1. Introduction
Hippeastrum (
Amaryllidaceae) is a well-known bulbous plant, valued for its charm for centuries, with the first reports of it appearing in Europe as early as the 18th century. Over the years, there has been a growing interest in
Hippeastrum flowers, which has led to an increase in their cultivation area worldwide [
1,
2].
Hippeastrum bulbs are mainly reproduced in South America (including Brazil, with an annual production of 17 million bulbs [
3], South Africa and the Netherlands. In South Africa,
Hippeastrum, which is an introduced species there, has the largest cultivation area. The bulbs are first propagated in in vitro cultures and hardened in greenhouses and then reproduced in the open field [
4]. Dutch data indicate an increase in the area under
Hippeastrum cultivation in that country from 41 ha in 2020 to 52 ha in 2023 [
5]. This therefore necessitates an increase in productivity and the development of new micropropagation protocols for this genus.
In the case of
Hippeastrum, micropropagation consists of several stages, but—as with other geophytes—the end product is a bulb; hence the key stage is tuberisation, in which direct organogenesis occurs, and the acclimatisation stage. Previous studies have focused, among other things, on the overall improvement of micropropagation efficiency and bulb formation, e.g., through the use of methyl jasmonate, spermine, casein hydrolysate and progesterone, but also included the supplementation of media with cytokinins in combination with auxins [
6,
7,
8]. The initial material is twin-scale explants, which can be placed on Murashige and Skoog (MS) [
9] medium with the addition of plant growth regulators (PGRs). Regeneration occurs a few weeks later. Bulbs form on the inner side of the scale arch, right next to the basal plate [
10,
11]. The regeneration yield correlates with the size of the explants, as evidenced by the fact that one 2 cm long twin-scale explant yielded an average of 2.1 new bulbs, while explants smaller than 1 cm did not regenerate at all [
12].
The process of somatic embryogenesis in
Hippeastrum has been developed and described for bulbs and bulb scales [
13,
14], in vitro microbulbs [
15,
16], ovules [
17] and pedicels [
18]. The induction of somatic embryos from
Hippeastrum perianth segments has also been confirmed [
19]. Due to increasingly clear ecological trends, experiments evaluating the effect of adding biostimulants to in vitro media seem very interesting. To date, the effect of supplementing MS medium with the biostimulant Goteo on the efficiency of
Hippeastrum micropropagation has been investigated as an alternative to traditional PGRs, especially synthetic ones. The positive effect of Goteo on increased regeneration, as well as on the increased number of roots and mass of microplants, was observed at the stage of bulb formation of
H. hybridum ‘Double Roma’ and
H. ×
chmielii clone No. 18 [
16].
In 2019, an EU regulation defined biostimulants as agents that stimulate plant nutrition processes regardless of the nutrient content of the product, whose sole purpose is to improve at least one of the plant’s properties. These properties include: nutrient use efficiency, resistance to abiotic stress, quality characteristics and the assimilation of nutrients that are difficult to access from the soil or rhizosphere [
20]. Biostimulants are obtained from plants, peat, and also animals. They are usually single substances or multi-component preparations, which may contain: polymers with a large number of peptide bonds, amino acids, endogenous growth regulators, vitamins, enzymes and carbohydrates. Biostimulants are very often prepared based on algae extracts, but they can also be produced from crustacean shells containing a substance called chitosan [
21,
22,
23].
Geophytes with characteristic survival organs, i.e., tubers, rhizomes or bulbs, are characterised by high carbohydrate and water content in their tissues [
24]. These reserves are stored in the fleshy bases of leaves, bulb scales or tubers [
25]. Starch is the most abundant storage carbohydrate in this group of plants [
26]. It is the main source of carbon and is an insoluble glucan consisting of two glucose polymers—amylose and amylopectin, which are responsible for storing and supplying energy [
27]. Another group of storage sugars—alternatives to starch—are fructans, linear or branched polymers of fructose with a terminal glucose unit [
28]. In geophytes, the primary role of carbohydrates is to provide the energy needed for plant growth after a period of dormancy, despite often unfavourable conditions, and in particular they are used for respiration and as a structural component for cell wall construction [
29,
30].
The aim of the research was to evaluate the possibility of replacing synthetic PGRs added to the in vitro media with biostimulants of natural origin (Goteo and Folium) on the micropropagation and acclimatisation efficiency of several Hippeastrum genotypes. The effect of the biostimulants on the starch and fructan content of the bulbils after a 10-week in vitro culture was also investigated.
The use of both biostimulants as a supplement to the nutrient medium is a novelty in the micropropagation of ornamental geophytes, although Goteo has already been tested preliminary by us for
Hippeastrum [
16]. However, there are no reports in the available literature on studies concerning the use of the Folium biostimulant, and our research will partially fill the gap in this area of research. The first of these—Goteo—has been produced using PhysioActivator technology and contains amino acids, vitamins, oligosaccharides and phytohormones extracted from
Ascophyllum nodosum seaweed [
31]. The second one—Folium—is a liquid (foliar) biostimulant containing natural, free L-α amino acids produced by enzymatic hydrolysis, enriched with microelements: boron, manganese and zinc [
32].
4. Discussion
Our research has focused on the possibility of using environmentally friendly biostimulant preparations at various stages of
Hippeastrum micropropagation and ex vitro acclimatisation. Depending on their origin, biostimulants contain various hormonal components or substances that can interact with plants. These natural, organic preparations often reduce stress, increase the physiological activity of plants or improve plant growth and development, e.g., root development. Their use in ex vitro conditions is quite common [
21,
35,
36]. However, compared to the traditional use of biostimulants, there are only few reports on their use in in vitro cultures of ornamental geophytes as an alternative to commonly used PGRs. Thorpe et al. (2008) [
37], Molnár et al. (2011) [
38] and Sochacki et al. (2018) [
16] confirm the possibility of using natural substances in plant micropropagation. Among other things, this refers to the addition of seaweed extract, which can be a source of amino acids, peptides, fatty acids, carbohydrates, vitamins and phytohormones in various concentrations. This is confirmed by studies conducted on peas (
Pisum sativum), beetroot (
Beta vulgaris), tobacco (
Nicotiana tabacum) and amaryllis (
Hippeastrum). Stirk et al. (2002) [
39] and Ördög et al. (2004) [
40] proved in their studies the presence of plant growth regulators in extracts from seaweed and bacteria (cyanobacteria), which can be a useful source of these regulators in tissue cultures.
Our research has shown the effect of supplementing MS medium with Goteo (0.2%) and Folium (0.5%) biostimulants on most of the studied characteristics of
Hippeastrum. The use of Goteo increased plant weight in 40% of the studied population by an average of 0.25 g. Similar results were also obtained for
Hippeastrum earlier [
16], when studying the effect of supplementing the medium with the Goteo (0.1%) on the biometric parameters of clone 18
H. ×
chmielii. Plants on the medium with the biostimulant reached a mass of 2.2 g, while those on the control medium weighed only 1.2 g, i.e., almost half as much.
The effect of both biostimulants on leaf length was also demonstrated. The biostimulants Goteo and Folium demonstrated analogous efficacy, each promoting a mean increase of 1.21 cm in the target parameter in 40% of the studied genotypes. It is worth noting that the research using Folium as an in vitro nutrient supplement was definitely pioneering, as there are no reports on this subject in the literature. However, the use of a biostimulant based on free amino acids in in vitro cultures seems to be a very good direction for research, as very often, media have been enriched with various amino acids. Considering the manufacturer’s recommendations, this biostimulant enhances photosynthesis activity. The study by Radkowski et al. (2020) [
41] provided empirical evidence confirming that foliar application of amino acids significantly influences the accumulation of micro- and macroelements in grassland phytomass. They showed that the Folium biostimulant caused a 14% increase in potassium content in the tested meadow plants compared to unsprayed plants. In addition, the content of microelements such as copper and zinc in plants sprayed with the biostimulant increased by 15% compared to the control. These elements determine the activity of photosynthetic enzymes. Potassium and zinc deficiency can reduce the intensity of photosynthesis and, in extreme cases, even inhibit it. Amino acids such as glycine, glutamine, proline and serine can increase chlorophyll content, improve photosystem II efficiency and protect the photosynthetic apparatus from oxidative stress [
42,
43]. These mechanisms lead to increased production of assimilates. According to Heidarzadeh (2025) [
44], amino acids support the integrity of chloroplast membranes and the activity of photosynthetic enzymes.
In our research, supplementing the medium with the Goteo in 100% of the population resulted in an increase in the number of roots by an average of 1.4. Sochacki et al. (2018) [
16] also observed an increase in the number of roots after adding Goteo to the nutrient solutions for the 18
H. ×
chmielii clone and the ‘Double Roma’ cultivar. The reason may be the presence of natural growth regulators, including auxins, contained in seaweed extracts (
Ascophyllum nodosum). According to Craigie (2011) [
45], preparations containing exogenous hormones from seaweed, such as auxins or cytokinins, may have a beneficial effect on rhizogenesis processes in in vitro cultures. This effect is attributed to the natural auxins, cytokinins and gibberellins, which stimulate cell division, cell elongation and the development of plant organs, including roots [
46]. These processes can be supported by the content of other organic substances. Recent studies have shown that the biostimulating effects of seaweed extracts are mainly due to the action of complex non-hormonal compounds, such as oligosaccharides and polyphenols, which modulate gene expression and activate metabolic pathways. This points to a new, hormone-independent model of action for these biostimulants [
47,
48]. Some have previously suggested that the levels of phytohormones present in seaweed extracts were insufficient to have a significant effect on plants when used at the recommended doses and dilutions in field crops [
49].
It was also observed that both biostimulants tested in relation to MS medium supplemented with standard PGRs caused an increase in root length in all genotypes tested. However, no effect of either biostimulant on bulbil weight or multiplication rate was observed in all five genotypes tested.
The final and crucial stage of plant production via in vitro culture is acclimatisation. Its success depends on various factors, such as environmental conditions in the growth room or greenhouse, substrate properties [
50], and supplementation with compounds that aid adaptation [
51]. These include biostimulants, which help plants adapt to new conditions, often by mitigating stress responses. Our studies with Goteo and Folium confirmed that biostimulant effects are genotype specific. Goteo caused an increase in plant weight of 0.2 g compared to control plants in one genotype, an increase in the number of roots by 1.3 in four genotypes (80%) and their length by nearly 0.9 cm in two genotypes (40%). A similar relationship for root traits was presented in the studies by Lindsey et al. (1998) [
52], but they used a different preparation containing seaweed extract (
Ecklonia maxima)—Kelpak. They obtained a higher root mass of
Kniphofia pauciflora and
Scilla kraussii, which may mean that there were more roots or that they were longer in relation to plants not treated with this biostimulant. Grzelak et al. (2024) [
53] found that the Goteo lengthened the roots of
Echinacea purpurea during acclimatisation but did not affect their number compared to the control. In addition, Goteo did not increase the number of acclimatised plant leaves in relation to the control. In our own studies, similar results were obtained for 4 out of 5 genotypes tested, which represents 80% of the tested population.
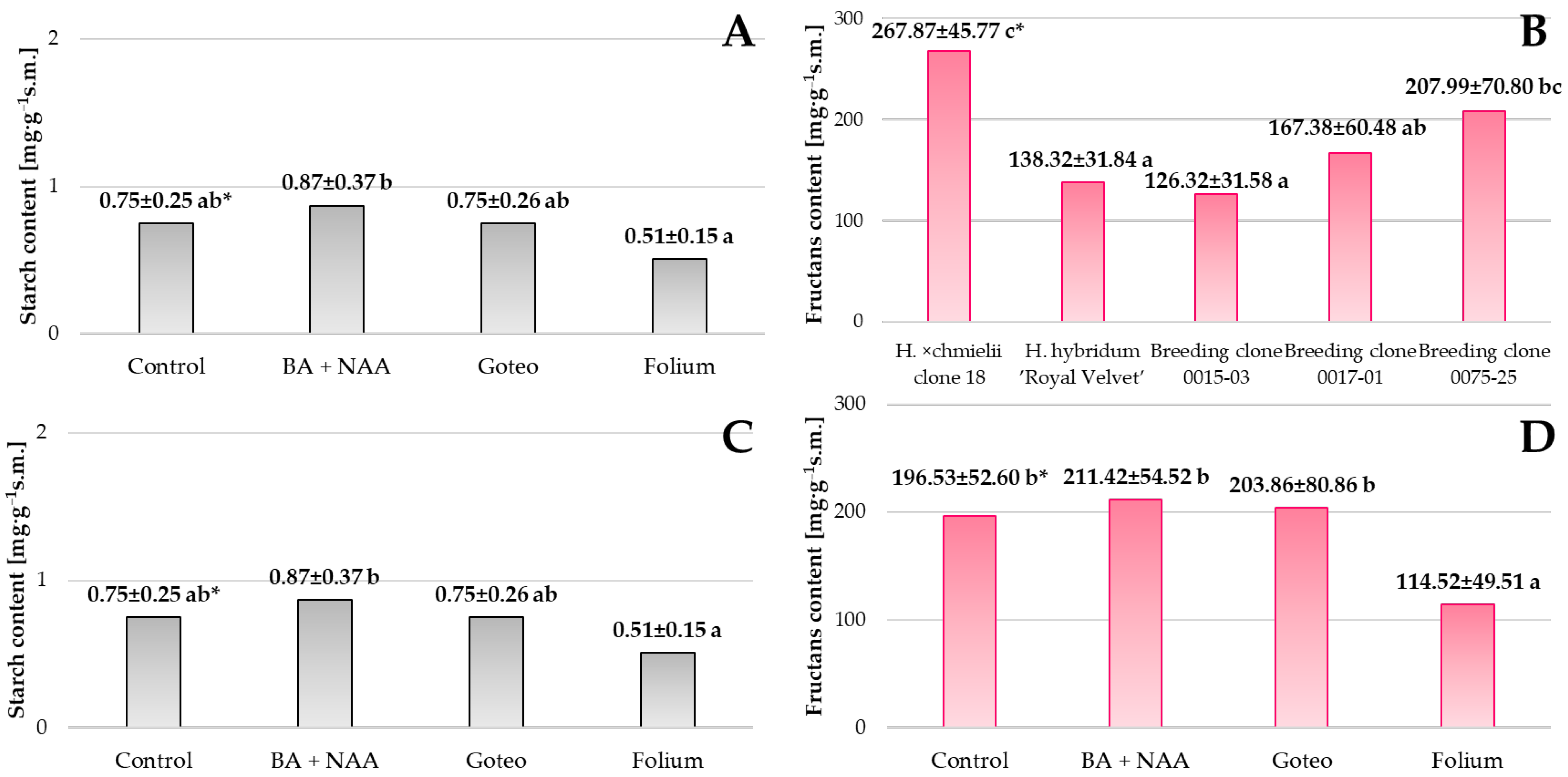
Hippeastrum has a multi-annual bulb, which is a reservoir of storage carbohydrates that can play key roles in growth and development processes [
54]. Such a relationship has been detected in the bulbs of
Lachenalia minima [
55] and
Galanthus nivalis, which belongs to the same botanical family [
56]. Our research focused on the influence of various factors on the content of storage carbohydrates—starch and fructans. Apart from their structure, starch and fructans differ in their cellular location and solubility. Starch is insoluble and located in plastids, while fructans are water-soluble and stored in vacuoles [
57]. This is extremely important in the case of geophytes, because a higher concentration of sugars improves the storage capacity of reserve organs and, as a consequence, stimulates the plant to grow intensively and flower profusely. Research suggests that fructans participate in osmotic regulation mechanisms that protect plants from drought, low temperatures and high salinity [
58,
59,
60]. Studies conducted by Jirakiattikul (1999) [
61] confirmed that fructans are a key storage carbohydrate in
Hippeastrum bulbs, and their decrease during flowering, enzymatic hydrolysis and transport of simple sugars indicate their involvement in the development of inflorescences and flowers. This discovery confirms the results previously published by Lambrechts et al. (1994) [
62] concerning the accumulation of fructans in the stems of pre-cooled tulip bulbs. Based on our own analyses of starch accumulation in the three cultivated clones and their parent forms, it can be seen that this sugar accumulated in greater quantities in the bulbs of clone 0017-01 compared to the other genotypes. For fructans, a similar relationship was only observed for clone 18
H. ×
chmielii.
The application of exogenous factors such as low nitrogen availability, low temperature or drought can affect changes in the content of starch and fructans in plants and their storage organs [
63,
64,
65]. Also using the biostimulants cointaining amino acids, as Folium used by us, can reduce the accumulation of reserve sugars, which may indicate a change in plant metabolism towards increased photosynthetic efficiency rather than storage of reserve substances. Such a mechanism may result, according to Al-Karaki et al. (2023) [
66], from the fact that amino acids increase the efficiency of photosynthesis and mineral uptake, which promotes the use of assimilates for current needs rather than storage. According to Treder (2002) [
67], the use of supplementary lighting in the growing of the
Lilium ‘Stargazer’ resulted in an increase in starch content in both the scales of the mother bulbs and the scales of the daughter bulbs. A similar relationship was demonstrated by Moscatello et al. (2023) [
68] in their study of the fructan content in the storage roots of
Cichorium endivia. Treatment of plants with calcium nitrate resulted in an increase in fructan content compared to control plants. In our research, the accumulation of starch in the bulbs of plants from the medium with PGRs was higher than in the bulbs from the medium with the Folium biostimulant. This may be due to the fact that a plant with constant access to amino acids, sterols and lipid compounds continues to grow and develop intensively, without focusing on the accumulation of reserve carbohydrates in the bulb. Similarly, for fructans, the average content of this sugar in the bulbs of the tested genotypes was lowest after the application of the Folium, but not lower in bulbs treated with the Goteo.
5. Conclusions
The research focused on assessing the impact of Goteo and Folium biostimulants on the micropropagation and ex vitro acclimatisation of Hippeastrum plants. These biostimulants, containing, among others, seaweed extracts and free amino acids, have been shown to improve plant growth and development in in vitro cultures and during the acclimatisation process by increasing plant mass, leaf length, and root number and length. Continuing research on the use of biostimulants in in vitro cultures, with particular emphasis on their effect on different plant species, may be an interesting direction for future research as an alternative to synthetic PGRs added to culture media.
Supplementing the MS medium with biostimulants had a positive effect on the morphometric characteristics of the plants, although no effect was observed on bulb weight and multiplication rate. During the acclimatisation phase, the Goteo improved the overall condition of the plants, increasing root growth parameters, which confirms its potential to support this stage of micropropagation. Further optimisation of the doses, components of biopreparations, labour costs, as well as costs of biostimulants themselves are needed to increase their effectiveness. Nevertheless, the potential to replace the synthetic PGRs used to date with natural biostimulants is already evident. This could significantly support the development of more environmentally friendly methods of plant micropropagation.
Analysis of the content of storage carbohydrates, including starch and fructans, in Hippeastrum bulbs showed differences between the genotypes studied and dependence on the biostimulants and growth regulators used. Starch, as an insoluble polysaccharide stored in plastids, and fructans, soluble sugars stored in vacuoles, play a key role in the ability of plants to adapt to environmental conditions and in the processes of growth and development, but also in subsequent flowering. The use of the Folium reduced the accumulation of reserve sugars, which may indicate a change in plant metabolism towards increased photosynthetic efficiency rather than storage of reserve substances. Further study of the effect of biostimulants and in-depth molecular analysis of their impact on the metabolism of reserve sugars in geophytes will contribute to the optimisation of plant growth and flowering.
When considering further research into the effectiveness of biostimulants in plant micropropagation, including ornamental geophytes, molecular and metabolism-related studies should be considered. An in-depth molecular analysis is recommended, bearing in mind the expression of genes related to nitrogen and carbon metabolism. Monitoring the effect of biostimulants on hormone production and metabolism would allow for a better understanding of their mechanisms of action during the in vitro plant propagation process. The development of metabolism modelling using isotopic markers and detailed analysis of metabolic pathways in plant cells will enable the prediction of cell responses to environmental changes and the optimisation of culture conditions. In addition, research on the long-term impact of biostimulants added to culture media on possible somaclonal variation induced in in vitro cultures would be valuable.