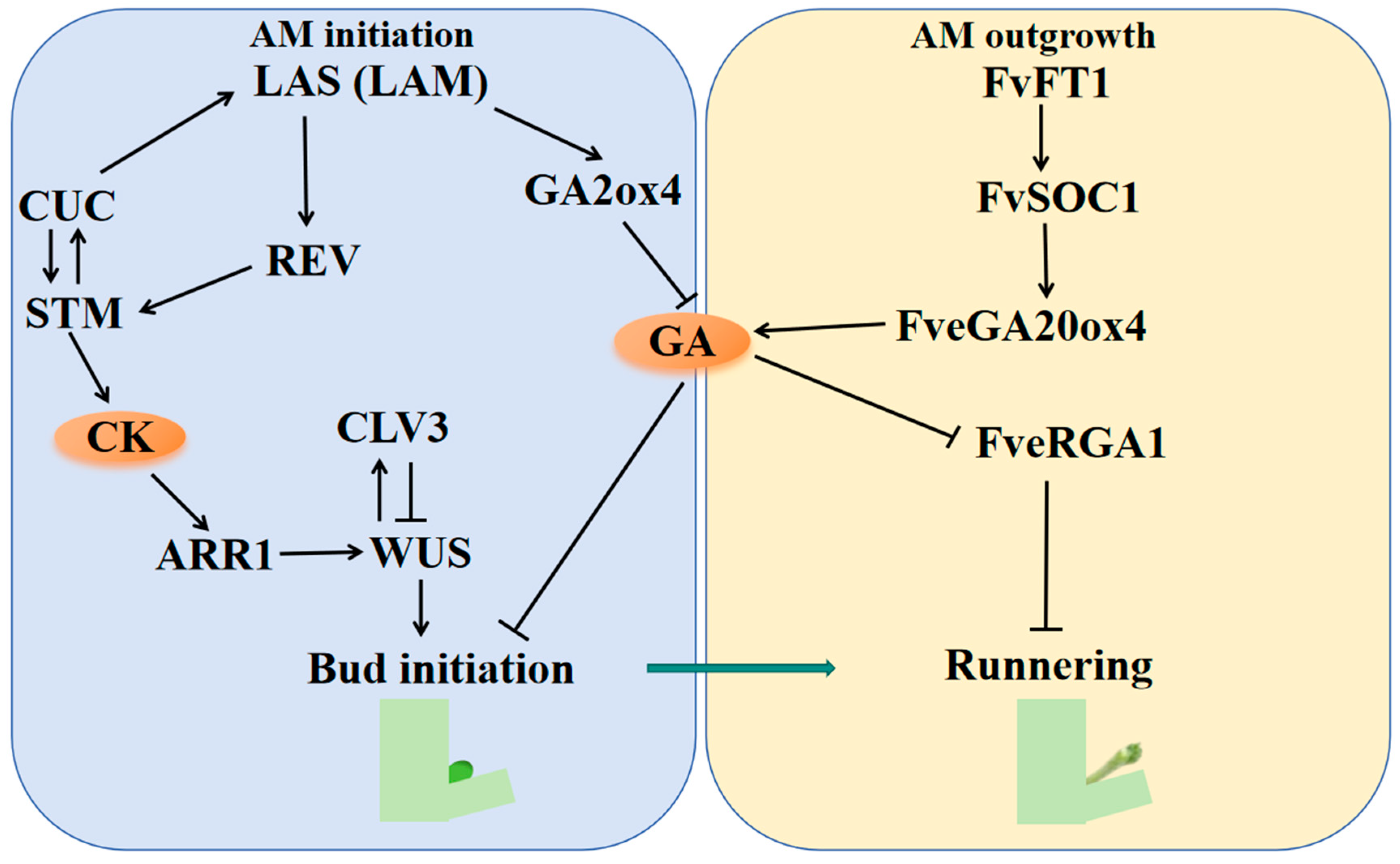
4. Genetic Control of Runner Formation
4.1. Runner Formation in Woodland Strawberry
4.2. Runner Formation in Cultivated Strawberry
5. An Integrated Conceptual Framework for Runner Formation
6. Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, L.; Plunkert, M.; Luo, X.; Liu, Z. Developmental regulation of stolon and rhizome. Curr. Opin. Plant Biol. 2021, 59, 101970. [Google Scholar] [CrossRef] [PubMed]
- Savini, G.; Giorgi, V.; Scarano, E.; Neri, D. Strawberry plant relationship through the stolon. Physiol. Plant. 2008, 134, 421–429. [Google Scholar] [CrossRef]
- Díaz-García, G.; Enciso-Maldonado, G.A.; Díaz-García, L.A.; Legaria-Solano, J.P.; Bamberg, J.; Lozoya-Saldaña, H. Field Screening of Solanum demissum confirms its late blight resistance in the Toluca valley, Mexico. Am. J. Potato Res. 2024, 101, 122–131. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Byers, R.A.; Skinner, R.H.; Elwinger, G.F. Growth and complexity of white clover stolons in response to biotic and abiotic stress. Crop Sci. 2003, 43, 2197–2205. [Google Scholar] [CrossRef]
- Pornaro, C.; Macolino, S.; Menegon, A.; Richardson, M. WinRHIZO technology for measuring morphological traits of bermudagrass stolons. Agron. J. 2017, 109, 3007–3010. [Google Scholar] [CrossRef]
- Sishu, N.K.; Selvara, C.I.; Parasurama, D.S. A review of micropropagation of Glycyrrhiza glabra L. (Licorice). In Micropropagation of Medicinal Plants; Bentham Science Publishers: Sharjah, United Arab Emirates, 2024; pp. 267–282. [Google Scholar]
- Gaston, A.; Perrotte, J.; Lerceteau-Köhler, E.; Rousseau-Gueutin, M.; Petit, A.; Hernould, M.; Rothan, C.; Denoyes, B. PFRU, a single dominant locus regulates the balance between sexual and asexual plant reproduction in cultivated strawberry. J. Exp. Bot. 2013, 64, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.G.; Muneer, S.; Kim, S.; Hwang, S.J.; Jeong, B.R. Foliar or subirrigational silicon supply modulates salt stress in strawberry during vegetative propagation. Hortic. Environ. Biotechnol. 2018, 59, 11–18. [Google Scholar] [CrossRef]
- Tsuruyama, J.; Shibuya, T. Growth and flowering responses of seed-propagated strawberry seedlings to different photoperiods in controlled environment chambers. HortTechnology 2018, 28, 453–458. [Google Scholar] [CrossRef]
- Hammami, I.; Jellali, M.; Ksontini, M.; Rejeb, M. Propagation of the strawberry tree through seed (Arbutus unedo). Int. J. Agric. Biol. 2005, 7, 457–459. [Google Scholar]
- Rho, I.R.; Woo, J.G.; Jeong, H.J.; Jeon, H.Y.; Lee, C.H. Characteristics of F1 hybrids and inbred lines in octoploid strawberry (Fragaria × ananassa Duchesne). Plant Breed. 2012, 131, 550–554. [Google Scholar] [CrossRef]
- Zheng, J.; Ji, F.; He, D.; Niu, G. Effect of light intensity on rooting and growth of hydroponic strawberry runner plants in a LED plant factory. Agronomy 2019, 9, 875. [Google Scholar] [CrossRef]
- Walter, M.; Snelling, C.; Boyd-Wilson, K.S.; Langford, G.I.; Williams, G. Evaluation of organic strawberry runner production. HortTechnology 2005, 15, 787–796. [Google Scholar] [CrossRef]
- Momenpour, A.; Taghavi, T.S.; Manochehr, S. Effects of banzyladenine and gibberellin on runner production and some vegetative traits of three strawberry cultivars. Afr. J. Agric. Res. 2011, 6, 4357–4361. [Google Scholar]
- Amin, B.; Qureshi, K.M.; Ghani, A.; Mahmood, A.; Shah, S.A.S.; Khan, M.F.; Khalid, S.; Iqbal, S.; Anwar, I. Effect of Different Environments and GA3 on Growth and Runners Production of Strawberry (Frageria Ananasa). J. Pharm. Negat. Results 2023, 14, 208–218. [Google Scholar]
- Chawla, W.; Singh, S.K.; Bal, S. Evaluation of performance of strawberry cultivars for vegetative attributes and runner production. Plant Arch. 2020, 20, 3759–3762. [Google Scholar]
- El-Deeb, A.; Mohamed, F. Runner production of strawberry plants in soilless suspended system: Nitrogen rate, GA3 and genotype effects. Hortscience J. Suez Canal Univ. 2018, 7, 35–46. [Google Scholar] [CrossRef]
- Avdiu, V.; Dragusha, B.; Hajra, E.; Hondolli, G. Effects of different substrates on the runner production of the June-bearing strawberry cv.‘Senga Sengana’. Hortic. Sci. 2022, 49, 197–204. [Google Scholar] [CrossRef]
- Savini, G.; Neri, D.; Zucconi, F.; Sugiyama, N. Strawberry growth and flowering: An architectural model. Int. J. Fruit Sci. 2005, 5, 29–50. [Google Scholar] [CrossRef]
- Tenreira, T.; Lange, M.J.P.; Lange, T.; Bres, C.; Labadie, M.; Monfort, A.; Hernould, M.; Rothan, C.; Denoyes, B. A specific gibberellin 20-oxidase dictates the flowering-runnering decision in diploid strawberry. Plant Cell 2017, 29, 2168–2182. [Google Scholar] [CrossRef]
- Feng, J.; Cheng, L.; Zhu, Z.; Yu, F.; Dai, C.; Liu, Z.; Guo, W.-W.; Wu, X.-M.; Kang, C. GRAS transcription factor LOSS OF AXILLARY MERISTEMS is essential for stamen and runner formation in wild strawberry. Plant Physiol. 2021, 186, 1970–1984. [Google Scholar] [CrossRef]
- Andrés, J.; Andrés, F.; Hytönen, T.; Koskela, E. Regulation of Axillary Bud Fate in Wild Strawberries; Helsingin yliopisto: Helsinki, Finland, 2025. [Google Scholar]
- Zhang, C.; Fan, L.; Le, B.H.; Ye, P.; Mo, B.; Chen, X. Regulation of ARGONAUTE10 expression enables temporal and spatial precision in axillary meristem initiation in Arabidopsis. Dev. Cell 2020, 55, 603–616.e5. [Google Scholar] [CrossRef] [PubMed]
- Aspuria, J.R.; Fujime, Y. Eco-physiological studies in the analysis of dormancy in strawberry. In Proceedings of the Dormancy and the Related Problems of Deciduous Fruit Trees 395, Kyoto, Japan, 21–27 August 1994; pp. 97–104. [Google Scholar]
- Hytönen, T.; Elomaa, P. Genetic and environmental regulation of flowering and runnering in strawberry. Genes Genomes Genom. 2011, 5, 56–64. [Google Scholar]
- Hytönen, T.; Kurokura, T. Control of flowering and runnering in strawberry. Hortic. J. 2020, 89, 96–107. [Google Scholar] [CrossRef]
- Honjo, M.; Kataoka, S.; Yui, S.; Morishita, M.; Yano, T.; Hamano, M.; Yamazaki, H. Varietal differences and selection indicators for flowering pattern in everbearing strawberry. J. Jpn. Soc. Hortic. Sci. 2011, 80, 38–44. [Google Scholar] [CrossRef][Green Version]
- Yoshida, Y.; Nishimoto, T. Propagation and floral induction of transplant for forcing long-term production of seasonal flowering strawberries in Japan. Hortic. J. 2020, 89, 87–95. [Google Scholar] [CrossRef]
- Heide, O.; Stavang, J.; Sønsteby, A. Physiology and genetics of flowering in cultivated and wild strawberries–a review. J. Hortic. Sci. Biotechnol. 2013, 88, 1–18. [Google Scholar] [CrossRef]
- Hytönen, T.; Elomaa, P.; Moritz, T.; Junttila, O. Gibberellin mediates daylength-controlled differentiation of vegetative meristems in strawberry (Fragaria× ananassa Duch). BMC Plant Biol. 2009, 9, 18. [Google Scholar] [CrossRef]
- Mouhu, K.; Kurokura, T.; Koskela, E.A.; Albert, V.A.; Elomaa, P.; Hytönen, T. The Fragaria vesca homolog of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 represses flowering and promotes vegetative growth. Plant Cell 2013, 25, 3296–3310. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, J.; Hu, J.; Jeong, B.R. Critical photoperiod and optimal quality of night interruption light for runner induction in June-bearing strawberries. Agronomy 2021, 11, 1996. [Google Scholar] [CrossRef]
- Hossain, M.R.; Natarajan, S.; Kim, H.-T.; Jesse, D.M.I.; Lee, C.-G.; Park, J.-I.; Nou, I.-S. High density linkage map construction and QTL mapping for runner production in allo-octoploid strawberry Fragaria× ananassa based on ddRAD-seq derived SNPs. Sci. Rep. 2019, 9, 3275. [Google Scholar] [CrossRef]
- Rivero, R.; Remberg, S.F.; Heide, O.M.; Sønsteby, A. Environmental regulation of dormancy, flowering and runnering in two genetically distant everbearing strawberry cultivars. Sci. Hortic. 2021, 290, 110515. [Google Scholar] [CrossRef]
- Sonsteby, A.; Nes, A. Short days and temperature effects on growth and flowering in strawberry (Fragaria× ananassa Duch.). J. Hortic. Sci. Biotechnol. 1998, 73, 730–736. [Google Scholar] [CrossRef]
- Smeets, L. Runner formation on strawberry plants in autumn and winter: II. Influence of the light intensity on the photoperiodical behaviour. Euphytica 1955, 4, 240–244. [Google Scholar] [CrossRef]
- Xu, X.; Hernandez, R. The effect of light intensity on vegetative propagation efficacy, growth, and morphology of “Albion” strawberry plants in a precision indoor propagation system. Appl. Sci. 2020, 10, 1044. [Google Scholar] [CrossRef]
- Wu, C.; Hsu, S.; Chang, M.; Fang, W. Effect of light environment on runner plant propagation of strawberry. In Proceedings of the VI International Symposium on Light in Horticulture 907, Tsukuba, Japan, 15–19 November 2009; pp. 297–302. [Google Scholar]
- Zheng, J.; He, D.; Ji, F. Effects of light intensity and photoperiod on runner plant propagation of hydroponic strawberry transplants under LED lighting. Int. J. Agric. Biol. Eng. 2019, 12, 26–31. [Google Scholar] [CrossRef]
- Park, S.W.; Kwack, Y.; Chun, C. Growth of runner plants grown in a plant factory as affected by light intensity and container volume. Hortic. Sci. Technol. 2017, 35, 439–445. [Google Scholar] [CrossRef]
- Aubé, M.; Roby, J.; Kocifaj, M. Evaluating potential spectral impacts of various artificial lights on melatonin suppression, photosynthesis, and star visibility. PLoS ONE 2013, 8, e67798. [Google Scholar] [CrossRef] [PubMed]
- Dufault, R.J.; Ward, B.K. Further attempts to enhance forced ‘Sweet Charlie’ strawberry yield through manipulation of light quality in high tunnels. Int. J. Fruit Sci. 2009, 9, 409–418. [Google Scholar] [CrossRef]
- Uddin, A.; Hoq, M.; Rini, S.; Urme, F.; Ahmad, H. Influence of supplement LED spectrum on growth and yield of Strawberry. J. Biosci. Agr. Res 2018, 16, 1348–1355. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Flowering and runnering of seasonal strawberry under different photoperiods are affected by intensity of supplemental or night-interrupting blue light. Plants 2024, 13, 375. [Google Scholar] [CrossRef] [PubMed]
- Heide, O.M.; Sønsteby, A. Interactions of temperature and photoperiod in the control of flowering of latitudinal and altitudinal populations of wild strawberry (Fragaria vesca). Physiol. Plant. 2007, 130, 280–289. [Google Scholar] [CrossRef]
- Morrison, D.M.; Blankenship, E.E.; Read, P.E.; Paparozzi, E.T. Stolon development and cultural production practices of winter-grown strawberries. Int. J. Fruit Sci. 2018, 18, 138–152. [Google Scholar] [CrossRef]
- Yanagi, T.; Oda, Y. Effects of chilling history on successive flowering and runner development of everbearing and non-everbearing strawberry cultivars. J. Jpn. Soc. Hortic. Sci. 1990, 59, 357–363. [Google Scholar] [CrossRef]
- Watanabe, G.; Yanagi, T.; Okuda, N.; Saito, Y. Effect of cold storage duration on runner production in strawberry plants in winter. In Proceedings of the VI International Strawberry Symposium 842, Huelva, Spain, 3–7 March 2008; pp. 729–732. [Google Scholar]
- Al-Madhagi, I.A.; Al-Munibary, M.; Al-Doubibi, M. Effect of chilling and accumulative photo-thermal units on flowering of strawberry (Fragaria× Ananassa Duch.). J. Hortic. Res. 2018, 26, 25–35. [Google Scholar] [CrossRef]
- Sønsteby, A.; Heide, O.M. Dormancy relations and flowering of the strawberry cultivars Korona and Elsanta as influenced by photoperiod and temperature. Sci. Hortic. 2006, 110, 57–67. [Google Scholar] [CrossRef]
- Hokanson, S.C.; Takeda, F.; Enns, J.M.; Black, B.L. Influence of plant storage duration on strawberry runner tip viability and field performance. HortScience 2004, 39, 1596–1600. [Google Scholar] [CrossRef]
- Hamano, M.; Yamazaki, H.; Morishita, M.; Imada, S. Effect of chilling and day length on runner of everbearing type strawberry. In Proceedings of the VI International Strawberry Symposium 842, Huelva, Spain, 3–7 March 2008; pp. 671–674. [Google Scholar]
- Lee, I.; Kim, H.-S.; Nam, M.H.; Oh, S.-K. Effect of cold storage method for ‘Sulhyang’ strawberry mother plants on mother plant growth and the number of runners and daughters. Korean J. Agric. Sci. 2020, 47, 625–632. [Google Scholar] [CrossRef]
- Li, Y.; Hu, J.; Wei, H.; Jeong, B.R. A long-day photoperiod and 6-benzyladenine promote runner formation through upregulation of soluble sugar content in strawberry. Int. J. Mol. Sci. 2020, 21, 4917. [Google Scholar] [CrossRef] [PubMed]
- Kurokura, T.; Iwama, T.; Inaba, Y.; Sugiyama, N. Effect of day-length on the developmental pattern of axillary buds in June-bearing strawberry plants. J. Hortic. Sci. Biotechnol. 2005, 80, 139–142. [Google Scholar] [CrossRef]
- Sønsteby, A.; Heide, O. Flowering performance and yield of established and recent strawberry cultivars (Fragaria× ananassa) as affected by raising temperature and photoperiod. J. Hortic. Sci. Biotechnol. 2017, 92, 367–375. [Google Scholar] [CrossRef]
- Chen, J.; Ji, F.; Gao, R.; He, D. Reducing red light proportion in full-spectrum LEDs enhances runner plant propagation by promoting the growth and development of mother plants in strawberry. Front. Plant Sci. 2024, 15, 1465004. [Google Scholar] [CrossRef]
- Samad, S.; Rivero, R.; Kalyandurg, P.B.; Vetukuri, R.R.; Heide, O.M.; Sønsteby, A.; Khalil, S. Characterization of environmental effects on flowering and plant architecture in an everbearing strawberry F1-hybrid by meristem dissection and gene expression analysis. Horticulturae 2022, 8, 626. [Google Scholar] [CrossRef]
- Durner, E.F. Photoperiod affects floral ontogeny in strawberry (Fragaria× ananassa Duch.) plug plants. Sci. Hortic. 2015, 194, 154–159. [Google Scholar] [CrossRef]
- Sidhu, V.; Bernier-English, V.; Lamontagne-Drolet, M.; Gravel, V. Effect of light quality and extended photoperiod on flower bud induction during transplant production of day-neutral strawberry cultivars. Can. J. Plant Sci. 2021, 102, 356–367. [Google Scholar] [CrossRef]
- Van Delm, T.; Melis, P.; Stoffels, K.; Baets, W. The effect of long-day treatment on runners and inflorescences on everbearing strawberry cultivar’Capri’. In Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): II 1117, Brisbane, Australia, 17 August 2014; pp. 285–290. [Google Scholar]
- Bradford, E.; Hancock, J.F.; Warner, R.M. Interactions of temperature and photoperiod determine expression of repeat flowering in strawberry. J. Am. Soc. Hortic. Sci. 2010, 135, 102–107. [Google Scholar] [CrossRef]
- Pipattanawong, N.; Fujishige, N.; Yamane, K.; Ijiro, Y.; Ogata, R. Effects of growth regulators and fertilizer on runner production, flowering, and growth in day-neutral strawberries [Fragaria ananassa]. Jpn. J. Trop. Agric. 1996, 40, 101–105. [Google Scholar]
- Farjana, S.; Park, I.S.; Choi, J.M. Macro-Elements in liquid fertilization influence differently in occurrence and growth of runner plants in strawberry propagation. Hortic. Sci. Technol. 2025, 43, 21–32. [Google Scholar] [CrossRef]
- Bakshi, P.; Jasrotia, A.; Sharma, A.; Rai, P.; Wali, V.; Kumar, R. Pre-harvest application of iron and zinc influences growth, yield, quality and runner production of strawberry (Fragaria ananassa) cv Chandler. Indian J. Agric. Sci. 2013, 83, 678–684. [Google Scholar]
- Mohamed, R.; Abd El-Aal, H.; Abd El-Aziz, M. Effect of phosphorus, zinc and their interactions on vegetative growth characters, yield and fruit quality of strawberry. J. Hortic. Sci. Ornam. Plants 2011, 3, 106–114. [Google Scholar]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta--analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Kurokura, T.; Samad, S.; Koskela, E.; Mouhu, K.; Hytönen, T. Fragaria vesca CONSTANS controls photoperiodic flowering and vegetative development. J. Exp. Bot. 2017, 68, 4839–4850. [Google Scholar] [CrossRef]
- Gaston, A.; Potier, A.; Alonso, M.; Sabbadini, S.; Delmas, F.; Tenreira, T.; Cochetel, N.; Labadie, M.; Prévost, P.; Folta, K.M. The FveFT2 florigen/FveTFL1 antiflorigen balance is critical for the control of seasonal flowering in strawberry while FveFT3 modulates axillary meristem fate and yield. New Phytol. 2021, 232, 372–387. [Google Scholar] [CrossRef]
- Stewart, P.J.; Folta, K.M. A review of photoperiodic flowering research in strawberry (Fragaria spp.). Crit. Rev. Plant Sci. 2010, 29, 1–13. [Google Scholar] [CrossRef]
- Rantanen, M.; Kurokura, T.; Jiang, P.; Mouhu, K.; Hytönen, T. Strawberry homologue of TERMINAL FLOWER 1 integrates photoperiod and temperature signals to inhibit flowering. Plant J. 2015, 82, 163–173. [Google Scholar] [CrossRef]
- Whitaker, V.M.; Knapp, S.J.; Hardigan, M.A.; Edger, P.P.; Slovin, J.P.; V Bassil, N.; Hytönen, T.; Mackenzie, K.K.; Lee, S.; Jung, S. A roadmap for research in octoploid strawberry. Hortic. Res. 2020, 7, 33. [Google Scholar] [CrossRef]
- Pietikäinen, L. Flower Induction and Vegetative Growth Characteristics in Fragaria× ananassa Cultivars ‘Calypso’ and ‘Hapil’. Master’s Thesis, University of Helsinki, Helsinki, Finland, October 2021. [Google Scholar]
- Kumar, R.; Bakshi, M.; Singh, D. Influence of plant growth regulators on growth, yield and quality of strawberrry (Fragaria x ananassa Duch.) under UP sub tropics. Asian J. Hortic. 2012, 7, 434–436. [Google Scholar]
- Jamal Uddin, A.; Hossan, M.; Islam, M.; Ahsan, M.; Mehraj, H. Strawberry growth and yield responses to gibberellic acid concentrations. J. Exp. Biosci. 2012, 3, 51–56. [Google Scholar]
- Palei, S.; Das, K.; Sahoo, K.; Dash, D.K.; Swain, S. Influence of plant growth regulators on strawberry Cv. Chandler under Odisha condition. Int. J. Sci. Res 2016, 7, 9945–9948. [Google Scholar]
- Greene, D.W.; Schloemann, S.G. Prohexadione-calcium inhibits runner formation and enhances yield of strawberry. J. Am. Pomol. Soc. 2010, 64, 125–139. [Google Scholar]
- Kim, H.M.; Lee, H.R.; Kang, J.H.; Hwang, S.J. Prohexadione-calcium application during vegetative growth affects growth of mother plants, runners, and runner plants of Maehyang strawberry. Agronomy 2019, 9, 155. [Google Scholar] [CrossRef]
- Surapornpiboon, P.; Surapornpiboon, P. Effect of benzyladenine and gibberellic acid on runner production in strawberry. In Proceedings of the 19th Rajamangala University of Technology Conference, Pathum Thani, Thailand, 22–27 January 2002. [Google Scholar]
- Bagheri, M.; Mansouri, H. Effect of induced polyploidy on some biochemical parameters in Cannabis sativa L. Appl. Biochem. Biotechnol. 2015, 175, 2366–2375. [Google Scholar] [CrossRef]
- Dai, F.; Wang, Z.; Luo, G.; Tang, C. Phenotypic and transcriptomic analyses of autotetraploid and diploid mulberry (Morus alba L.). Int. J. Mol. Sci. 2015, 16, 22938–22956. [Google Scholar] [CrossRef]
- Qiu, Y.; Guan, S.C.; Wen, C.; Li, P.; Gao, Z.; Chen, X. Auxin and cytokinin coordinate the dormancy and outgrowth of axillary bud in strawberry runner. BMC Plant Biol. 2019, 19, 528. [Google Scholar] [CrossRef]
- Booker, J.; Chatfield, S.; Leyser, O. Auxin acts in xylem-associated or medullary cells to mediate apical dominance. Plant Cell 2003, 15, 495–507. [Google Scholar] [CrossRef]
- Kour, S.; Kumar, R.; Wali, V.; Sharma, A.; Bakshi, P. Impact of benzyladenine and gibberellic acid on quality and economics of runner production in Chandler strawberry (Fragaria× ananassa) under subtropical climate. Indian J. Agric. Sci 2017, 87, 964–967. [Google Scholar] [CrossRef]
- Kahangi, E.; Fujime, Y.; Nakamura, E. Effects of chilling and growth regulators on runner production of three strawberry cultivars under tropical conditions. J. Hortic. Sci. 1992, 67, 381–384. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Z.; Park, Y.G.; Wei, H.; Jeong, B.R. PGR and its application method affect number and length of runners produced in ‘Maehyang’ and ‘Sulhyang’ strawberries. Agronomy 2019, 9, 59. [Google Scholar] [CrossRef]
- Li, Y.; Hu, J.; Xiao, J.; Guo, G.; Jeong, B.R. Foliar thidiazuron promotes the growth of axillary buds in Strawberry. Agronomy 2021, 11, 594. [Google Scholar] [CrossRef]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.-P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.-C. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef]
- Leyser, H.; de Saint Germain, A.; Waldie, T.; Troadec, C.; Citerne, S.; Kadakia, N.; Pillot, J.-P.; Prigge, M.; Aubert, G.; Bendahmane, A. The pea branching RMS2 gene encodes the PsAFB4/5 auxin receptor and is involved in an auxin-strigolactone regulation loop. PLoS Genet. 2018, 13, e1007089. [Google Scholar]
- Zhu, Y.; Wagner, D. Plant inflorescence architecture: The formation, activity, and fate of axillary meristems. Cold Spring Harb. Perspect. Biol. 2020, 12, a034652. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Jiang, Y.; He, C.; She, M.; Li, M.; Chen, Q.; Zhang, Y.; Lin, Y.; Zhang, Y.; Wang, Y. Exogenous GR24 inhibits strawberry tillering by affecting the phytohormone signaling and sugar metabolism pathways. Agronomy 2023, 13, 3078. [Google Scholar] [CrossRef]
- Kender, W.; Carpenter, S.; Braun, J. Runner formation in everbearing strawberry as influenced by growth-promoting and inhibiting substances. Ann. Bot. 1971, 35, 1045–1052. [Google Scholar] [CrossRef]
- Yao, C.; Finlayson, S.A. Abscisic acid is a general negative regulator of Arabidopsis axillary bud growth. Plant Physiol. 2015, 169, 611–626. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Yu, H.; Guo, H.; Lin, T.; Kou, L.; Wang, A.; Shao, N.; Ma, H.; Xiong, G. Transcriptional regulation of strigolactone signalling in Arabidopsis. Nature 2020, 583, 277–281. [Google Scholar] [CrossRef]
- Luo, Z.; Janssen, B.J.; Snowden, K.C. The molecular and genetic regulation of shoot branching. Plant Physiol. 2021, 187, 1033–1044. [Google Scholar] [CrossRef]
- Aguilar-Martínez, J.A.; Poza-Carrion, C.; Cubas, P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 2007, 19, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, B.; Sun, H.; Zhang, Z. Transcriptome profiling of runner formation induced by exogenous gibberellin in Fragaria vesca. Sci. Hortic. 2021, 281, 109966. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.M.; Kim, H.M.; Hwang, S.J. Growth and runner production of ‘Maehyang’ strawberry as affected by application method and concentration of cytokinin. J. Bio-Environ. Control 2017, 26, 72–77. [Google Scholar] [CrossRef]
- Deyton, D.E.; Sams, C.E.; Cummins, J.C. Strawberry growth and photosynthetic responses to paclobutrazol. HortScience 1991, 26, 1178–1180. [Google Scholar] [CrossRef]
- Lan, G.; Wu, M.; Zhang, Q.; Yuan, B.; Shi, G.; Zhu, N.; Zheng, Y.; Cao, Q.; Qiao, Q.; Zhang, T. Transcriptomic and physiological analyses for the role of hormones and sugar in axillary bud development of wild strawberry stolon. Plants 2024, 13, 2241. [Google Scholar] [CrossRef]
- Wang, M.; Pérez-Garcia, M.-D.; Davière, J.-M.; Barbier, F.; Ogé, L.; Gentilhomme, J.; Voisine, L.; Péron, T.; Launay-Avon, A.; Clément, G. Outgrowth of the axillary bud in rose is controlled by sugar metabolism and signalling. J. Exp. Bot. 2021, 72, 3044–3060. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Doust, A.N. Activation of apoplastic sugar at the transition stage may be essential for axillary bud outgrowth in the grasses. Front. Plant Sci. 2022, 13, 1023581. [Google Scholar] [CrossRef]
- Liu, W.; Peng, B.; Song, A.; Jiang, J.; Chen, F. Sugar transporter, CmSWEET17, promotes bud outgrowth in Chrysanthemum morifolium. Genes 2020, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, J.; Schneider, A.; Ledroit, L.; Perez-Garcia, M.-D.; Boudon, F.; Godin, C.; Sakr, S. Axillary bud outgrowth regulation by light intensity: Modelling hormone and sugar interactions. In Proceedings of the International Conference on Functional-Structural Plant Models (FSPM 2020), Germany, Hannover, 5–9 October 2020. [Google Scholar]
- Bertheloot, J.; Barbier, F.; Boudon, F.; Perez-Garcia, M.D.; Péron, T.; Citerne, S.; Dun, E.; Beveridge, C.; Godin, C.; Sakr, S. Sugar availability suppresses the auxin--induced strigolactone pathway to promote bud outgrowth. New Phytol. 2020, 225, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Salam, B.B.; Barbier, F.; Danieli, R.; Teper-Bamnolker, P.; Ziv, C.; Spíchal, L.; Aruchamy, K.; Shnaider, Y.; Leibman, D.; Shaya, F. Sucrose promotes stem branching through cytokinin. Plant Physiol. 2021, 185, 1708–1721. [Google Scholar] [CrossRef]
- Bird, K.A.; Hardigan, M.A.; Ragsdale, A.P.; Knapp, S.J.; VanBuren, R.; Edger, P.P. Diversification, spread, and admixture of octoploid strawberry in the Western Hemisphere. Am. J. Bot. 2021, 108, 2269–2281. [Google Scholar] [CrossRef]
- Edger, P.P.; Poorten, T.J.; VanBuren, R.; Hardigan, M.A.; Colle, M.; McKain, M.R.; Smith, R.D.; Teresi, S.J.; Nelson, A.D.; Wai, C.M. Origin and evolution of the octoploid strawberry genome. Nat. Genet. 2019, 51, 541–547. [Google Scholar] [CrossRef]
- Koskela, E.A.; Hytönen, T. Control of flowering in strawberries. In The Genomes of Rosaceous Berries and Their Wild Relatives; Springer: Cham, Switzerland, 2018; pp. 35–48. [Google Scholar][Green Version]
- Caruana, J.C.; Sittmann, J.W.; Wang, W.; Liu, Z. Suppressor of runnerless encodes a DELLA protein that controls runner formation for asexual reproduction in strawberry. Mol. Plant 2018, 11, 230–233. [Google Scholar] [CrossRef]
- Joldersma, D.; Sadowski, N.; Timp, W.; Liu, Z. Assembly and annotation of Fragaria vesca ‘Yellow Wonder’ genome, a model diploid strawberry for molecular genetic research. Fruit Res. 2022, 2, 13. [Google Scholar] [CrossRef]
- Newton, A.; Duncan, J.; Augustin, N.H.; Guy, D.; Cooke, D. Survival, distribution and genetic variability of inoculum of the strawberry red core pathogen, Phytophthora fragariae var. fragariae, in soil. Plant Pathol. 2010, 59, 472–479. [Google Scholar] [CrossRef]
- Alger, E.I.; Platts, A.E.; Deb, S.K.; Luo, X.; Ou, S.; Cao, Y.; Hummer, K.E.; Xiong, Z.; Knapp, S.J.; Liu, Z. Chromosome-scale genome for a red-fruited, perpetual flowering and runnerless woodland strawberry (Fragaria vesca). Front. Genet. 2021, 12, 671371. [Google Scholar] [CrossRef] [PubMed]
- Hjalmarsson, I. Strawberry cultivars preserved in the Swedish National Gene Bank. In Proceedings of the Latvian Academy of Sciences, Lomma, Sweden, 15 July 2022; pp. 402–408. [Google Scholar]
- Martins, A.O.; Nunes-Nesi, A.; Araújo, W.L.; Fernie, A.R. To bring flowers or do a runner: Gibberellins make the decision. Mol. Plant 2018, 11, 4–6. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Sun, H.; Wang, S.; Chen, K.; Liu, Y.; Li, H.; Ma, Y.; Zhang, Z. FveRGA1, encoding a DELLA protein, negatively regulates runner production in Fragaria vesca. Planta 2018, 247, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Wang, J.G.; Wang, L.Y.; Wang, J.F.; Wang, Q.; Yu, P.; Bai, M.Y.; Fan, M. Gibberellin repression of axillary bud formation in Arabidopsis by modulation of DELLA-SPL9 complex activity. J. Integr. Plant Biol. 2020, 62, 421–432. [Google Scholar] [CrossRef]
- Li, Y.; Xia, T.; Gao, F.; Li, Y. Control of plant branching by the CUC2/CUC3-DA1-UBP15 regulatory module. Plant Cell 2020, 32, 1919–1932. [Google Scholar] [CrossRef]
- Zheng, C.; Kwame Acheampong, A.; Shi, Z.; Halaly, T.; Kamiya, Y.; Ophir, R.; Galbraith, D.W.; Or, E. Distinct gibberellin functions during and after grapevine bud dormancy release. J. Exp. Bot. 2018, 69, 1635–1648. [Google Scholar] [CrossRef]
- Raman, S.; Greb, T.; Peaucelle, A.; Blein, T.; Laufs, P.; Theres, K. Interplay of miR164, CUP-SHAPED COTYLEDON genes and LATERAL SUPPRESSOR controls axillary meristem formation in Arabidopsis thaliana. Plant J. 2008, 55, 65–76. [Google Scholar] [CrossRef]
- Zheng, G.; Wei, W.; Li, Y.; Kan, L.; Wang, F.; Zhang, X.; Li, F.; Liu, Z.; Kang, C. Conserved and novel roles of miR164--CUC 2 regulatory module in specifying leaf and floral organ morphology in strawberry. New Phytol. 2019, 224, 480–492. [Google Scholar] [CrossRef]
- Cao, X.; Jiao, Y. Control of cell fate during axillary meristem initiation. Cell. Mol. Life Sci. 2020, 77, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Sooriyapathirana, S.S.; Mookerjee, S.; Weebadde, C.K.; Finn, C.E.; Lewers, K.; Bushakra, J.M.; Luby, J.J.; Stewart, P.; Neils, S.; Hancock, J.F. Identification of QTL associated with flower and runner production in octoploid strawberry (Fragaria × ananassa). J. Berry Res. 2015, 5, 107–116. [Google Scholar] [CrossRef]
- Cockerton, H.M.; Nellist, C.F.; Hytönen, T.; Litthauer, S.; Hopson, K.; Whitehouse, A.; Sobczyk, M.; Harrison, R.J. Epistatic modifiers influence the expression of continual flowering in strawberry. Plants People Planet 2023, 5, 70–81. [Google Scholar] [CrossRef]
- Samad, S.; Kurokura, T.; Koskela, E.; Toivainen, T.; Patel, V.; Mouhu, K.; Sargent, D.J.; Hytönen, T. Additive QTLs on three chromosomes control flowering time in woodland strawberry (Fragaria vesca L.). Hortic. Res. 2017, 4, 17020. [Google Scholar] [CrossRef]
- Saiga, S.; Tada, M.; Segawa, T.; Nishikawa, M.; Makita, N.; Sakamoto, M.; Tanaka, K.; Wada, T.; Takagi, H. NGS-based genome wide association study helps to develop co-dominant marker for the physical map-based locus of PFRU controlling flowering in cultivated octoploid strawberry. Euphytica 2023, 219, 6. [Google Scholar] [CrossRef]
- Liang, J.; Wu, Z.; Zheng, J.; Koskela, E.A.; Fan, L.; Fan, G.; Gao, D.; Dong, Z.; Hou, S.; Feng, Z. The GATA factor HANABA TARANU promotes runner formation by regulating axillary bud initiation and outgrowth in cultivated strawberry. Plant J. 2022, 110, 1237–1254. [Google Scholar] [CrossRef]
- Park, S.W. Establishment of a Propagation System for Strawberry Using a Plant Factory with Artificial Lighting. Doctoral Thesis, Seoul National University, Seoul, Republic of Korea, February 2018. [Google Scholar]
- Lee, H.; Park, S.W.; Cui, M.; Lee, B.; Minh Pham, D.; Hwang, H.; Chun, C. Improvement of strawberry transplant production efficiency by supplementary blue light in a plant factory using white LEDs. Hortic. Environ. Biotechnol. 2023, 64, 233–244. [Google Scholar] [CrossRef]
- Roszak, P.; Heo, J.O.; Blob, B.; Toyokura, K.; Sugiyama, Y.; de Luis Balaguer, M.A.; Lau, W.W.Y.; Hamey, F.; Cirrone, J.; Madej, E.; et al. Cell-by-cell dissection of phloem development links a maturation gradient to cell specialization. Science 2021, 374, eaba5531. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Jeong, B.R.; Huang, P.; Qiu, X.; Zhu, F.; He, J.; Zhao, L.; Wang, S.; Meng, X.; Ding, M. Factors Controlling Runner Formation in Strawberries. Agronomy 2025, 15, 2235. https://doi.org/10.3390/agronomy15092235
Li Y, Jeong BR, Huang P, Qiu X, Zhu F, He J, Zhao L, Wang S, Meng X, Ding M. Factors Controlling Runner Formation in Strawberries. Agronomy. 2025; 15(9):2235. https://doi.org/10.3390/agronomy15092235
Chicago/Turabian StyleLi, Yali, Byoung Ryong Jeong, Ping Huang, Xia Qiu, Feiyu Zhu, Jiaxian He, Liang Zhao, Si Wang, Xin Meng, and Mingzhong Ding. 2025. "Factors Controlling Runner Formation in Strawberries" Agronomy 15, no. 9: 2235. https://doi.org/10.3390/agronomy15092235
APA StyleLi, Y., Jeong, B. R., Huang, P., Qiu, X., Zhu, F., He, J., Zhao, L., Wang, S., Meng, X., & Ding, M. (2025). Factors Controlling Runner Formation in Strawberries. Agronomy, 15(9), 2235. https://doi.org/10.3390/agronomy15092235







