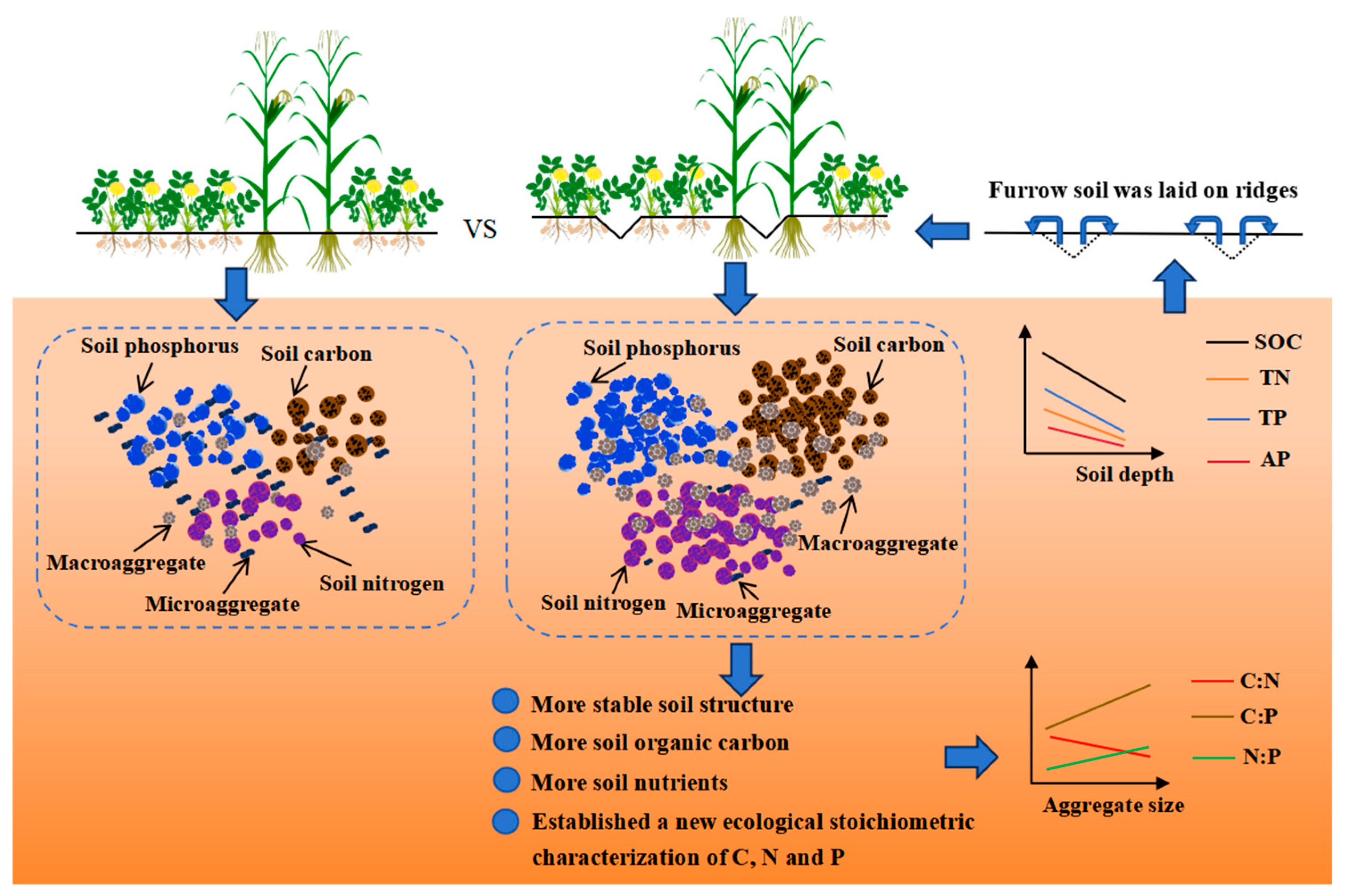
Co-Ridge Planting Enhances Yield Advantages of Maize Intercropping with Peanut by Improving Soil Aggregate Stability and the Ecological Stoichiometric Characteristics of Carbon, Nitrogen, and Phosphorus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Soil Sampling
2.4. Determination of Soil Aggregate
2.5. Analysis of Soil Physical and Chemical Properties
2.6. Determination of Crop Yields
2.7. Calculations
2.7.1. Stability Index of Soil Aggregates
2.7.2. Soil Bulk Density
2.7.3. Soil Porosity
2.7.4. Soil Carbon, Nitrogen, and Phosphorus Storage
2.7.5. Intercropping Yield Advantage
2.8. Statistical Analyses
3. Results
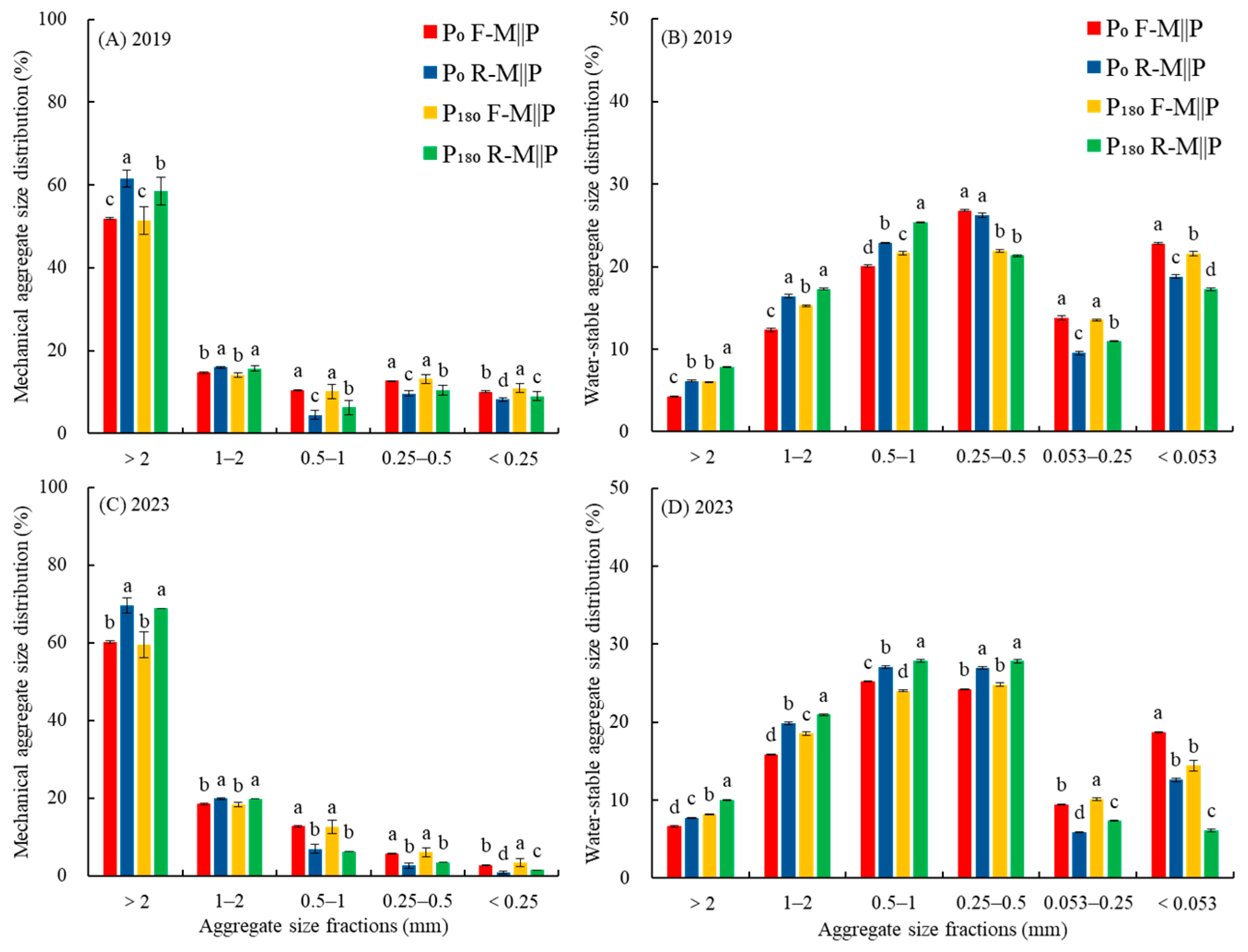
3.1. Effects of Co-Ridge Planting on Soil Aggregate Proportion, Stability, Bulk Density, and Porosity
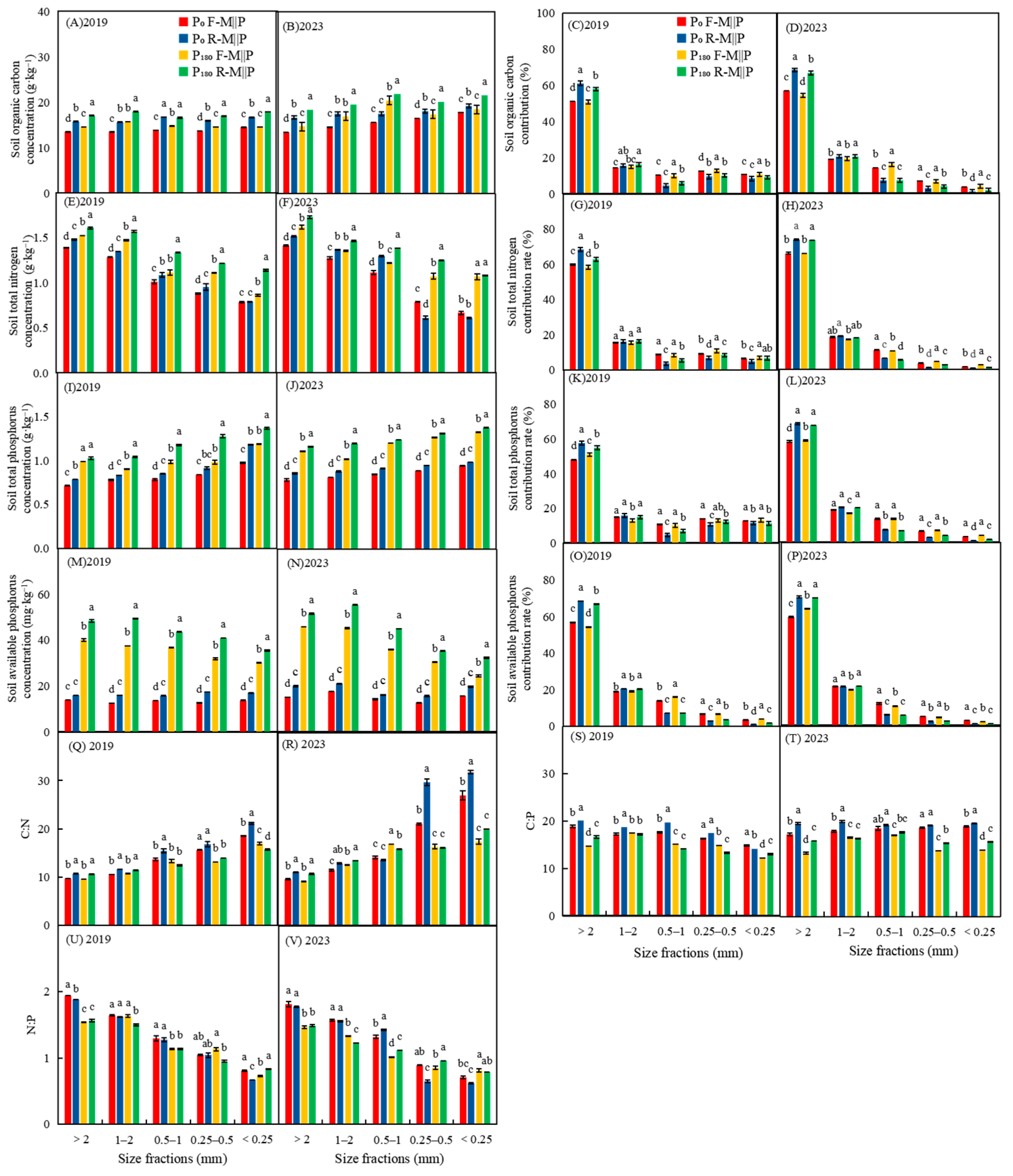
3.2. Effects of Co-Ridge Planting on the Concentration and Contribution Rate of Soil Organic Carbon and Nutrients, and Ecological Stoichiometric Characteristics of Carbon, Nitrogen, and Phosphorus
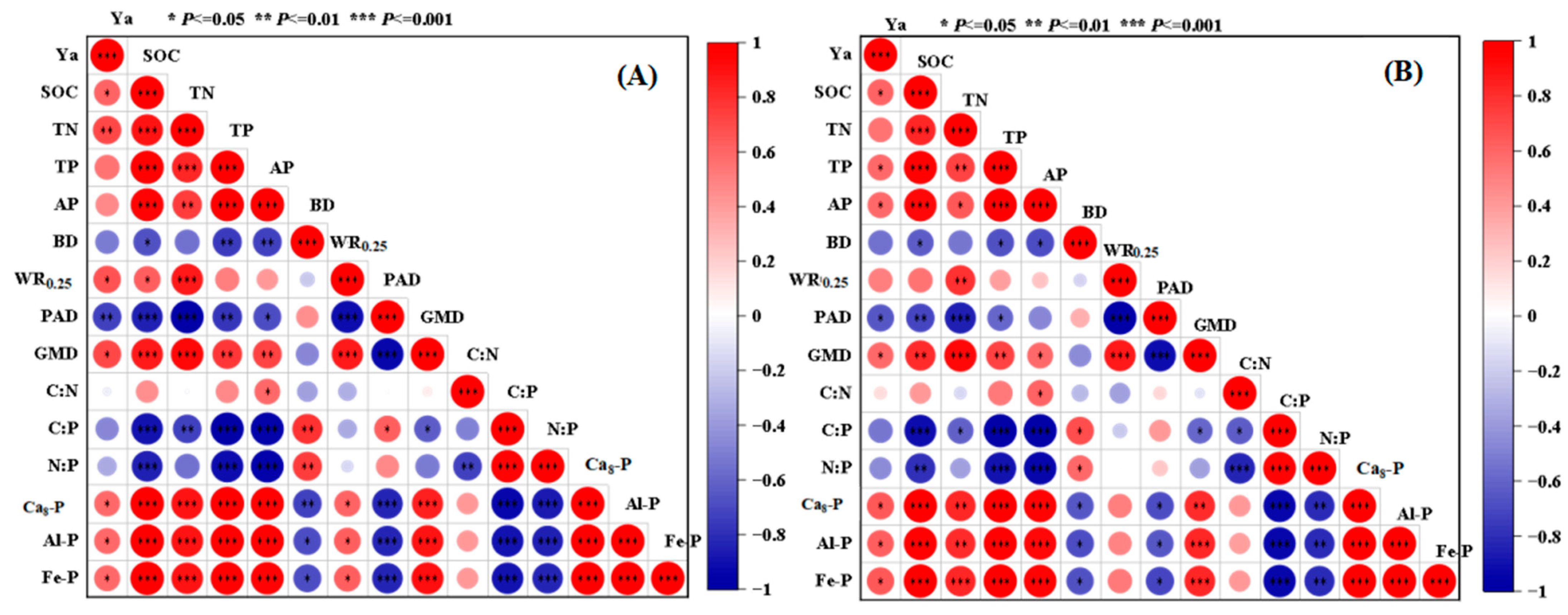
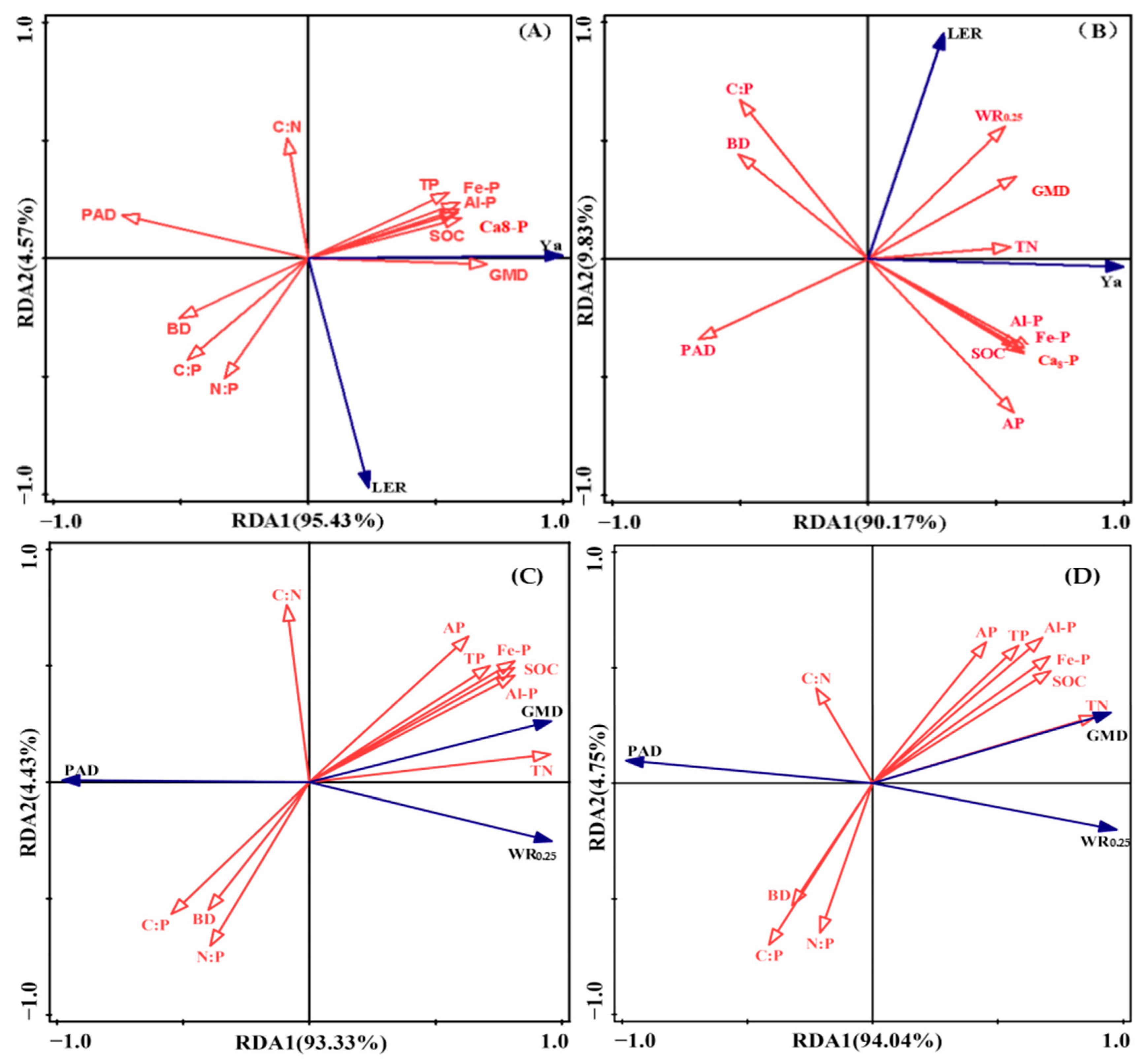
3.3. Relationship Between Intercropping Advantage in Yield and Soil Physical–Chemical Properties
4. Discussion
4.1. Co-Ridge Planting Improves Soil Aggregate Stability and Soil Porosity While Reducing Bulk Density in Maize Intercropping with the Peanut System
4.2. Co-Ridge Planting Improves Soil Organic Carbon and Nutrient Concentrations in Maize Intercropping with the Peanut System
4.3. Co-Ridge Planting Shapes New Ecological Stoichiometric Characteristics of Carbon, Nitrogen, and Phosphorus in Topsoil in Maize Intercropping with Peanut System
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Planting Pattern | Name | Explains (%) | Contribution (%) | Pseudo-F | p Value |
|---|---|---|---|---|---|
| F-M||P | SOC | 34.7 | 34.7 | 5.3 | * |
| PAD | 18.4 | 18.4 | 3.5 | ns | |
| BD | 8.2 | 8.2 | 1.7 | ns | |
| Ca8-P | 3.3 | 3.3 | 0.7 | ns | |
| C:P | 3.4 | 3.4 | 0.6 | ns | |
| TP | 8.9 | 8.9 | 1.9 | ns | |
| Fe-P | 14.8 | 14.8 | 7.3 | ns | |
| GMD | 3.6 | 3.6 | 2.4 | ns | |
| C:N | 0.9 | 0.9 | 0.5 | ns | |
| N:P | 1.8 | 1.8 | 1 | ns | |
| Al-P | 1.8 | 1.8 | <0.1 | ns | |
| R-M||P | SOC | 33.2 | 33.2 | 5 | * |
| AP | 22.7 | 22.7 | 6.9 | * | |
| PAD | 18 | 18 | 3.3 | ns | |
| Ca8-P | 2.2 | 2.2 | 0.6 | ns | |
| WR0.25 | 6.2 | 6.2 | 2.1 | ns | |
| C:P | 7.2 | 7.2 | 3.4 | ns | |
| Fe-P | 2.2 | 2.2 | 1.1 | ns | |
| MWD | 1.7 | 1.7 | 0.8 | ns | |
| Al-P | 1.9 | 1.9 | 0.8 | ns | |
| TN | 1.1 | 1.1 | 0.3 | ns | |
| BD | 3.6 | 3.6 | <0.1 | ns |
| Planting Pattern | Name | Explains (%) | Contribution (%) | Pseudo-F | p Value |
|---|---|---|---|---|---|
| F-M||P | SOC | 62.7 | 62.8 | 16.8 | ** |
| TN | 25.5 | 25.6 | 19.6 | ** | |
| N:P | 7.7 | 7.7 | 15 | ** | |
| Al-P | 2 | 2 | 6.7 | ** | |
| C:P | 0.7 | 0.7 | 3 | * | |
| AP | 0.3 | 0.3 | 1.5 | ns | |
| TP | 0.3 | 0.3 | 1.5 | ns | |
| Fe-P | 0.4 | 0.4 | 2.9 | ns | |
| BD | 0.2 | 0.2 | 2.8 | ns | |
| C:N | <0.1 | <0.1 | 1 | ns | |
| R-M||P | SOC | 48.8 | 48.8 | 9.5 | * |
| C:N | 30.2 | 30.2 | 12.9 | ** | |
| AP | 8 | 8 | 4.9 | * | |
| Fe-P | 4.4 | 4.4 | 3.6 | ns | |
| Al-P | 6.6 | 6.6 | 19.5 | ** | |
| N:P | 0.3 | 0.3 | 0.8 | ns | |
| TP | 0.7 | 0.7 | 2.6 | ns | |
| TN | 0.5 | 0.5 | 2.5 | ns | |
| BD | 0.4 | 0.4 | 6 | ns | |
| C:P | <0.1 | <0.1 | 1.2 | ns |
| Year | P | Maize Yield (t·ha−1) | Peanut Yield (t·ha−1) | Intercropping Advantage (t·ha−1) | LER | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SM | IM | R-IM | SP | IP | R-IP | M||P | R-M||P | M||P | R-M||P | ||
| 2018 | P0 | 5.25 ± 0.05 a | 4.53 ± 0.03 b | 5.03 ± 0.10 a | 3.08 ± 0.22 a | 0.95 ± 0.10 b | 1.02 ± 0.02 b | 1.18 ± 0.15 b | 1.74 ± 0.05 a | 1.18 ± 0.05 b | 1.29 ± 0.01 a |
| P180 | 7.44 ± 0.46 a | 5.82 ± 0.03 b | 6.22 ± 0.13 b | 4.00 ± 0.13 a | 1.07 ± 0.04 b | 1.18 ± 0.07 b | 0.95 ± 0.23 b | 1.47 ± 0.34 a | 1.05 ± 0.04 b | 1.14 ± 0.07 a | |
| 2019 | P0 | 5.63 ± 0.14 a | 4.69 ± 0.15 b | 5.32 ± 0.10 a | 2.44 ± 0.01 a | 0.72 ± 0.01 c | 0.82 ± 0.01 b | 1.18 ± 0.22 b | 1.90 ± 0.08 a | 1.13 ± 0.05 b | 1.28 ± 0.02 a |
| P180 | 9.62 ± 0.42 a | 7.07 ± 0.12 b | 8.02 ± 0.27 b | 3.79 ± 0.02 a | 1.06 ± 0.02 c | 1.15 ± 0.02 b | 1.07 ± 0.21 b | 2.12 ± 0.24 a | 1.02 ± 0.03 b | 1.14 ± 0.03 a | |
| 2020 | P0 | 6.36 ± 0.09 a | 5.21 ± 0.08 c | 5.83 ± 0.04 b | 2.37 ± 0.03 a | 0.85 ± 0.01 b | 0.90 ± 0.01 b | 1.45 ± 0.12 b | 2.13 ± 0.07 a | 1.18 ± 0.02 b | 1.30 ± 0.01 a |
| P180 | 9.63 ± 0.23 a | 8.29 ± 0.09 b | 9.10 ± 0.38 ab | 3.52 ± 0.04 a | 0.98 ± 0.01 b | 1.00 ± 0.01 b | 2.32 ± 0.20 b | 3.16 ± 0.38 a | 1.14 ± 0.03 b | 1.23 ± 0.04 a | |
| 2021 | P0 | 5.24 ± 0.13 a | 4.49 ± 0.04 b | 5.20 ± 0.08 a | 3.17 ± 0.08 a | 0.84 ± 0.01 b | 0.98 ± 0.01 b | 1.00 ± 0.12 b | 1.85 ± 0.13 a | 1.12 ± 0.03 b | 1.30 ± 0.03 a |
| P180 | 7.43 ± 0.10 a | 6.08 ± 0.09 c | 6.87 ± 0.23 b | 4.13 ± 0.05 a | 1.18 ± 0.04 b | 1.36 ± 0.06 b | 1.27 ± 0.10 b | 2.25 ± 0.18 a | 1.10 ± 0.02 b | 1.25 ± 0.02 a | |
| 2022 | P0 | 4.28 ± 0.04 a | 3.51 ± 0.06 b | 4.09 ± 0.13 a | 4.35 ± 0.09 a | 1.55 ± 0.04 c | 2.17 ± 0.08 b | 0.75 ± 0.10 b | 1.95 ± 0.23 a | 1.18 ± 0.02 b | 1.46 ± 0.05 a |
| P180 | 7.39 ± 0.21 a | 5.99 ± 0.09 c | 6.51 ± 0.08 b | 5.23 ± 0.06 a | 1.86 ± 0.03 c | 2.47 ± 0.05 b | 1.41 ± 0.17 b | 2.54 ± 0.13 a | 1.17 ± 0.03 b | 1.35 ± 0.03 a | |
| 2023 | P0 | 6.54 ± 0.23 a | 5.31 ± 0.42 c | 6.01 ± 0.42 b | 4.17 ± 0.03 a | 1.67 ± 0.03 c | 2.03 ± 0.02 b | 1.56 ± 0.42 b | 2.61 ± 0.23 a | 1.21 ± 0.02 b | 1.41 ± 0.21 a |
| P180 | 9.85 ± 0.42 a | 7.41 ± 0.04 c | 7.91 ± 0.32 b | 5.70 ± 0.21 a | 2.17 ± 0.02 c | 2.78 ± 0.04 b | 1.69 ± 0.31 b | 2.8 ± 0.32 a | 1.14 ± 0.06 b | 1.29 ± 0.06 a | |
| P | *** | *** | *** | *** | *** | *** | * | ** | ** | *** | |
| Year | *** | *** | *** | *** | *** | *** | *** | * | ** | *** | |
| P*Year | ** | *** | *** | ns | * | * | * | ns | ns | ns | |
References
- Jiao, N.Y.; Wang, F.; Ma, C.; Zhang, F.S.; Jensen, E.S. Interspecific interactions of iron and nitrogen use in peanut (Arachis hypogaea L.)-maize (Zea mays L.) intercropping on a calcareous soil. Eur. J. Agron. 2021, 128, 126303. [Google Scholar] [CrossRef]
- Chen, J.N.; Jiang, W.Y.; Zan, Z.M.; Wang, J.T.; Zheng, B.; Liu, L.; Liu, J.; Jiao, N.Y. Effects of maize and peanut co-ridge intercropping on crop photosynthetic characteristics and intercropping advantages. CJAE 2023, 34, 2672–2682. (In Chinese) [Google Scholar] [CrossRef]
- Lu, M.; Zhao, J.X.; Lu, Z.R.; Li, M.J.; Yang, J.F.; Fullen, M.; Li, Y.M.; Fan, M.F. Maize–soybean intercropping increases soil nutrient availability and aggregate stability. Plant Soil 2023, 506, 441–456. [Google Scholar] [CrossRef]
- Seidel, E.P.; dos Reis, W.; Mottin, M.C.; Fey, E.; Schneider, A.P.R.; Sustakowski, M.C. Evaluation of aggregate distribution and selected soil physical properties under maizejack bean intercropping and gypsum rates. Afr. J. Agr. Res. 2017, 12, 1209–1216. [Google Scholar] [CrossRef]
- Cong, W.F.; Hoffland, E.; Li, L.; Six, J.; Sun, J.H.; Bao, X.G.; Zhang, F.S.; van der Werf, W. Intercropping enhances organic carbon and nitrogen in soil. Global Change Biol. 2015, 21, 1715–1726. [Google Scholar] [CrossRef]
- Yin, X.G.; Song, Z.W.; Shi, S.H.; Bai, Z.Y.; Jiang, Y.L.; Zheng, A.X.; Huang, W.H.; Chen, N.N.; Chen, F.C. Developments and prospects of multiple cropping in China. Farm. Syst. 2024, 2, 100083. [Google Scholar] [CrossRef]
- Zan, Z.M.; Jiao, N.Y.; Ma, R.T.; Wang, J.T.; Wang, Y.; Ning, T.Y.; Zheng, B.; Liu, L.; Zhao, X.P.; Cong, W.F. Long-term maize intercropping with peanut and phosphorus application maintains sustainable farmland productivity by improving soil aggregate stability and P availability. Agronomy 2023, 13, 2846. [Google Scholar] [CrossRef]
- Ma, R.T.; Yu, N.; Zhao, S.W.; Kou, T.J.; Jiao, N.Y. Effects of long-term maize-peanut Intercropping and Phosphorus Application on CNP Content and Stoichiometry in soil Aggregates. J. Soil Sci. Plant Nut. 2024, 25, 40–52. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Zhang, Y.P.; Luo, G.W. Regulation of soil C–N–P stoichiometry by intercropping mitigates microbial resource limitations and contributes to maize productivity. Plant Soil 2024, 498, 21–38. [Google Scholar] [CrossRef]
- Katsvairo, T.; Cox, W.J.; van Es, H. Tillage and rotation effects on soil physical characteristics. Agron. J. 2002, 94, 299–304. [Google Scholar] [CrossRef]
- Tang, X.H.; Luo, Y.J.; Ren, Z.J.; Lü, J.K.; Wei, C.F. Distribution characteristics of soil humus fractions stable carbon isotope natural abundance (delta 13C) in paddy field under long-term ridge culture. J. Appl. Ecol. 2011, 22, 985–991. Available online: https://europepmc.org/article/med/21774322 (accessed on 20 January 2024). (In Chinese).
- Zhang, J.; Liu, Y.; Zheng, T.; Zhao, X.; Liu, H.; Zhang, Y. Nutrient and stoichiometric characteristics of aggregates in a sloping farmland area under different tillage practices. Sustainability 2021, 13, 890. [Google Scholar] [CrossRef]
- Gan, Y.G.; Siddique, K.H.M.; Turner, N.C.; Li, X.G.; Niu, J.Y.; Yang, C.; Liu, L.P.; Chai, Q. Ridge-furrow mulching systems–an innovative technique for boosting crop productivity in semiarid rain-fed environments. Adv. Agron. 2013, 118, 429–476. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Li, L.T.; Liu, B.; Liu, J.; Chen, F.X.; Ni, J.P.; Wei, C.F.; Zhong, S.Q. Effect of ridge tillage with rice-rape rotation on the root system and yield of crops. Plant Soil 2024, 510, 291–304. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Xiong, K.N.; Chi, Y.K. Effects of intercropping on fractal dimension and physicochemical properties of soil in karst areas. Forests 2021, 12, 1422. [Google Scholar] [CrossRef]
- Beshir, S.; Abdulkerim, J. Effect of maize/haricot bean intercropping on soil fertility improvement under different tied ridges and planting methods, Southeast Ethiopia. JGEP 2017, 5, 63–70. [Google Scholar] [CrossRef]
- Luan, C.; He, W.; Su, X.; Wang, X.M.; Bai, Y.K.; Wang, L.X. Effects of biochar on soil water and temperature, nutrients, and yield of maize/soybean and maize/peanut intercropping systems. Int. Agroghys 2021, 35, 365–373. [Google Scholar] [CrossRef]
- Li, L.; Zhang, F.S.; Li, X.L.; Christie, P.; Sun, J.H.; Yang, S.C.; Tang, C.X. Interspecific facilitation of nutrient uptake by intercropped maize and faba bean. Nutr. Cycl. Agroecosystems 2003, 65, 61–71. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, G.X.; Cui, N.; Liu, E.K.; Zhang, Y.; Liu, D.H.; Ren, X.L.; Jia, Z.K.; Zhang, P. Suitable fertilization can improve maize growth and nutrient utilization in ridge-furrow rainfall harvesting cropland in semiarid area. Front. Plant Sci. 2023, 14, 1198366. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Xu, S.Z.; Liu, G.Y.; Lian, T.X.; Li, Z.H.; Liang, T.T.; Zhang, D.M.; Cui, Z.P.; Zhan, L.J.; Sun, L.; et al. Ridge intertillage alters rhizosphere bacterial communities and plant physiology to reduce yield loss of waterlogged cotton. Field Crops Res. 2023, 293, 108849. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Wei, C.; Yang, C.; Zhong, S. Effect of Mechanized Ridge Tillage with Rice-Rape Rotation on Paddy Soil Structure. Agriculture 2022, 12, 2147. [Google Scholar] [CrossRef]
- Zou, C.; Ding, L.L.; Zhang, Y.J.; Wang, P.C.; Chen, C.; Long, Z.F. Intercropping effects of sophora davidii and silage maize on soil physicochemical properties, Enzyme Activities and Yield. IOP Conf. Ser. EES 2021, 769, 032032. [Google Scholar] [CrossRef]
- Balesdent, J.; Chenu, C.; Balabane, M. Relationship of soil organic matter dynamics to physical protection and tillage. Soil Tillage Res. 2000, 53, 215–230. [Google Scholar] [CrossRef]
- Li, X.F.; Wang, Z.G.; Bao, X.G.; Sun, J.H.; Yang, S.C.; Wang, P.; Wang, C.B.; Wu, J.P.; Liu, X.R.; Tian, X.L.; et al. Long-term increased grain yield and soil fertility from intercropping. Nat. Sustain. 2021, 4, 943–950. [Google Scholar] [CrossRef]
- Are, M.; Kaart, T.; Selge, A.; Astover, A.; Reintam, E. The interaction of soil aggregate stability with other soil properties as influenced by manure and nitrogen fertilization. Zemdir Agric. 2018, 105, 195–202. [Google Scholar] [CrossRef]
- Gautam, A.; Guzman, J.; Kovacs, P.; Kumar, S. Manure and inorganic fertilization impacts on soil nutrients, aggregate stability, and organic carbon and nitrogen in different aggregate fractions. Arch. Agron. Soil. Sci. 2022, 68, 1261–1273. [Google Scholar] [CrossRef]
- Roohi, M.; Arif, M.S.; Guillaume, T.; Yasmeen, T.; Riaz, M.; Shakoor, A.; Farooq, T.H.; Shahzad, S.M.; Bragazza, L. Role of fertilization regime on soil carbon sequestration and crop yield in a maize-cowpea intercropping system on low fertility soils. Geoderma 2022, 428, 116152. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Env. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, H.; Yi, H. Impact of fertilizer on crop yield and C:N:P stoichiometry in arid and semi-arid soil. IJERPH 2021, 18, 4341. [Google Scholar] [CrossRef]
- Huang, Y.P.; Wang, Q.Q.; Zhang, W.J.; Zhu, P.; Xiao, Q.; Wang, C.J.; Wu, L.; Tian, Y.F.; Xu, M.G.; Gunina, A. Stoichiometric imbalance of soil carbon and nutrients drives microbial community structure under long-term fertilization. Appl. Soil Ecol. 2021, 168, 104119. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, M.; Huang, Z.; Lin, T.C.; Vadeboncoeur, M.A.; Searle, E.B.; Chen, H.Y.H. Temporal changes in soil C-N-P stoichiometry over the past 60 years across subtropical China. Glob. Change Biol. 2018, 24, 1308–1320. [Google Scholar] [CrossRef]
- Gong, X.W.; Dang, K.; Lv, S.M.; Zhao, G.; Wang, H.L.; Feng, B.L. Interspecific competition and nitrogen application alter soil ecoenzymatic stoichiometry, microbial nutrient status, and improve grain yield in broomcorn millet/mung bean intercropping systems. Field Crops Res. 2021, 270, 108227. [Google Scholar] [CrossRef]
- Saudy, H.S. Maize–cowpea intercropping as an ecological approach for nitrogen-use rationalization and weed suppression. Arch. Agron. Soil. Sci. 2015, 61, 1–14. [Google Scholar] [CrossRef]
- Usman, M.; Nangere, M.G.; Audu, S.D. Advantages of intercropping cowpea with maize crop under different tillage methods and inorganic fertilizer rates. Int. J. Res. Agric. Food Sci. 2018, 4, 18–33. Available online: https://www.researchgate.net/publication/360525903 (accessed on 21 January 2024).
- Mwende, N.; Danga, B.O.; Mugwe, J.; Kwena, K. Effect of integrating tied ridging, fertilizers and cropping systems on maize performance’in arid and semi-arid lands of Eastern Kenya. AJEST 2019, 5, 87–104. [Google Scholar] [CrossRef]
- Saidia, P.S.; Asch, F.; Kimaro, A.A.; Germer, J.; Kahimba, F.C.; Graef, F.; Semoka, J.M.R.; Rweyemamu, C.L. Soil moisture management and fertilizer micro-dosing on yield and land utilization efficiency of inter-cropping maize-pigeon-pea in sub humid Tanzania. Agr. Water Manag. 2019, 223, 105712. [Google Scholar] [CrossRef]
- Bai, Y.X.; Zhou, Y.C.; He, H.Z. Effects of rehabilitation through afforestation on soil aggregate stability and aggregate-associated carbon after forest fires in subtropical China. Geoderma 2020, 376, 114548. [Google Scholar] [CrossRef]
- Soinne, H.; Hovi, J.; Tammeorg, P.; Turtola, E. Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma 2014, 219, 162–167. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agriculture Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2015. [Google Scholar]
- Dou, Y.X.; Yang, Y.; An, S.S.; Zhu, Z. Effects of different vegetation restoration measures on soil aggregate stability and erodibility on the Loess Plateau, China. Catena 2020, 185, 104294. [Google Scholar] [CrossRef]
- Zuo, F.L.; Li, X.Y.; Yang, X.F.; Wang, Y.; Ma, Y.J.; Huang, Y.H.; Wei, C.F. Soil particle-size distribution and aggregate stability of new reconstructed purple soil affected by soil erosion in overland flow. J. Soils Sediments 2020, 20, 272–283. [Google Scholar] [CrossRef]
- Blake, G.R. Bulk density. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling; American Society of Agronomy: Madison, WI, USA, 1965; Volume 9, pp. 374–390. [Google Scholar] [CrossRef]
- Das, B.; Chakraborty, D.; Singh, V.K.; Aggarwal, P.; Singh, R.; Dwivedi, B.S.; Mishra, R.P. Effect of integrated nutrient management practice on soil aggregate properties, its stability and aggregate-associated carbon content in an intensive rice-wheat system. Soil Tillage Res. 2014, 136, 9–18. [Google Scholar] [CrossRef]
- Tian, X.L.; Wang, C.B.; Bao, X.G.; Wang, P.; Li, X.F.; Yang, S.C.; Ding, G.C.; Christie, P.; Li, L. Crop diversity facilitates soil aggregation in relation to soil microbial community composition driven by intercropping. Plant Soil 2019, 436, 173–192. [Google Scholar] [CrossRef]
- Beedy, T.L.; Snapp, S.S.; Akinnifesi, F.K.; Sileshi, G.W. Impact of Gliricidia sepium intercropping on soil organic matter fractions in a maize-based cropping system. Agr. Ecosyst. Environ. 2010, 138, 139–146. [Google Scholar] [CrossRef]
- Konopinski, M.; Kesik, T.; Blazewicz-Wozniak, M. The effect of intercrops and ploughing term on the structure of yield and some qualities of salsify (Tragopogon porrifolius L.) roots. Acta Sci. Pol. Hortorum Cultus 2013, 12, 35–45. Available online: https://czasopisma.up.lublin.pl/asphc/article/view/2878 (accessed on 23 January 2024).
- Bravo-Garza, M.R.; Voroney, P.; Bryan, R.B. Particulate organic matter in water stable aggregates formed after the addition of 14C-labeled maize residues and wetting and drying cycles in vertisols. Soil Biol. Biochem. 2010, 42, 953–959. [Google Scholar] [CrossRef]
- Guo, F.; Wang, M.L.; Si, T.; Wang, Y.F.; Zhao, H.J.; Zhang, X.J.; Yu, X.N.; Wan, S.B.; Zou, X.X. Maize-peanut intercropping led to an optimization of soil from the perspective of soil microorganism. Arch. Agron. Soil. Sci. 2021, 67, 1986–1999. [Google Scholar] [CrossRef]
- Lian, T.X.; Mu, Y.H.; Jin, J.; Ma, Q.B.; Cheng, Y.B.; Cai, Z.D.; Nian, H. Impact of intercropping on the coupling between soil microbial community structure, activity, and nutrient-use efficiencies. PeerJ 2019, 7, e6412. Available online: https://peerj.com/articles/6412/ (accessed on 23 January 2024). [CrossRef]
- Brandao, E.D.; da Silva, I.D.F. Formation and stabilization of aggregates by the grass root system in an Oxisol/Formacao e estabilizacao de agregados pelo sistema radicular de braquiaria em um Nitossolo Vermelho. Rural. Sci. 2012, 42, 1193–1200. [Google Scholar]
- Shi, M.F.; Kang, Y.C.; Zhang, W.N.; Yang, X.Y.; Fan, Y.L.; Yu, H.F.; Zhang, R.Y.; Guo, A.X.; Qin, S.H. Plastic film mulching with ridge planting alters soil chemical and biological properties to increase potato yields in semiarid Northwest China. Chem. Biol. Technol. Agric. 2022, 9, 16. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Silva, E.A.; Oliveira GCde Carducci, C.E.; Silva, B.M.; Oliveira LMde Costa, J.C. Increasing doses of gypsum aggregate stability organic carbon in the Cerrado Latosol under coffee crop. J. Agrar. Sci. 2013, 56, 25–32. [Google Scholar] [CrossRef]
- García-Orenes, F.; Guerrero, C.; Mataix-Solera, J.; Navarro-Pedreño, J.; Gómez, I.; Mataix-Beneyto, J. Factors controlling the aggregate stability and bulk density in two different degraded soils amended with biosolids. Soil Tillage Res. 2005, 82, 65–76. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, C.; Xie, D.; Shi, X.L.; Jiang, X.J. Effects of Ridge and No-Tillage on Fertility Change of Purple Paddy Soil. J. Southwest Univ. Nat. Sci. Ed. 2023, 45, 138–146. (In Chinese) [Google Scholar] [CrossRef]
- Liu, H.; Wei, B.H.; Dang, K.K.; Peng, J.W.; Li, J.G.; Dong, Y.H. Effect of deep vertical rotary tillage on microbial community in sugar cane soil. Chin. J. Trop. Crops 2022, 43, 597–605. (In Chinese) [Google Scholar] [CrossRef]
- Adingo, S. Review on the fully mulched ridge–furrow system for sustainable maize production on the semi-arid Loess Plateau. J. Integr. Agric. 2023, 22, 1277–1290. [Google Scholar] [CrossRef]
- Du, S.P.; Ma, Z.M.; Chen, J.; Xue, L.; Tang, C.N.; MEShareef, T.; HMSiddique, K. Effects of organic fertilizer proportion on the distribution of soil aggregates and their associated organic carbon in a field mulched with gravel. Sci. Rep. 2022, 12, 11513. [Google Scholar] [CrossRef]
- Xiao, S.S.; Zhang, W.; Ye, Y.Y.; Zhao, J.; Wang, K.L. Soil aggregate mediates the impacts of land uses on organic carbon, total nitrogen, and microbial activity in a Karst ecosystem. Sci. Rep. 2017, 7, 41402. [Google Scholar] [CrossRef]
- Dignac, M.F.; Derrien, D.; Barré, P.; Barot, S.; Cécillon, L.; Chenu, C.; Chevallier, T.; Freschet, G.T.; Garnier, P.; Guenet, B.; et al. Increasing soil carbon storage: Mechanisms, effects of agricultural practices and proxies. A review. Agron. Sustain. Dev. 2017, 37, 14. [Google Scholar] [CrossRef]
- Feng, C.; Sun, Z.X.; Zhang, L.Z.; Feng, L.S.; Zheng, J.M.; Bai, W.; Gu, C.F.; Wang, Q.; van der Werf, W. Maize/peanut intercropping increases land productivity: A meta-analysis. Field Crops Res. 2021, 270, 108208. [Google Scholar] [CrossRef]
- Fonte, S.J.; Nesper, M.; Hegglin, D.; Velásquez, J.E.; Ramirez, B.; Rao, I.M.; Bernasconi, S.M.; Bünemann, E.K.; Frossard, E.; Oberson, A. Pasture degradation impacts soil phosphorus storage via changes to aggregate-associated soil organic matter in highly weathered tropical soils. Soil Biol. Biochem. 2014, 68, 150–157. [Google Scholar] [CrossRef]
- Kurothe, R.S.; Kumar, G.; Singh, R.; Singh, H.B.; Tiwari, S.P.; Vishwakarma, A.K.; Pande, V.C. Effect of tillage and cropping systems on runoff, soil loss and crop yields under semiarid rainfed agriculture in India. Soil Tillage Res. 2014, 140, 126–134. [Google Scholar] [CrossRef]
- Liu, C.; Pan, J.S.; Dong, Z.; Li, Y.B.; Zhang, Q.; Wu, Q.C. Characteristics of soil physical property improvement and carbon sequestration for agroforestry in yellow river flood plain. Bull. Soil Water Conserv. 2022, 42, 318–323. (In Chinese) [Google Scholar] [CrossRef]
- Materechera, S.A.; Mloza-Banda, H.R. Soil penetration resistance, root growth and yield of maize as influenced by tillage system on ridges in Malawi. Soil Tillage Res. 1997, 41, 13–24. [Google Scholar] [CrossRef]
- Zheng, H.B.; Huang, H.; Liu, J.X.; Yao, L.; He, H. Recent progress and prospects in the development of ridge tillage cultivation technology in China. Soil Tillage Res. 2014, 142, 1–7. [Google Scholar] [CrossRef]
- Li, L.; Li, S.M.; Sun, J.H.; Zhou, L.L.; Bao, X.G.; Zhang, H.G.; Zhang, F.S. Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. PNAS 2007, 104, 11192–11196. [Google Scholar] [CrossRef]
- Jobbagy, E.G.; Jackson, R.B. The distribution of soil nutrients with depth: Global patterns and the imprint of plants. Biogeochemistry 2001, 53, 51–77. [Google Scholar] [CrossRef]
- Shi, X.H.; Yang, X.M.; Drury, C.F.; Reynolds, W.D.; McLaughlin, N.B.; Zhang, X.P. Impact of ridge tillage on soil organic carbon and selected physical properties of a clay loam in southwestern Ontario. Soil Tillage Res. 2012, 120, 1–7. [Google Scholar] [CrossRef]
- Akinnifesi, F.K.; Makumba, W.; Sileshi, G.; Ajayi, O.C.; Mweta, D. Synergistic effect of inorganic N and P fertilizers and organic inputs from Gliricidia sepium on productivity of intercropped maize in Southern Malawi. Plant Soil 2007, 294, 203–217. [Google Scholar] [CrossRef]
- Cui, Y.X.; Moorhead, D.L.; Guo, X.B.; Peng, S.S.; Wang, Y.Q.; Zhang, X.C.; Fang, L.C. Stoichiometric models of microbial metabolic limitation in soil systems. Global Ecol. Biogeogr. 2021, 30, 2297–2311. [Google Scholar] [CrossRef]
- Singh, G.; Williard, K.W.J.; Schoonover, J.E. Cover Crops and Tillage Influence on Nitrogen Dynamics in Plant-Soil-Water Pools. SSSA J. 2018, 82, 1572–1582. [Google Scholar] [CrossRef]
- Ren, Z.J.; Han, X.J.; Feng, H.X.; Wang, L.F.; Ma, G.; Li, J.H.; Lv, J.J.; Tian, W.Z.; He, X.H.; Zhao, Y.N.; et al. Long-term conservation tillage improves soil stoichiometry balance and crop productivity based on a 17-year experiment in a semi-arid area of northern China. Sci. Total Environ. 2024, 908, 168283. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Zhang, H.; OuYang, Z.C.; Zhao, X.M. Effects of different land use types on ecological stoichiometry characteristics of Carbon, Nitrogen and Phosphorus in farmland soils in Jiangxi Province, China. Acta Sci. Cir. 2019, 39, 939–951. Available online: https://html.rhhz.net/hjkxxb/html/20180806001.htm (accessed on 21 January 2024). (In Chinese).
- Liu, J.; Wang, Y.; Li, Y.; Peñuelas, J.; Zhao, Y.; Sardans, J.; Tetzlaff, D.; Liu, J.; Liu, X.L.; Yuan, H.Z.; et al. Soil ecological stoichiometry synchronously regulates stream nitrogen and phosphorus concentrations and ratios. Catena 2023, 231, 107357. [Google Scholar] [CrossRef]
- Bimüller, C.; Kreyling, O.; Kölbl, A.; von Lützow, M.; Kögel-Knabner, I. Carbon and nitrogen mineralization in hierarchically structured aggregates of different size. Soil Tillage Res. 2016, 160, 23–33. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C: N: P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Song, X.L.; Lu, X.G.; Xue, Z.S. Ecological stoichiometry of carbon, nitrogen, and phosphorus in estuarine wetland soils: Influences of vegetation coverage, plant communities, geomorphology, and seawalls. J. Soils Sediments 2013, 13, 1043–1051. [Google Scholar] [CrossRef]
- Tessier, J.T.; Raynal, D.J. Use of nitrogen to phosphorus ratios in plant tissue as an indicator of nutrient limitation and nitrogen saturation. J. Appl. Ecol. 2003, 40, 523–534. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. Available online: https://www.jstor.org/stable/2404783 (accessed on 21 January 2024). [CrossRef]
- Kan, H.; Xu, H.; Zhang, G.; Chen, C.; Pang, Z.; Fan, X.; Wu, J. Stoichiometric characteristics drive the soil aggregate stability after 5 years of vegetation restoration in China. Front. Ecol. Evol. 2023, 11, 1280303. [Google Scholar] [CrossRef]
- Zhong, X.L.; Li, J.T.; Li, X.J.; Ye, Y.C.; Liu, S.S.; Hallett, P.D.; Ogden, M.R.; Naveed, M. Physical protection by soil aggregates stabilizes soil organic carbon under simulated N deposition in a subtropical forest of China. Geoderma 2017, 285, 323–332. [Google Scholar] [CrossRef]
- Liu, R.Q.; Zhou, X.H.; Wang, J.W.; Shao, J.J.; Fu, Y.L.; Liang, C.; Yan, E.R.; Chen, X.Y.; Wang, X.H.; Bai, S.H. Differential magnitude of rhizosphere effects on soil aggregation at three stages of subtropical secondary forest successions. Plant Soil 2019, 436, 365–380. [Google Scholar] [CrossRef]
- He, Y.Q.; Zhang, Q.C.; Jiang, C.Y.; Lan, Y.H.; Zhang, H.; Ye, S.M. Mixed planting improves soil aggregate stability and aggregate-associated C-N-P accumulation in subtropical China. Front. For. Global Change 2023, 6, 1141953. [Google Scholar] [CrossRef]
- An, S.; Mentler, A.; Mayer, H.; Blum, W.E. Soil aggregation, aggregate stability, organic carbon and nitrogen in different soil aggregate fractions under forest and shrub vegetation on the Loess Plateau, China. Catena 2010, 81, 226–233. [Google Scholar] [CrossRef]
| Year | P Level | PP | DR0.25 (%) | WR0.25 (%) | ELT (%) | PAD (%) | MWD (mm) | GMD (mm) | BD (g·cm−3) | Pt (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | P0 | F-M||P | 89.90 ± 0.14 c | 63.41 ± 0.24 c | 36.59 ± 0.24 a | 29.47 ± 0.25 a | 0.55 ± 0.00 d | 0.88 ± 0.00 c | / | / |
| R-M||P | 91.78 ± 0.16 a | 71.67 ± 0.19 a | 28.33 ± 0.19 c | 21.91 ± 0.11 c | 0.66 ± 0.00 b | 0.99 ± 0.00 b | / | / | ||
| P180 | F-M||P | 89.01 ± 0.09 d | 64.89 ± 0.16 b | 35.11 ± 0.16 b | 27.10 ± 0.20 b | 0.62 ± 0.00 c | 0.98 ± 0.00 b | / | / | |
| R-M||P | 91.00 ± 0.05 b | 71.81 ± 0.07 a | 28.19 ± 0.07 c | 21.09 ± 0.04 c | 0.71 ± 0.00 a | 1.05 ± 0.00 a | / | / | ||
| 2023 | P0 | F-M||P | 97.22 ± 0.03 c | 71.90 ± 0.02 d | 28.10 ± 0.02 a | 26.05 ± 0.00 a | 0.67 ± 0.00 d | 1.00 ± 0.00 c | 1.32 ± 0.01 a | 50.28 ± 0.23 c |
| R-M||P | 99.15 ± 0.02 a | 81.51 ± 0.25 b | 20.72 ± 0.25 b | 17.79 ± 0.25 c | 0.76 ± 0.00 b | 1.06 ± 0.00 b | 1.27 ± 0.01 bc | 52.06 ± 0.26 ab | ||
| P180 | F-M||P | 96.55 ± 0.03 d | 75.48 ± 0.55 c | 27.30 ± 0.41 a | 21.82 ± 0.58 b | 0.72 ± 0.00 c | 1.06 ± 0.00 b | 1.29 ± 0.00 b | 51.46 ± 0.14 b | |
| R-M||P | 98.48 ± 0.03 b | 86.59 ± 0.24 a | 20.36 ± 0.38 b | 12.08 ± 0.23 d | 0.81 ± 0.01 a | 1.11 ± 0.00 a | 1.24 ± 0.00 c | 53.12 ± 0.15 a | ||
| P level | *** | *** | *** | *** | *** | *** | *** | *** | ||
| PP | *** | *** | *** | *** | *** | *** | * | ** | ||
| P level×PP | *** | *** | *** | *** | ** | *** | *** | *** | ||
| Year | Size Fractions (mm) | P Level | PP | Ca2-P | Ca8-P | Al-P | Fe-P | Olsen-P | Ca10-P |
|---|---|---|---|---|---|---|---|---|---|
| 2019 | >2 | P0 | F-M||P | 2.20 ± 0.04 d | 147.43 ± 2.11 d | 29.34 ± 0.13 d | 58.06 ± 0.96 d | 2.98 ± 0.06 b | 159.15 ± 0.85 b |
| P0 | R-M||P | 3.54 ± 0.00 c | 177.92 ± 0.28 c | 41.06 ± 0.09 c | 95.02 ± 0.26 c | 1.37 ± 0.02 c | 135.48 ± 0.39 d | ||
| P180 | F-M||P | 7.65 ± 0.11 b | 197.24 ± 3.57 b | 52.20 ± 0.21 b | 102.41 ± 0.31 b | 5.22 ± 0.13 a | 181.63 ± 0.80 a | ||
| P180 | R-M||P | 10.84 ± 0.03 a | 255.14 ± 0.92 a | 68.40 ± 0.34 a | 140.94 ± 0.38 a | 2.61 ± 0.03 b | 150.28 ± 1.37 c | ||
| 1–2 | P0 | F-M||P | 2.69 ± 0.04 d | 147.43 ± 2.11 d | 29.34 ± 0.13 d | 58.06 ± 0.96 d | 2.98 ± 0.06 b | 182.08 ± 0.32 b | |
| P0 | R-M||P | 4.03 ± 0.03 c | 177.92 ± 0.28 c | 41.06 ± 0.09 c | 95.02 ± 0.26 c | 1.37 ± 0.02 c | 151.80 ± 1.25 c | ||
| P180 | F-M||P | 10.60 ± 0.21 b | 197.24 ± 3.57 b | 52.20 ± 0.21 b | 102.41 ± 0.31 b | 5.22 ± 0.13 a | 210.67 ± 0.95 a | ||
| P180 | R-M||P | 15.00 ± 0.21 a | 255.14 ± 0.92 a | 68.40 ± 0.34 a | 140.94 ± 0.38 a | 2.61 ± 0.03 b | 183.20 ± 0.45 b | ||
| 0.5–1 | P0 | F-M||P | 3.33 ± 0.04 d | 137.83 ± 0.53 d | 39.04 ± 0.08 d | 89.07 ± 0.49 d | 3.10 ± 0.02 c | 197.29 ± 2.09 c | |
| P0 | R-M||P | 4.70 ± 0.03 c | 171.92 ± 0.62 c | 55.65 ± 0.10 c | 108.86 ± 0.32 c | 1.55 ± 0.03 d | 171.57 ± 1.22 d | ||
| P180 | F-M||P | 12.69 ± 0.20 b | 217.72 ± 1.71 b | 65.99 ± 0.09 b | 134.40 ± 1.04 b | 6.74 ± 0.03 a | 231.73 ± 1.65 a | ||
| P180 | R-M||P | 18.18 ± 0.16 a | 297.05 ± 0.10 a | 81.54 ± 0.50 a | 168.70 ± 0.86 a | 3.92 ± 0.11 b | 211.57 ± 0.18 b | ||
| 0.25–0.5 | P0 | F-M||P | 3.92 ± 0.04 d | 150.27 ± 0.76 d | 49.30 ± 0.10 d | 103.54 ± 0.60 d | 5.96 ± 0.06 b | 228.18 ± 0.60 c | |
| P0 | R-M||P | 5.37 ± 0.04 c | 206.52 ± 2.76 c | 65.18 ± 0.08 c | 122.82 ± 0.11 c | 2.57 ± 0.09 c | 199.65 ± 1.21 d | ||
| P180 | F-M||P | 15.56 ± 0.17 b | 256.20 ± 0.96 b | 72.64 ± 0.55 b | 179.56 ± 0.67 b | 8.96 ± 0.15 a | 260.60 ± 0.48 a | ||
| P180 | R-M||P | 23.87 ± 0.13 a | 319.69 ± 2.00 a | 91.78 ± 0.34 a | 210.02 ± 0.69 a | 5.84 ± 0.16 b | 239.04 ± 0.21 b | ||
| <0.25 | P0 | F-M||P | 5.02 ± 0.09 d | 142.31 ± 0.53 d | 51.28 ± 0.63 d | 115.57 ± 0.15 d | 6.90 ± 0.20 b | 258.07 ± 0.46 c | |
| P0 | R-M||P | 6.48 ± 0.06 c | 207.51 ± 3.84 c | 73.14 ± 0.97 c | 143.32 ± 0.95 c | 3.04 ± 0.05 c | 227.90 ± 0.87 d | ||
| P180 | F-M||P | 15.84 ± 0.02 b | 298.42 ± 2.59 b | 87.01 ± 0.40 b | 215.99 ± 0.11 b | 12.86 ± 0.11 a | 286.97 ± 0.31 a | ||
| P180 | R-M||P | 21.81 ± 0.23 a | 353.84 ± 0.95 a | 105.26 ± 0.69 a | 248.12 ± 1.46 a | 7.35 ± 0.24 b | 269.59 ± 1.36 b | ||
| 2023 | >2 | P0 | F-M||P | 3.21 ± 0.02 c | 173.44 ± 1.48 d | 43.15 ± 0.06 d | 103.07 ± 0.30 d | 1.77 ± 0.01 b | 119.36 ± 0.64 b |
| P0 | R-M||P | 4.57 ± 0.02 c | 215.48 ± 1.48 c | 56.29 ± 0.30 c | 123.45 ± 0.56 c | 0.81 ± 0.01 d | 101.61 ± 0.29 d | ||
| P180 | F-M||P | 10.41 ± 0.41 b | 266.14 ± 1.66 b | 69.49 ± 0.19 b | 146.57 ± 0.55 b | 2.74 ± 0.03 a | 136.22 ± 0.60 a | ||
| P180 | R-M||P | 12.41 ± 0.29 a | 314.78 ± 2.24 a | 81.84 ± 0.23 a | 188.05 ± 0.54 a | 1.43 ± 0.02 c | 112.71 ± 1.03 c | ||
| 1–2 | P0 | F-M||P | 3.70 ± 0.02 d | 173.44 ± 1.48 d | 43.15 ± 0.06 d | 103.07 ± 0.30 d | 1.77 ± 0.01 b | 136.56 ± 0.24 b | |
| P0 | R-M||P | 5.06 ± 0.02 c | 215.48 ± 1.48 c | 56.29 ± 0.30 c | 123.45 ± 0.56 c | 0.81 ± 0.01 d | 113.85 ± 0.94 c | ||
| P180 | F-M||P | 13.15 ± 0.14 b | 266.14 ± 1.66 b | 69.49 ± 0.19 b | 146.57 ± 0.55 b | 2.74 ± 0.03 a | 158.00 ± 0.71 a | ||
| P180 | R-M||P | 16.04 ± 0.25 a | 314.78 ± 2.24 a | 81.84 ± 0.23 a | 188.05 ± 0.54 a | 1.43 ± 0.02 c | 137.40 ± 0.34 b | ||
| 0.5–1 | P0 | F-M||P | 4.28 ± 0.03 d | 173.43 ± 0.93 d | 54.92 ± 0.12 d | 116.35 ± 0.20 d | 2.44 ± 0.01 b | 147.97 ± 1.56 c | |
| P0 | R-M||P | 5.64 ± 0.03 c | 215.48 ± 0.93 c | 71.74 ± 0.15 c | 135.35 ± 0.21 c | 1.01 ± 0.01 d | 128.68 ± 0.92 d | ||
| P180 | F-M||P | 15.24 ± 0.15 b | 297.80 ± 0.32 b | 87.87 ± 0.32 b | 172.49 ± 0.64 b | 3.72 ± 0.03 a | 173.80 ± 1.24 a | ||
| P180 | R-M||P | 19.21 ± 0.17 a | 360.17 ± 1.02 a | 98.93 ± 0.27 a | 226.48 ± 1.06 a | 2.02 ± 0.03 c | 158.68 ± 0.13 b | ||
| 0.25–0.5 | P0 | F-M||P | 4.78 ± 0.02 d | 180.75 ± 2.66 d | 66.18 ± 0.26 d | 132.42 ± 0.41 d | 3.36 ± 0.03 b | 171.13 ± 0.45 c | |
| P0 | R-M||P | 6.14 ± 0.02 c | 222.79 ± 2.66 c | 82.78 ± 0.22 c | 150.62 ± 0.32 c | 1.44 ± 0.01 d | 149.74 ± 0.91 d | ||
| P180 | F-M||P | 18.11 ± 0.09 b | 323.29 ± 0.81 b | 101.19 ± 0.48 b | 220.11 ± 0.62 b | 4.80 ± 0.08 a | 195.45 ± 0.36 a | ||
| P180 | R-M||P | 24.90 ± 0.21 a | 391.21 ± 1.65 a | 112.16 ± 0.90 a | 252.54 ± 1.39 a | 3.03 ± 0.05 c | 179.28 ± 0.16 b | ||
| <0.25 | P0 | F-M||P | 5.88 ± 0.03 c | 187.17 ± 3.61 d | 77.67 ± 0.15 d | 150.16 ± 0.38 d | 4.37 ± 0.00 b | 193.55 ± 0.35 c | |
| P0 | R-M||P | 7.24 ± 0.03 c | 229.21 ± 3.61 c | 98.11 ± 0.10 c | 168.29 ± 0.21 c | 1.95 ± 0.01 d | 170.93 ± 0.65 d | ||
| P180 | F-M||P | 25.45 ± 0.36 b | 370.30 ± 3.65 b | 117.43 ± 0.82 b | 259.80 ± 1.26 b | 6.38 ± 0.08 a | 215.23 ± 0.23 a | ||
| P180 | R-M||P | 32.20 ± 0.39 a | 426.16 ± 1.57 a | 126.32 ± 0.87 a | 290.37 ± 1.00 a | 4.04 ± 0.02 c | 202.19 ± 1.02 b | ||
| P level | *** | *** | *** | *** | *** | *** | |||
| PP | *** | *** | *** | *** | *** | *** | |||
| P level×PP | *** | *** | *** | *** | *** | * | |||
| Year | P | PP | SOC g·kg−1 | TN g·kg−1 | TP g·kg−1 | AP mg·kg−1 | Ca2-P mg·kg−1 | Ca8-P mg·kg−1 | Al-P mg·kg−1 | Fe-P mg·kg−1 | Olsen-P mg·kg−1 | Ca10-P mg·kg−1 | SCS g·m−2 | SNS g·m−2 | SPS g·m−2 | C:N | C:P | N:P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | P0 | F-M||P | 13.21 ± 0.02 d | 1.21 ± 0.00 c | 0.77 ± 0.00 d | 13.46 ± 0.03 b | 2.89 ± 0.03 b | 147.36 ± 1.15 b | 39.05 ± 0.11 b | 82.01 ± 0.51 b | 4.45 ± 0.03 b | 185.29 ± 0.28 c | 3.48 ± 0.01 d | 0.32 ± 0.00 c | 0.20 ± 0.00 d | 10.95 ± 0.04 c | 17.10 ± 0.06 b | 1.56 ± 0.01 a |
| R-M||P | 15.68 ± 0.08 b | 1.33 ± 0.01 b | 0.84 ± 0.00 c | 16.04 ± 0.02 a | 4.09 ± 0.01 a | 182.79 ± 0.56 a | 51.51 ± 0.12 a | 108.26 ± 0.12 a | 1.85 ± 0.01 c | 153.53 ± 0.69 d | 3.99 ± 0.02 b | 0.34 ± 0.00 b | 0.21 ± 0.00 c | 11.81 ± 0.02 ab | 18.66 ± 0.15 a | 1.58 ± 0.01 a | ||
| P180 | F-M||P | 15.13 ± 0.09 c | 1.30 ± 0.01 b | 1.00 ± 0.00 b | 37.14 ± 0.28 b | 10.52 ± 0.10 b | 235.12 ± 2.52 b | 67.40 ± 0.13 b | 146.98 ± 0.28 b | 8.03 ± 0.09 c | 212.82 ± 0.83 c | 3.89 ± 0.03 c | 0.34 ± 0.00 b | 0.26 ± 0.00 b | 11.61 ± 0.12 b | 15.20 ± 0.11 d | 1.31 ± 0.01 b | |
| R-M||P | 17.61 ± 0.08 a | 1.43 ± 0.01 a | 1.10 ± 0.01 a | 46.24 ± 0.28 a | 14.30 ± 0.04 a | 290.65 ± 0.49 a | 81.43 ± 0.15 a | 174.84 ± 0.42 a | 4.11 ± 0.03 d | 179.29 ± 0.68 d | 4.38 ± 0.03 a | 0.36 ± 0.00 a | 0.27 ± 0.00 a | 12.30 ± 0.13 a | 16.07 ± 0.07 c | 1.31 ± 0.02 b | ||
| 2023 | P0 | F-M||P | 14.02 ± 0.04 c | 1.31 ± 0.01 c | 0.81 ± 0.01 d | 15.63 ± 0.06 b | 3.60 ± 0.02 b | 175.73 ± 1.65 b | 51.67 ± 0.09 b | 113.94 ± 0.22 b | 2.35 ± 0.01 c | 131.25 ± 0.29 c | 3.69 ± 0.02 c | 0.35 ± 0.00 c | 0.21 ± 0.00 b | 10.68 ± 0.09 b | 17.39 ± 0.21 b | 1.63 ± 0.01 a |
| R-M||P | 16.14 ± 0.07 b | 1.44 ± 0.01 b | 0.86 ± 0.01 c | 20.03 ± 0.23 a | 4.80 ± 0.01 a | 216.52 ± 1.46 a | 62.80 ± 0.24 a | 129.47 ± 0.34 a | 0.94 ± 0.00 d | 107.82 ± 0.37 d | 4.10 ± 0.04 b | 0.37 ± 0.00 b | 0.22 ± 0.00 b | 11.19 ± 0.02 b | 18.72 ± 0.06 a | 1.67 ± 0.00 a | ||
| P180 | F-M||P | 16.26 ± 0.04 b | 1.46 ± 0.01 b | 1.12 ± 0.00 b | 43.08 ± 0.07 b | 12.51 ± 0.23 b | 291.12 ± 1.42 b | 81.84 ± 0.15 b | 173.11 ± 0.49 b | 3.59 ± 0.04 c | 151.30 ± 0.66 c | 4.18 ± 0.01 b | 0.38 ± 0.00 b | 0.29 ± 0.00 a | 11.11 ± 0.11 b | 14.50 ± 0.01 d | 1.31 ± 0.01 b | |
| R-M||P | 18.91 ± 0.03 a | 1.59 ± 0.03 a | 1.18 ± 0.00 a | 51.40 ± 0.17 a | 14.30 ± 0.16 a | 335.42 ± 1.60 a | 89.68 ± 0.22 a | 205.73 ± 0.61 a | 1.80 ± 0.02 d | 124.21 ± 0.61 d | 4.70 ± 0.01 a | 0.40 ± 0.01 a | 0.29 ± 0.00 a | 11.88 ± 0.18 a | 16.01 ± 0.04 c | 1.35 ± 0.02 b | ||
| P | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ||
| PP | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ns | ||
| P × PP | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zan, Z.; Ma, R.; Wang, J.; Liu, L.; Ning, T.; Jiao, N. Co-Ridge Planting Enhances Yield Advantages of Maize Intercropping with Peanut by Improving Soil Aggregate Stability and the Ecological Stoichiometric Characteristics of Carbon, Nitrogen, and Phosphorus. Agronomy 2025, 15, 2227. https://doi.org/10.3390/agronomy15092227
Zan Z, Ma R, Wang J, Liu L, Ning T, Jiao N. Co-Ridge Planting Enhances Yield Advantages of Maize Intercropping with Peanut by Improving Soil Aggregate Stability and the Ecological Stoichiometric Characteristics of Carbon, Nitrogen, and Phosphorus. Agronomy. 2025; 15(9):2227. https://doi.org/10.3390/agronomy15092227
Chicago/Turabian StyleZan, Zhiman, Rentian Ma, Jiangtao Wang, Ling Liu, Tangyuan Ning, and Nianyuan Jiao. 2025. "Co-Ridge Planting Enhances Yield Advantages of Maize Intercropping with Peanut by Improving Soil Aggregate Stability and the Ecological Stoichiometric Characteristics of Carbon, Nitrogen, and Phosphorus" Agronomy 15, no. 9: 2227. https://doi.org/10.3390/agronomy15092227
APA StyleZan, Z., Ma, R., Wang, J., Liu, L., Ning, T., & Jiao, N. (2025). Co-Ridge Planting Enhances Yield Advantages of Maize Intercropping with Peanut by Improving Soil Aggregate Stability and the Ecological Stoichiometric Characteristics of Carbon, Nitrogen, and Phosphorus. Agronomy, 15(9), 2227. https://doi.org/10.3390/agronomy15092227






