Comparative Analysis of Morphological, Histological, and Metabolic Differences of In Vitro- and Ex Vitro-Grown Panax ginseng
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. In Vitro Cultivation of Ginseng
2.3. Ex Vitro Cultivation of Ginseng
2.4. Experimental Design and Replication
2.5. Histological Analysis of Aerial and Underground Parts
2.6. Chlorophyll Content Analysis
2.7. Stomatal Traits Analysis
2.8. Growth Measurements
2.9. Freeze-Drying
2.10. Ginsenoside Profiling
2.11. Volatile Compound Analysis
2.12. Statistical Analysis
3. Results
3.1. Phenotypic Differences in Ginseng Under Different Cultivation Environments
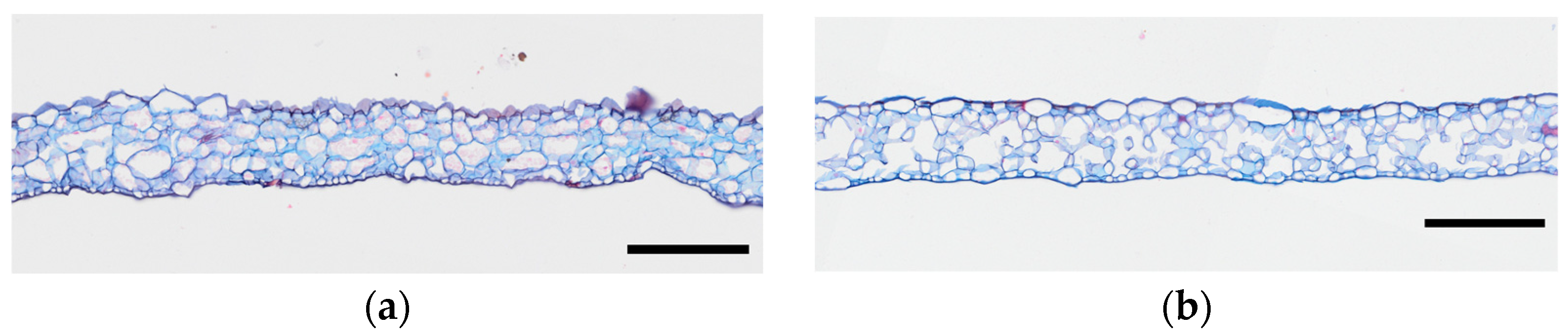
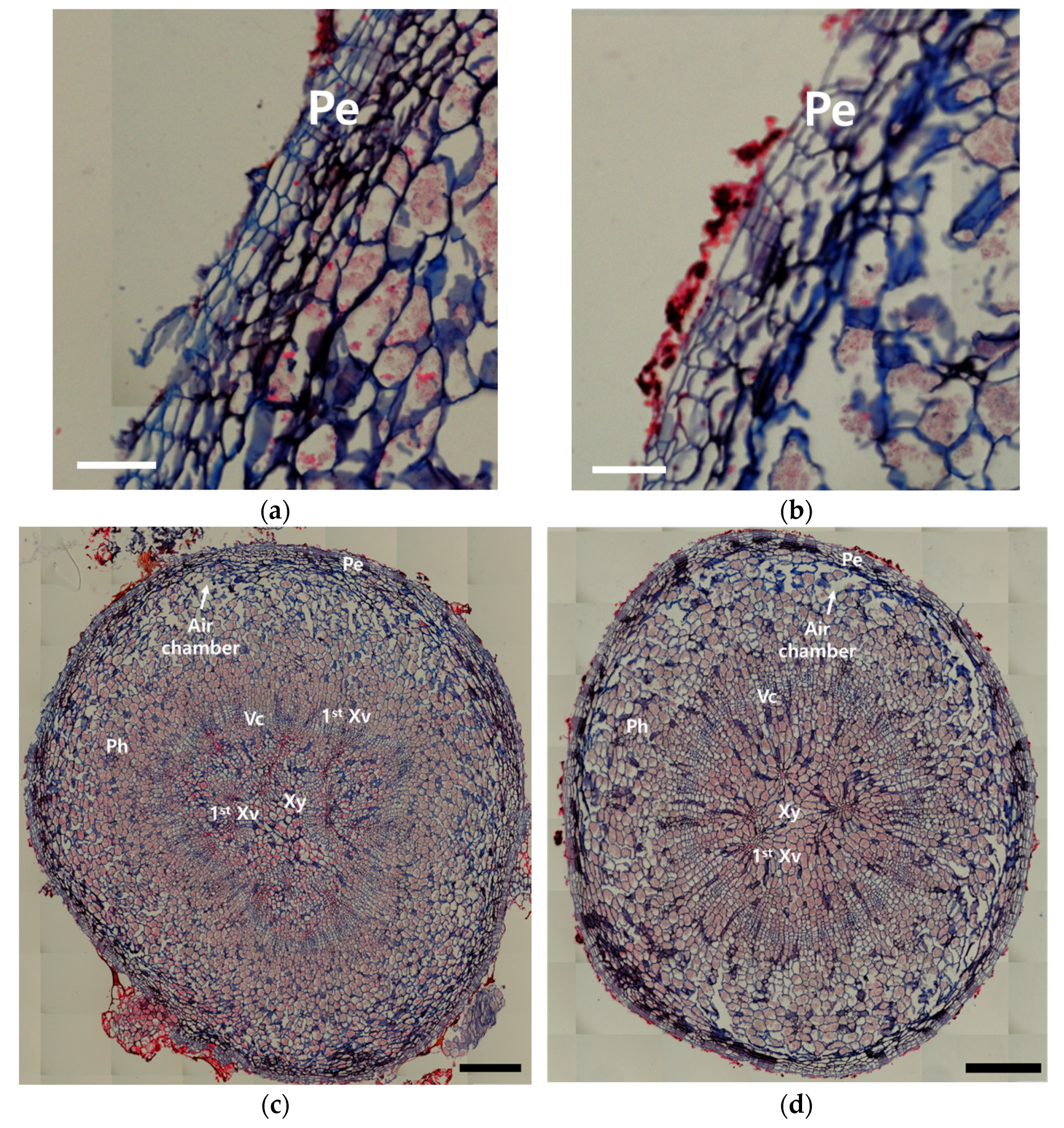
3.2. Histological Differences in Ginseng Under Different Cultivation Environments
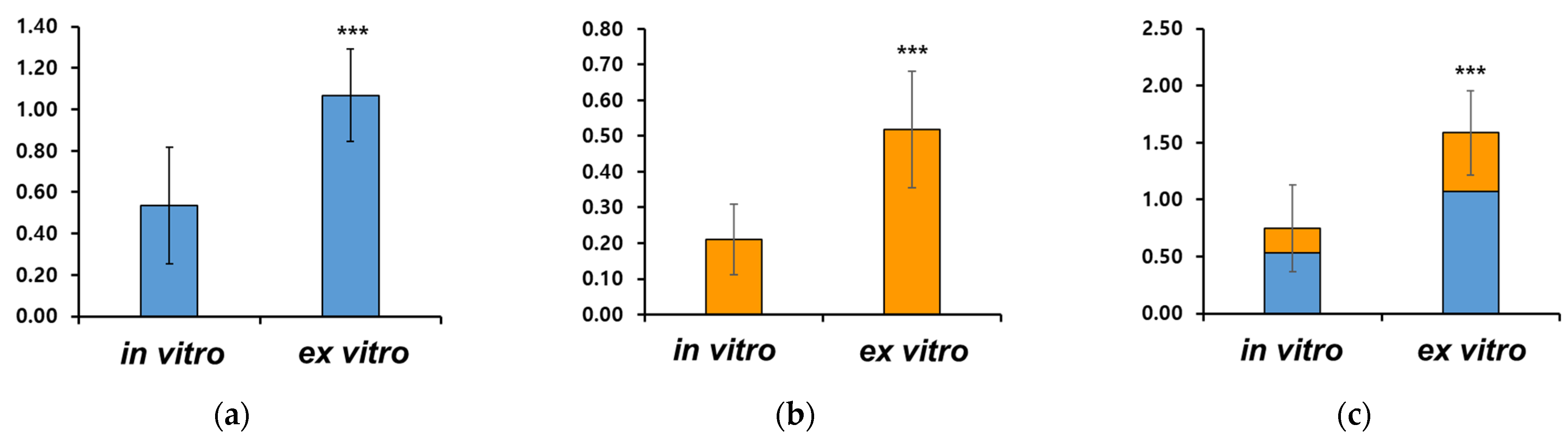
3.3. Chlorophyll Content Under Different Cultivation Environments
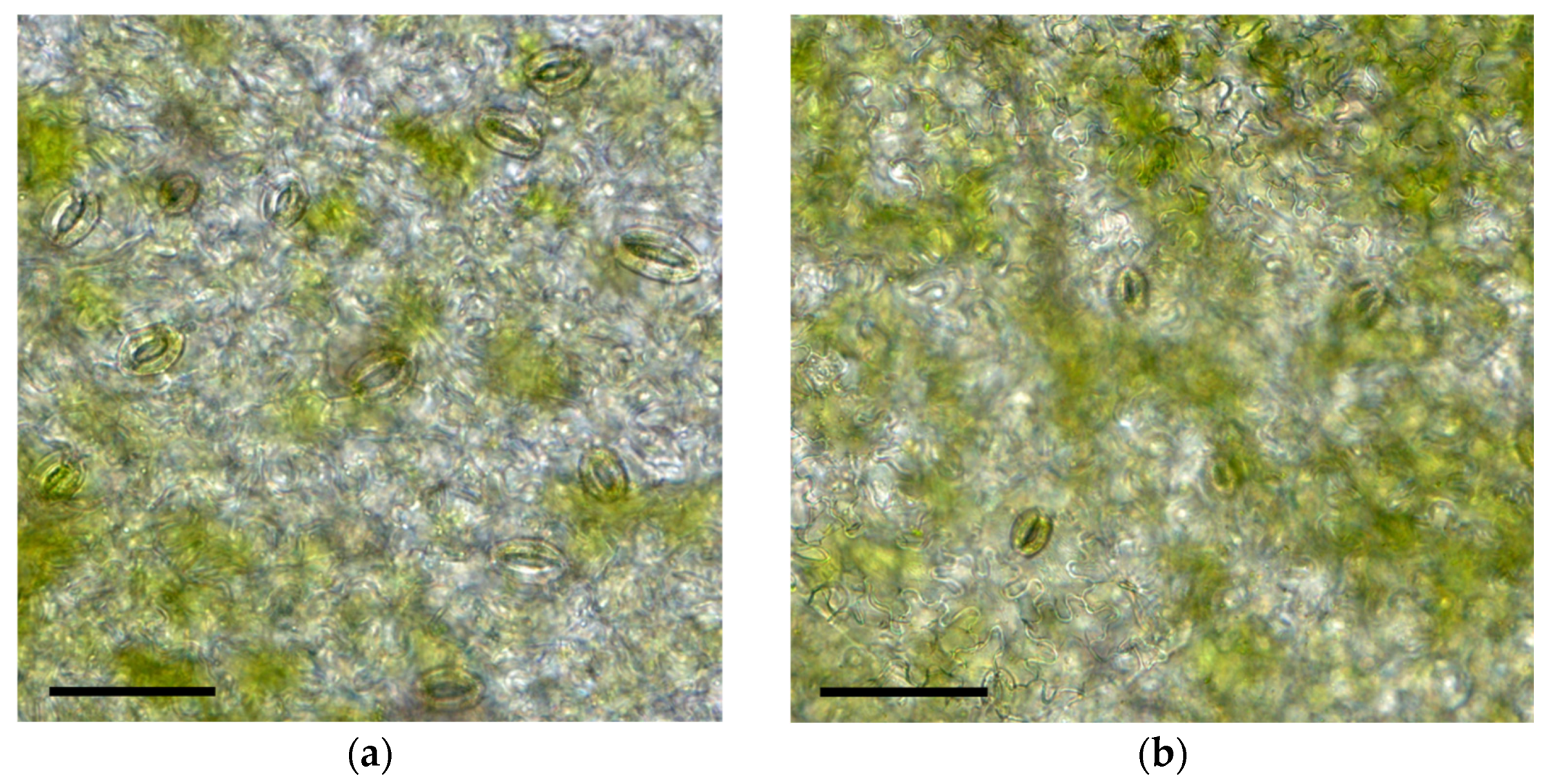
3.4. Stomatal Characteristics Under Different Cultivation Environments
3.5. Growth Differences Under Different Cultivation Environments
3.6. Ginsenoside Analysis
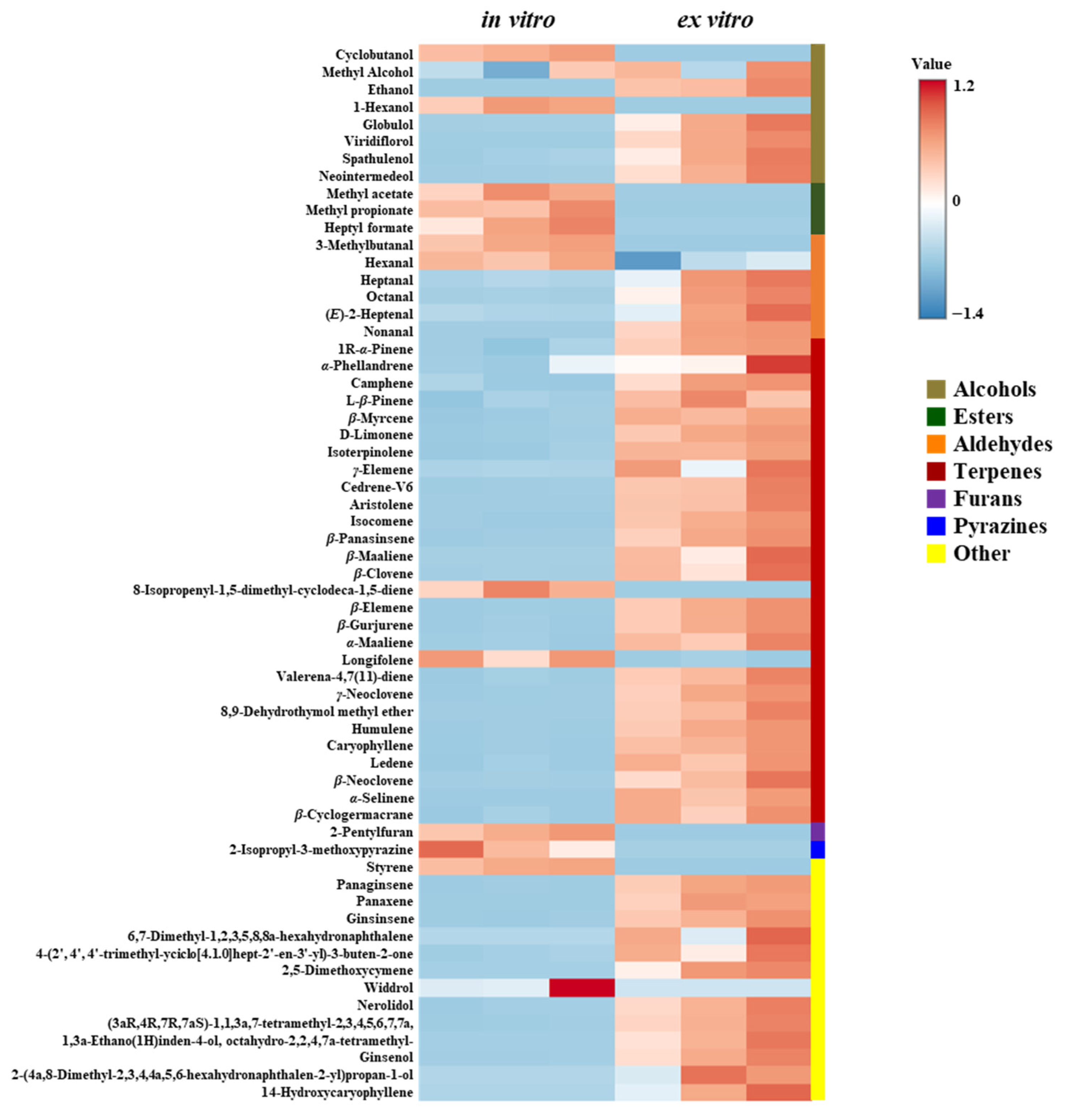
3.7. Volatile Compound Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PPD | Protopanaxadiol |
| PPT | Protopanaxatriol |
| PCA | Principal Component Analysis |
References
- Ratan, Z.A.; Haidere, M.F.; Hong, Y.H.; Park, S.H.; Lee, J.O.; Lee, J.; Cho, J.Y. Pharmacological potential of ginseng and its major component ginsenosides. J. Ginseng Res. 2020, 45, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Geem, K.R.; Kim, J.; Jo, I.H.; Yang, T.J.; Shim, D.; Ryu, H. Prolonged exposure to high temperature inhibits shoot primary and root secondary growth in Panax ginseng. Int. J. Mol. Sci. 2022, 23, 11647. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Food and Rural Affairs (MAFRA). Ginseng Statistics Yearbook 2023; MAFRA: Sejong, Korea, 2024; Available online: https://lib.mafra.go.kr/skyblueimage/32579.pdf (accessed on 8 September 2025). (In Korean)
- Lee, J.S.; Kim, Y.S.; Shim, S.L.; Park, C.S. ‘Sunmyoung’: A variety with high production stability due to its resistance against high temperature and lodging stresses. Korean J. Breed. Sci. 2021, 53, 475–481. [Google Scholar] [CrossRef]
- Kim, J.U.; Kim, Y.C.; Kim, D.H.; Bang, K.H.; Kwon, N.; Jung, S.M.; Lee, S.W.; Lee, J.W.; Kim, K.M. New Korean ginseng variety ‘Jinwon’ with high-yielding and high-temperature stress tolerance. Korean J. Med. Crop Sci. 2024, 32, 73–79. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, Y.C.; Bang, K.H.; Kim, J.U.; Lee, J.W.; Cho, I.H.; Kim, Y.B.; Lim, J.Y.; Kim, K.H. Flowering and fruits formation characteristics in major varieties of Panax ginseng. Korean J. Med. Crop Sci. 2016, 24, 207–213. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, J.U.; Bang, K.H.; Kim, D.H.; Jo, I.H.; Kim, Y.C. Somatic embryogenesis and acclimatization from anther culture of Panax ginseng C.A. Meyer. Korean J. Med. Crop Sci. 2023, 31, 73–80. [Google Scholar]
- Mahato, A.; Chaudhari, G.; Banjade, G.; Uprety, S. Tissue culture and its application in modern agriculture. INWASCON Technol. Mag. (i-TECH MAG) 2023, 5, 9–11. [Google Scholar] [CrossRef]
- Atkins, P.A.; Voytas, D.F. Overcoming bottlenecks in plant gene editing. Curr. Opin. Plant Biol. 2020, 54, 79–84. [Google Scholar] [CrossRef]
- Butenko, R.G.; Popov, A.S.; Volkova, L.A.; Chernyak, N.D.; Nosov, A.M. Recovery of cell cultures and their biosynthetic capacity after storage of Dioscorea deltoidea and Panax ginseng cells in liquid nitrogen. Plant Sci. Lett. 1984, 33, 285–292. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, J.U.; Bang, K.H.; Kwon, N.; Kim, Y.C.; Jo, I.H.; Park, Y.D. Efficient somatic embryogenesis, regeneration and acclimatization of Panax ginseng Meyer: True-to-type conformity of plantlets as confirmed by ISSR analysis. Plants 2023, 12, 1270. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, J.U.; Bang, K.H.; Kwon, N.; Kim, Y.C.; Jo, I.H.; Park, Y.D. Efficient micropropagation of genetically stable Panax ginseng Meyer by somatic embryogenesis. Agronomy 2023, 13, 1139. [Google Scholar] [CrossRef]
- Choi, H.S.; Koo, H.B.; Jeon, S.W.; Han, J.Y.; Kim, J.S.; Jun, K.M.; Choi, Y.E. Modification of ginsenoside saponin composition via the CRISPR/Cas9-mediated knockout of protopanaxadiol 6-hydroxylase gene in Panax ginseng. J. Ginseng Res. 2022, 46, 505–514. [Google Scholar] [CrossRef]
- Lee, J.W.; Kwon, N.; Kim, J.U.; Bang, K.H.; Jung, S.M.; Lee, S.W.; Park, Y.D. In vitro micropropagation of commercial ginseng cultivars (Panax ginseng Meyer) via somatic embryogenesis compared to traditional seed production. Horticulturae 2023, 9, 435. [Google Scholar] [CrossRef]
- Choi, Y.E.; Yang, D.C.; Choi, K.T. Induction of somatic embryos by macrosalt stress from mature zygotic embryos of Panax ginseng. Plant Cell Tissue Organ. Cult. 1998, 52, 177–181. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, D.H.; Kim, Y.C.; Kim, K.H.; Han, J.Y.; Choi, Y.E. In vitro grown thickened taproots, a new type of soil transplanting source in Panax ginseng. J. Ginseng Res. 2016, 40, 409–414. [Google Scholar] [CrossRef]
- Hazarika, B.N. Morpho-physiological disorders in in vitro culture of plants. Sci. Hortic. 2006, 108, 105–120. [Google Scholar] [CrossRef]
- Nicolova, R.L. The derivation of diminutives from abstract noun bases in Bulgarian. Juznoslov. Filolog 2013, 69, 149–168. [Google Scholar] [CrossRef]
- Barry-Etienne, D.; Bertrand, B.; Vasquez, N.; Etienne, H. Comparison of somatic embryogenesis-derived coffee (Coffea arabica L.) plantlets regenerated in vitro or ex vitro: Morphological, mineral and water characteristics. Ann. Bot. 2002, 90, 77–85. [Google Scholar] [CrossRef]
- Debnath, S.C.; Arigundam, U. In vitro propagation strategies of medicinally important berry crop, lingonberry (Vaccinium vitis-idaea L.). Agronomy 2020, 10, 744. [Google Scholar] [CrossRef]
- Moyo, M.; Finnie, J.F.; Van Staden, J. Microculture effects on leaf epidermis and root structure in Sclerocarya birrea subsp. caffra. S. Afr. J. Bot. 2012, 78, 170–177. [Google Scholar] [CrossRef]
- Ševčíková, H.; Lhotáková, Z.; Hamet, J.; Lipavská, H. Mixotrophic in vitro cultivations: The way to go astray in plant physiology. Physiol. Plant 2019, 167, 365–377. [Google Scholar] [CrossRef]
- Firmin, A.; Selosse, M.A.; Dunand, C.; Elger, A. Mixotrophy in aquatic plants, an overlooked ability. Trends Plant Sci. 2022, 27, 147–157. [Google Scholar] [CrossRef]
- Wang, F.; Xin, X.; Wei, H.; Qiu, X.; Liu, B. In vitro regeneration, ex vitro rooting and foliar stoma studies of Pseudostellaria heterophylla (Miq.) Pax. Agronomy 2020, 10, 949. [Google Scholar] [CrossRef]
- Lee, J.W.; Jo, I.H.; Kim, J.U.; Hong, C.E.; Kim, Y.C.; Kim, D.H.; Park, Y.D. Improvement of seed dehiscence and germination in ginseng by stratification, gibberellin, and/or kinetin treatments. Hortic. Environ. Biotechnol. 2018, 59, 293–301. [Google Scholar] [CrossRef]
- Lee, J.W.; Do, G.R.; Jo, I.H.; Hong, C.E.; Bang, K.H.; Kim, J.U.; Park, Y.D. Zygotic embryo culture is an efficient way to optimize in vitro growth in Panax ginseng. Ind. Crops Prod. 2021, 167, 113497. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: San Diego, CA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Kumar, K.; Rao, I.U. Morphophysiological problems in acclimatization of micropropagated plants in ex vitro conditions—A review. J. Appl. Hortic. 2012, 14, 271–283. [Google Scholar]
- Gautam, N.; Faroda, P.; Agarwal, T.; Harish; Gupta, A.K. Effect of microclimatic physical factors on in vitro morphogenesis of plants: A systematic review. Discover Agric. 2024, 2, 13. [Google Scholar] [CrossRef]
- Shilpha, J.; Noh, K.; Yang, J.; Yeom, S.I.; Jeong, B.R. Lighting direction in controlled setting impacts the growth and quality of Panax ginseng C.A. Meyer sprouts. Hortic. Environ. Biotechnol. 2024, 66, 297–317. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.Y.; Lee, J.H.; Kim, J.U.; Lee, O.R. Establishment of a qualified ex vitro-acclimatized whole plant reproduction system using secondary somatic embryos in Panax ginseng. Plant Biotechnol. Rep. 2023, 17, 331–339. [Google Scholar] [CrossRef]
- Desjardins, Y.; Dubuc, J.F.; Badr, A. In vitro culture of plants: A stressful activity. In Proceedings of the III International Symposium on Acclimatization and Establishment of Micropropagated Plants, Faro, Portugal, 12–15 September 2007. [Google Scholar]
- Luna, C.V.; Gonzalez, A.M.; Mroginski, L.A.; Sansberro, P.A. Anatomical and histological features of Ilex paraguariensis leaves under different in vitro shoot culture systems. Plant Cell Tissue Organ. Cult. 2017, 129, 457–467. [Google Scholar] [CrossRef]
- Han, X.; Zhao, Y.; Chen, Y.; Xu, J.; Jiang, C.; Wang, X.; Zhuo, R.; Lu, M.Z.; Zhang, J. Lignin biosynthesis and accumulation in response to abiotic stresses in woody plants. For. Res. 2022, 2, 9. [Google Scholar] [CrossRef]
- Smith, M.A.L.; McClelland, M.T.; Timmermann, R. Anomalous root structure on woody plants in vitro. J. Environ. Hortic. 1991, 9, 61–64. [Google Scholar] [CrossRef]
- Gonçalves, J.E.L. A necessidade de reinventar as empresas. Rev. Adm. Empresas 1998, 38, 6–17. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Y.; Lan, Y.; Cheng, L.; Lv, Z.; Han, M.; Yang, L. Multiomics joint analysis reveals the potential mechanism of differences in the taproot thickening between cultivated ginseng and mountain-cultivated ginseng. BMC Genom. 2024, 25, 1228. [Google Scholar] [CrossRef]
- Singh, V.; Zemach, H.; Shabtai, S.; Aloni, R.; Yang, J.; Zhang, P.; Sergeeva, L.; Ligterink, W.; Firon, N. Proximal and distal parts of sweetpotato adventitious roots display differences in root architecture, lignin, and starch metabolism and their developmental fates. Front. Plant Sci. 2021, 11, 609923. [Google Scholar] [CrossRef]
- Gu, L.; Wu, Y.; Li, M.; Wang, F.; Li, Z.; Yuan, F.; Zhang, Z. Over-immunity mediated abnormal deposition of lignin arrests the normal enlargement of the root tubers of Rehmannia glutinosa under consecutive monoculture stress. Plant Physiol. Biochem. 2021, 165, 36–46. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, J.L.; Hao, B.; Lu, Y.C.; Qian, Z.L.; Li, Y.; Ye, S.; Tang, J.R.; Chen, M.; Long, G.Q.; et al. Comparative transcriptome and metabolome analyses provide new insights into the molecular mechanisms underlying taproot thickening in Panax notoginseng. BMC Plant Biol. 2019, 19, 451. [Google Scholar] [CrossRef]
- Kovalikova, Z.; Jiroutova, P.; Toman, J.; Dobrovolna, D.; Drbohlavova, L. Physiological responses of apple and cherry in vitro culture under different levels of drought stress. Agronomy 2020, 10, 1689. [Google Scholar] [CrossRef]
- Isah, T. Adjustments to in vitro culture conditions and associated anomalies in plants. Acta Biol. Cracov. Bot. 2015, 57, 9–28. [Google Scholar] [CrossRef]
- Asayesh, Z.M.; Vahdati, K.; Aliniaeifard, S.; Askari, N. Enhancement of ex vitro acclimation of walnut plantlets through modification of stomatal characteristics in vitro. Sci. Hortic. 2017, 220, 114–121. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Shilpha, J.; Jeong, B.R. Top and side lighting induce morphophysiological improvements in Korean ginseng sprouts (Panax ginseng C.A. Meyer) grown from one-year-old roots. Plants 2023, 12, 2849. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Sun, H.; Zhang, Z.; Li, M.; Shao, C.; Jin, Q.; Liang, H.; Wu, H.; Ruan, Y.; Zhang, Y. Effects of soil moisture on plant growth and ginsenoside production of Panax ginseng C.A. Meyer. Eur. J. Hortic. Sci. 2022, 87, 055. [Google Scholar]
- Silva, T.D.; Chagas, K.; Batista, D.S.; Felipe, S.H.S.; Louback, E.; Machado, L.T.; Fernandes, A.M.; Buttrós, V.H.T.; Koehler, A.D.; Farias, L.M.; et al. Morphophysiological in vitro performance of Brazilian ginseng (Pfaffia glomerata (Spreng.) Pedersen) based on culture medium formulations. In Vitro Cell Dev. Biol. Plant 2019, 55, 454–467. [Google Scholar] [CrossRef]
- Souza, D.M.S.C.; Fernandes, S.B.; Duarte, V.P.; Molinari, L.V.; Teixeira, G.L.; Brondani, G. Effect of light intensity and seal type on the in vitro elongation and adventitious rooting of Eucalyptus grandis × E. urophylla. N. Z. J. For. Sci. 2024, 54, 2. [Google Scholar]
- Sha Valli Khan, P.S.; Kozai, T.; Nguyen, Q.T.; Kubota, C.; Dhawan, V. Growth and water relations of Paulownia fortunei under photomixotrophic and photoautotrophic conditions. Biol. Plant 2003, 46, 161–166. [Google Scholar] [CrossRef]
- Yang, J.L.; Hu, Z.F.; Zhang, T.T.; Gu, A.D.; Gong, T.; Zhu, P. Progress on the studies of the key enzymes of ginsenoside biosynthesis. Molecules 2018, 23, 589. [Google Scholar] [CrossRef]
- Xu, F.; Valappil, A.K.; Mathiyalagan, R.; Tran, T.N.A.; Ramadhania, Z.M.; Awais, M.; Yang, D.C. In vitro cultivation and ginsenosides accumulation in Panax ginseng: A review. Plants 2023, 12, 3165. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, J.; Quan, X.; Quan, L.; Wu, S. Different chilling stresses stimulated the accumulation of different types of ginsenosides in Panax ginseng cells. Acta Physiol. Plant 2016, 38, 210. [Google Scholar] [CrossRef]
- Zhang, T.; Han, M.; Yang, L.; Han, Z.; Cheng, L.; Sun, Z.; Yang, L. The effects of environmental factors on ginsenoside biosynthetic enzyme gene expression and saponin abundance. Molecules 2018, 24, 14. [Google Scholar] [CrossRef]
- Kang, O.J.; Kim, J.S. Comparison of ginsenoside contents in different parts of Korean ginseng (Panax ginseng C.A. Meyer). Prev. Nutr. Food Sci. 2016, 21, 389. [Google Scholar] [CrossRef]
- Hou, M.; Wang, R.; Zhao, S.; Wang, Z. Ginsenosides in Panax genus and their biosynthesis. Acta Pharm. Sin. B 2021, 11, 1813–1834. [Google Scholar] [CrossRef] [PubMed]
- Murali-Baskaran, R.; Mooventhan, P.; Das, D.; Dixit, A.; Sharama, K.C.; Senthil-Nathan, S.; Kaushal, P.; Ghosh, P.K. The future of plant volatile organic compounds (pVOCs) research: Advances and applications for sustainable agriculture. Environ. Exp. Bot. 2022, 200, 104912. [Google Scholar] [CrossRef]
- Castellar, A.; Gagliardi, R.F.; Mansur, E.; Bizzo, H.R.; Souza, A.M.; Leitão, S.G. Volatile constituents from in vitro and ex vitro plants of Petiveria alliacea L. J. Essent. Oil Res. 2014, 26, 19–23. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kännaste, A.; Copolovici, L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front. Plant Sci. 2013, 4, 262. [Google Scholar] [CrossRef]
- Talaverano, I.; Ubeda, C.; Cáceres-Mella, A.; Valdés, M.E.; Pastenes, C.; Peña-Neira, Á. Water stress and ripeness effects on the volatile composition of Cabernet Sauvignon wines. J. Sci. Food Agric. 2018, 98, 1140–1152. [Google Scholar] [CrossRef]
- Cui, S.; Wang, J.; Yang, L.; Wu, J.; Wang, X. Qualitative and quantitative analysis on aroma characteristics of ginseng at different ages using E-nose and GC–MS combined with chemometrics. J. Pharm. Biomed. Anal. 2015, 102, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, J.W.; Jo, I.H.; Kwon, N.; Kim, D.; Chung, J.W.; Bang, K.H.; Sung, J. Volatile compositions of Panax ginseng and Panax quinquefolium grown for different cultivation years. Foods 2022, 12, 136. [Google Scholar] [CrossRef] [PubMed]

| Treat | Stomata Density (cm−2) | Stomata Length (μm) | Stomata Width (μm) | Pore Length (μm) | Pore Aperture (μm) | Stomata Length/Width |
|---|---|---|---|---|---|---|
| in vitro | 3918 ± 936 | 55.3 ± 7.3 | 35.6 ± 6.0 | 29.3 ± 6.3 | 8.8 ± 4.4 | 1.6 ± 0.3 |
| ex vitro | 2571 ± 595 | 34.4 ± 4.5 | 22.3 ± 2.6 | 17.0 ± 3.4 | 4.5 ± 1.6 | 1.6 ± 0.2 |
| *** | *** | *** | *** | *** | ns |
| Treat | Fresh Weight (mg) | Dry Weight (mg) | Stem Length (cm) | Stem Diameter (mm) | Leaf Length (cm) | Leaf Width (cm) |
|---|---|---|---|---|---|---|
| in vitro | 187.8 ± 27.2 | 29.1 ± 11.0 | 4.7 ± 1.7 | 1.08 ± 0.26 | 2.9 ± 0.6 | 1.3 ± 0.3 |
| ex vitro | 181.3 ± 68.5 | 40.6 ± 5.9 | 7.0 ± 0.7 | 0.94 ± 0.16 | 3.9 ± 0.4 | 1.9 ± 0.2 |
| ns | *** | *** | *** | *** | *** |
| Treat | Fresh Weight (mg) | Dry Weight (mg) | Root Length (cm) | Root Diameter (mm) |
|---|---|---|---|---|
| in vitro | 396.5 ± 115.7 | 109.4 ± 31.9 | 10.4 ± 3.6 | 4.68 ± 1.10 |
| ex vitro | 602.7 ± 73.6 | 192.9 ± 23.6 | 16.5 ± 3.5 | 3.66 ± 0.64 |
| *** | *** | *** | *** |
| Retention Time | In Vitro | Ex Vitro | |||
|---|---|---|---|---|---|
| PPD (μg/g) | Ginsenoside Rb2 | 25.69 | 162.97 ± 22.88 | 872.97 ± 9.96 | *** |
| Ginsenoside Rc | 24.13 | 195.91 ± 3.88 | 694.72 ± 30.62 | *** | |
| Ginsenoside Rd | 31.31 | 110.07 ± 3.98 | 529.26 ± 7.90 | *** | |
| Ginsenoside Rg3 | 35.59 | nd | nd | - | |
| Ginsenoside Rg5 | 36.62 | nd | nd | - | |
| Ginsenoside Rh2 | 37.15 | nd | nd | - | |
| PPT (μg/g) | Ginsenoside Re | 4.62 | 279.47 ± 9.18 | 359.31 ± 6.92 | *** |
| Ginsenoside Rf | 14.60 | 162.96 ± 6.37 | 163.55 ± 9.27 | ns | |
| Ginsenoside Rg1 | 4.82 | 414.72 ± 33.62 | 256.14 ± 17.93 | ** | |
| Ginsenoside Rh1 | 22.76 | nd | 44.02 ± 4.40 | - | |
| Quinquenoside R1 | 29.01 | nd | nd | - |
| Treat | PPD | PPT | PPD/PPT | Total Ginsenoside Content (mg/g) |
|---|---|---|---|---|
| in vitro | 468.95 ± 20.6 | 857.2 ± 33.7 | 0.55 ± 0.03 | 1326.1 ± 42.2 |
| ex vitro | 2097.0 ± 23.9 | 823.0 ± 33.5 | 2.55 ± 0.08 | 2920.0 ± 56.2 |
| *** | ns | *** | *** |
| RT A | RI B | In Vitro | Ex Vitro | Identification C | ||
|---|---|---|---|---|---|---|
| Alcohols | ||||||
| 1 | Cyclobutanol | 5.34 | - | 0.09 ± 0.01 | Nd D | MS, TI |
| 2 | Methyl Alcohol | 8.78 | 899 | 0.53 ± 0.06 | 0.59 ± 0.07 | MS, TI |
| 3 | Ethanol | 9.91 | 936 | nd | 0.24 ± 0.03 | MS, TI |
| 4 | 1-Hexanol | 22.66 | 1355 | 0.11 ± 0.02 | nd | MS, TI |
| 5 | Globulol | 33.99 | 2087 | 0.17 ± 0.01 | 2.85 ± 0.96 | MS, TI |
| 6 | Viridiflorol | 34.23 | 2111 | nd | 0.24 ± 0.05 | MS, TI |
| 7 | Spathulenol | 34.69 | 2153 | 0.12 ± 0.01 | 0.54 ± 0.14 | MS, TI |
| 8 | Neointermedeol | 34.86 | 2169 | 0.14 ± 0.01 | 1.24 ± 0.30 | MS, TI |
| Esters | ||||||
| 9 | Methyl acetate | 7.07 | 823 | 0.06 ± 0.01 | nd | MS, TI |
| 10 | Methyl propionate | 8.95 | 905 | 0.08 ± 0.01 | nd | MS, TI |
| 11 | Heptyl formate | 25.22 | 1459 | 0.06 ± 0.02 | nd | MS, TI |
| Aldehydes | ||||||
| 12 | 3-Methylbutanal | 9.24 | 914 | 0.07 ± 0.01 | nd | MS, TI |
| 13 | Hexanal | 14.49 | 1079 | 0.52 ± 0.03 | 0.24 ± 0.10 | MS, TI |
| 14 | Heptanal | 17.81 | 1184 | 0.17 ± 0.01 | 0.56 ± 0.22 | MS, TI |
| 15 | Octanal | 20.91 | 1290 | 0.43 ± 0.02 | 2.28 ± 0.67 | MS, TI |
| 16 | (E)-2-Heptenal | 21.94 | 1328 | 0.06 ± 0.00 | 0.13 ± 0.04 | MS, TI |
| 17 | Nonanal | 23.76 | 1391 | nd | 0.10 ± 0.02 | MS, TI |
| Terpenes | ||||||
| 18 | 1R-α-Pinene | 12.52 | 1019 | 0.94 ± 0.10 | 2.47 ± 0.26 | MS, TI |
| 19 | α-Phellandrene | 12.68 | 1024 | 0.10 ± 0.05 | 0.19 ± 0.01 | MS, TI |
| 20 | Camphene | 13.96 | 1063 | 0.31 ± 0.03 | 0.81 ± 0.12 | MS, TI |
| 21 | L-β-Pinene | 15.17 | 1100 | 0.26 ± 0.03 | 0.81 ± 0.10 | MS, TI |
| 22 | β-Myrcene | 17.02 | 1159 | 0.47 ± 0.03 | 1.62 ± 0.08 | MS, TI |
| 23 | D-Limonene | 18.18 | 1196 | 0.31 ± 0.02 | 1.50 ± 0.17 | MS, TI |
| 24 | Isoterpinolene | 20.75 | 1285 | 0.09 ± 0.01 | 0.34 ± 0.02 | MS, TI |
| 25 | γ-Elemene | 25.93 | 1490 | 0.04 ± 0.00 | 0.39 ± 0.04 | MS, TI |
| 26 | Cedrene-V6 | 26.35 | 1510 | 0.13 ± 0.01 | 1.75 ± 0.35 | MS, TI |
| 27 | Aristolene | 26.81 | 1535 | nd | 0.97 ± 0.16 | MS, TI |
| 28 | Isocomene | 26.87 | 1538 | 0.25 ± 0.02 | 3.53 ± 0.44 | MS, TI |
| 29 | β-Panasinsene | 26.97 | 1544 | 1.30 ± 0.07 | 15.32 ± 2.65 | MS, TI |
| 30 | β-Maaliene | 27.31 | 1562 | nd | 0.62 ± 0.21 | MS, TI |
| 31 | β-Clovene | 27.50 | 1573 | 0.06 ± 0.01 | 0.95 ± 0.30 | MS, TI |
| 32 | 8-Isopropenyl-1,5-dimethyl-cyclodeca-1,5-diene | 27.81 | 1590 | 0.05 ± 0.01 | nd | MS, TI |
| 33 | β-Elemene | 28.01 | 1601 | 0.72 ± 0.07 | 11.26 ± 1.77 | MS, TI |
| 34 | β-Gurjurene | 28.18 | 1612 | 0.59 ± 0.05 | 6.97 ± 1.05 | MS, TI |
| 35 | α-Maaliene | 28.36 | 1623 | 0.08 ± 0.01 | 0.80 ± 0.15 | MS, TI |
| 36 | Longifolene | 28.43 | 1628 | 0.23 ± 0.03 | 1.45 ± 0.29 | MS, TI |
| 37 | Valerena-4,7(11)-diene | 28.59 | 1638 | 0.52 ± 0.07 | 3.79 ± 0.67 | MS, TI |
| 38 | γ-Neoclovene | 29.06 | 1669 | 2.66 ± 0.20 | 28.83 ± 4.77 | MS, TI |
| 39 | 8,9-Dehydrothymol methyl ether | 29.32 | 1686 | nd | 0.98 ± 0.18 | MS, TI |
| 40 | Humulene | 29.41 | 1692 | 0.95 ± 0.08 | 10.99 ± 1.55 | MS, TI |
| 41 | Caryophyllene | 29.51 | 1698 | 0.64 ± 0.06 | 7.63 ± 0.85 | MS, TI |
| 42 | Ledene | 29.74 | 1716 | 0.18 ± 0.03 | 1.40 ± 0.18 | MS, TI |
| 43 | β-Neoclovene | 29.88 | 1726 | 0.28 ± 0.03 | 3.62 ± 0.98 | MS, TI |
| 44 | α-Selinene | 30.20 | 1751 | nd | 5.24 ± 0.53 | MS, TI |
| 45 | β-Cyclogermacrane | 30.31 | 1759 | 0.35 ± 0.08 | 2.82 ± 0.47 | MS, TI |
| Furans | ||||||
| 46 | 2-Pentylfuran | 19.17 | 1230 | 0.11 ± 0.01 | nd | MS, TI |
| Pyrazines | ||||||
| 47 | 2-Isopropyl-3-methoxypyrazine | 24.80 | 1441 | 0.11 ± 0.04 | nd | MS, TI |
| Others | ||||||
| 48 | Styrene | 20.00 | 1259 | 0.05 ± 0.00 | nd | MS, TI |
| 49 | Panaginsene | 24.44 | 1425 | 0.12 ± 0.01 | 1.95 ± 0.29 | MS, TI |
| 50 | Panaxene | 24.88 | 1444 | nd | 1.27 ± 0.19 | MS, TI |
| 51 | Ginsinsene | 25.11 | 1454 | 0.27 ± 0.03 | 4.98 ± 0.76 | MS, TI |
| 52 | 6,7-Dimethyl-1,2,3,5,8,8a-hexahydronaphthalene | 25.43 | 1468 | nd | 0.50 ± 0.09 | MS, TI |
| 53 | 4-(2′, 4′, 4′-trimethyl-yciclo [4.1.0]hept-2′-en-3′-yl)-3-buten-2-one | 25.60 | 1475 | 0.07 ± 0.02 | 1.03 ± 0.34 | MS, TI |
| 54 | 2,5-Dimethoxycymene | 31.85 | 1885 | nd | 0.25 ± 0.08 | MS, TI |
| 55 | Widdrol | 32.82 | 1974 | 0.02 ± 0.00 | nd | MS, TI |
| 56 | Nerolidol | 33.51 | 2041 | 0.02 ± 0.00 | 0.31 ± 0.07 | MS, TI |
| 57 | (3aR,4R,7R,7aS)-1,1,3a,7-tetramethyl-2,3,4,5,6,7,7a,7b-octahydro-1aH-cyclopropa [a]naphthalen-4-ol | 33.89 | 2078 | 0.02 ± 0.00 | 0.34 ± 0.08 | MS, TI |
| 58 | 1,3a-Ethano(1H)inden-4-ol, octahydro-2,2,4,7a-tetramethyl- | 35.07 | 2188 | nd | 0.33 ± 0.09 | MS, TI |
| 59 | Ginsenol | 35.17 | 2198 | 0.13 ± 0.01 | 2.53 ± 0.65 | MS, TI |
| 60 | 2-(4a,8-Dimethyl-2,3,4,4a,5,6-hexahydronaphthalen-2-yl)propan-1-ol | 35.54 | 2229 | nd | 0.13 ± 0.08 | MS, TI |
| 61 | 14-Hydroxycaryophyllene | 38.07 | 2414 | nd | 0.27 ± 0.15 | MS, TI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-J.; Jeon, Y.; Kim, J.-U.; Hong, J.; Koo, S.C.; Ha, J.Y.; Ma, K.H.; Sung, J.; Lee, J.-W. Comparative Analysis of Morphological, Histological, and Metabolic Differences of In Vitro- and Ex Vitro-Grown Panax ginseng. Agronomy 2025, 15, 2222. https://doi.org/10.3390/agronomy15092222
Kim S-J, Jeon Y, Kim J-U, Hong J, Koo SC, Ha JY, Ma KH, Sung J, Lee J-W. Comparative Analysis of Morphological, Histological, and Metabolic Differences of In Vitro- and Ex Vitro-Grown Panax ginseng. Agronomy. 2025; 15(9):2222. https://doi.org/10.3390/agronomy15092222
Chicago/Turabian StyleKim, So-Jeong, Yuna Jeon, Jang-Uk Kim, Jeongeui Hong, Sung Cheol Koo, Jun Young Ha, Kyung Ho Ma, Jeehye Sung, and Jung-Woo Lee. 2025. "Comparative Analysis of Morphological, Histological, and Metabolic Differences of In Vitro- and Ex Vitro-Grown Panax ginseng" Agronomy 15, no. 9: 2222. https://doi.org/10.3390/agronomy15092222
APA StyleKim, S.-J., Jeon, Y., Kim, J.-U., Hong, J., Koo, S. C., Ha, J. Y., Ma, K. H., Sung, J., & Lee, J.-W. (2025). Comparative Analysis of Morphological, Histological, and Metabolic Differences of In Vitro- and Ex Vitro-Grown Panax ginseng. Agronomy, 15(9), 2222. https://doi.org/10.3390/agronomy15092222






