Leguminous Cover Crops Promote Microbial Community Diversity in the Rhizosphere Soil of Tea Plants: Insights from 16S rRNA Microbiome Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Plant Material
2.2. Experimental Design and Treatments
2.3. Plot Layout
2.4. Soil Microbial Community
2.4.1. DNA Sequencing and Data Processing
2.4.2. Taxonomic and Diversity Analyses
2.4.3. Visualization and Community Structure
2.4.4. Functional and Metabolic Pathway Analysis
2.5. Data Analysis
3. Results
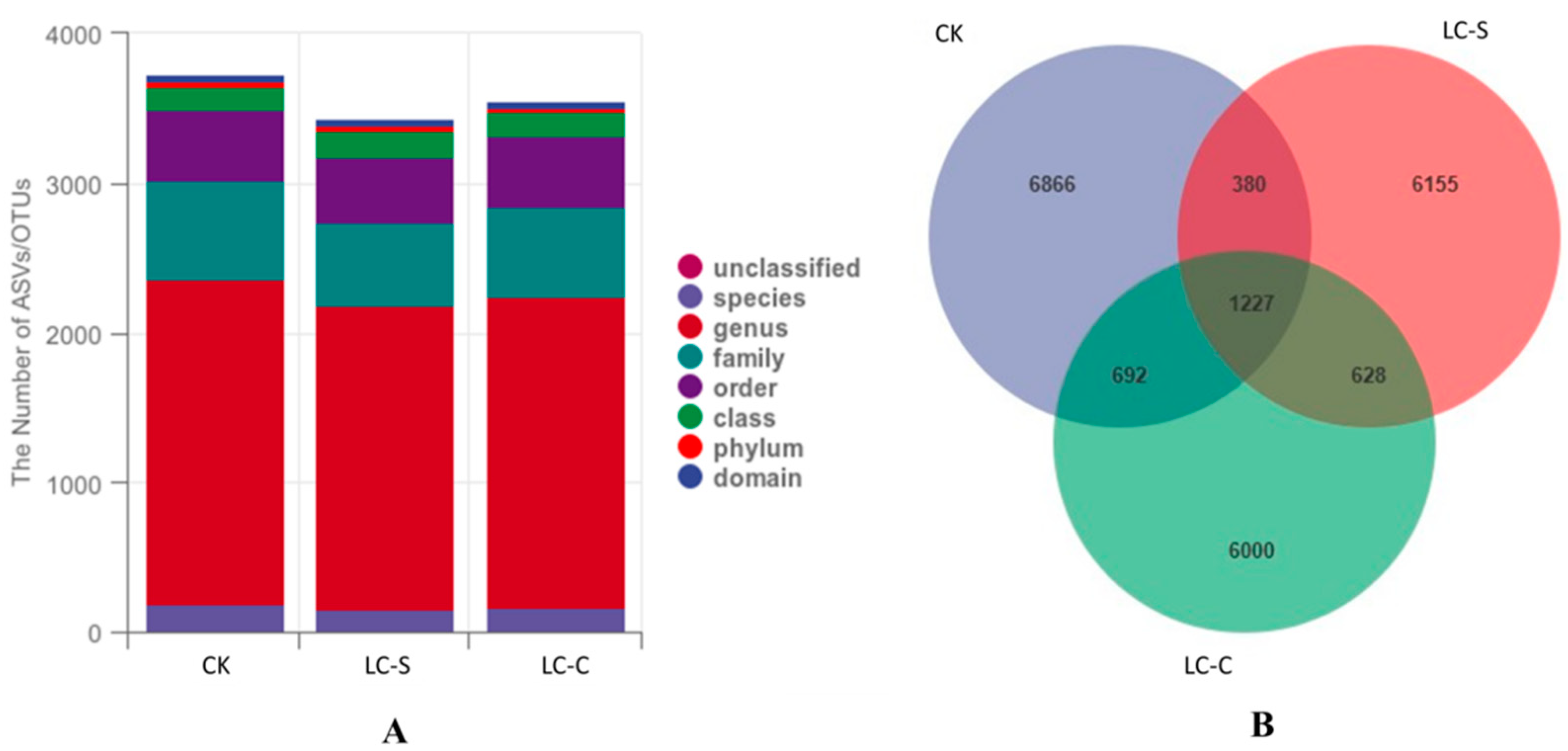
3.1. OTU/ASV Sequences of Microbes in the Collected Rhizosphere Soil Samples
3.2. Species Composition
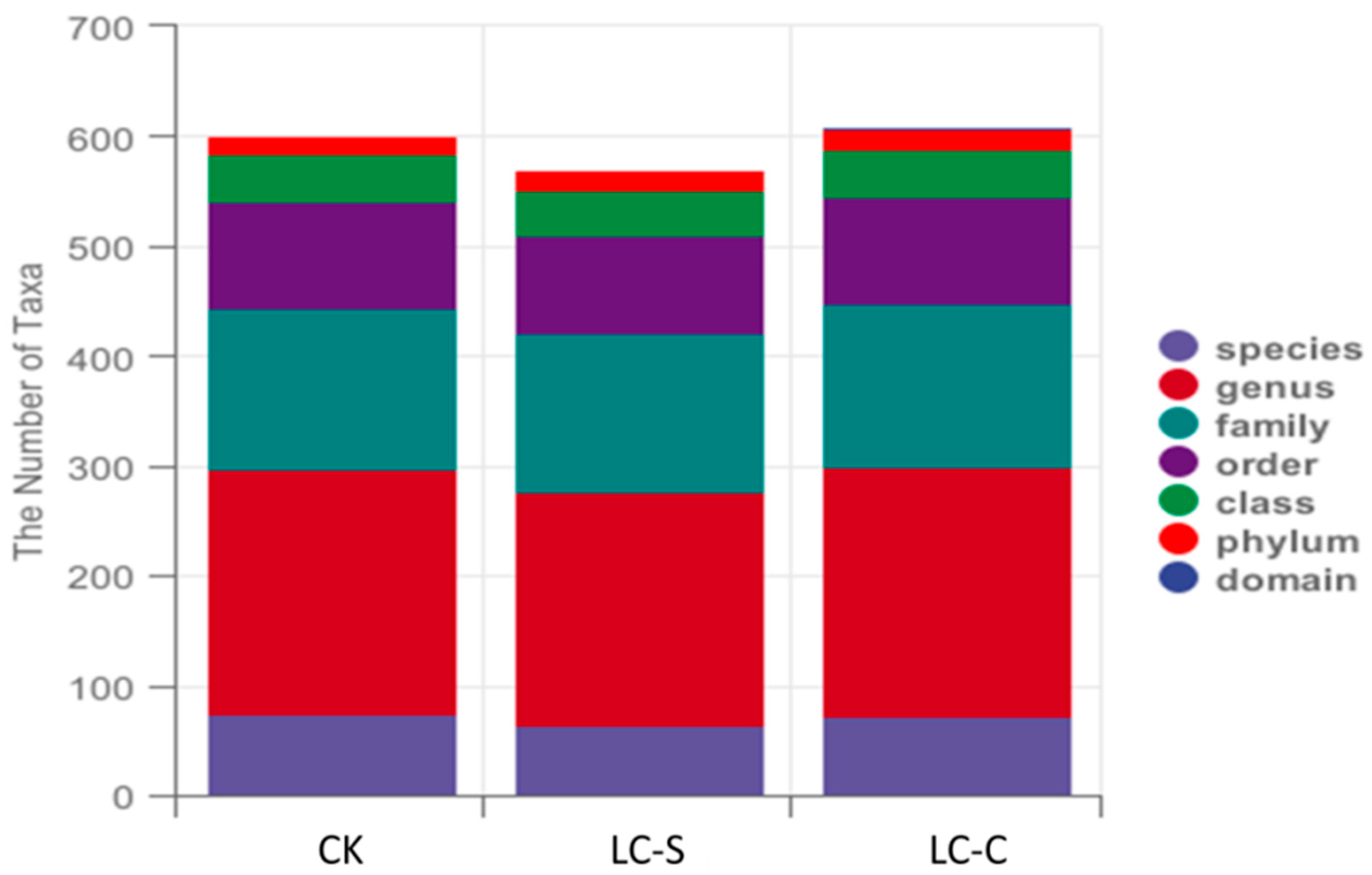
3.2.1. Species Taxonomic Annotation
3.2.2. Taxonomic Unit Counts
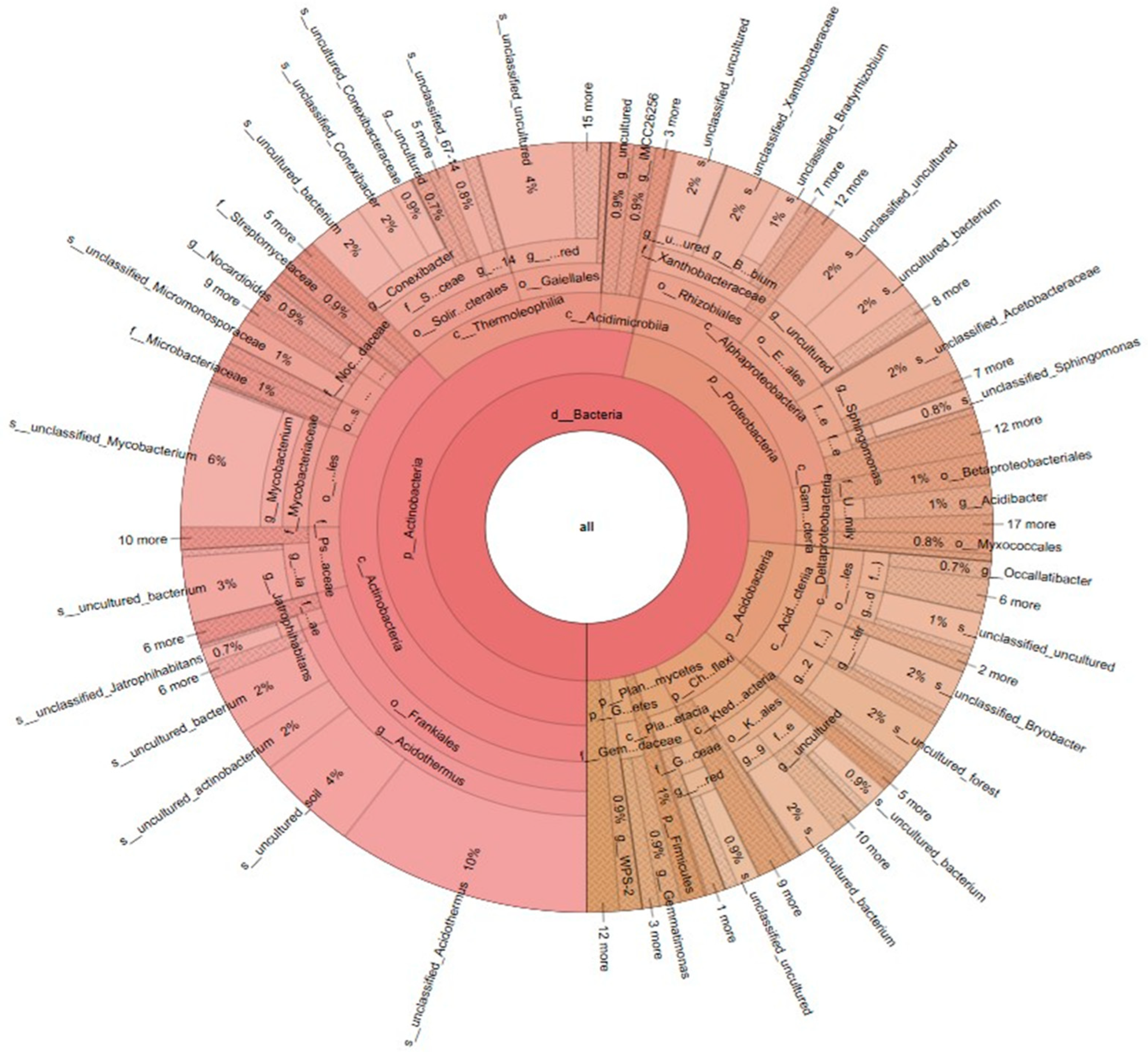
3.2.3. Krona Species Composition Map
3.3. Alpha Diversity Analysis
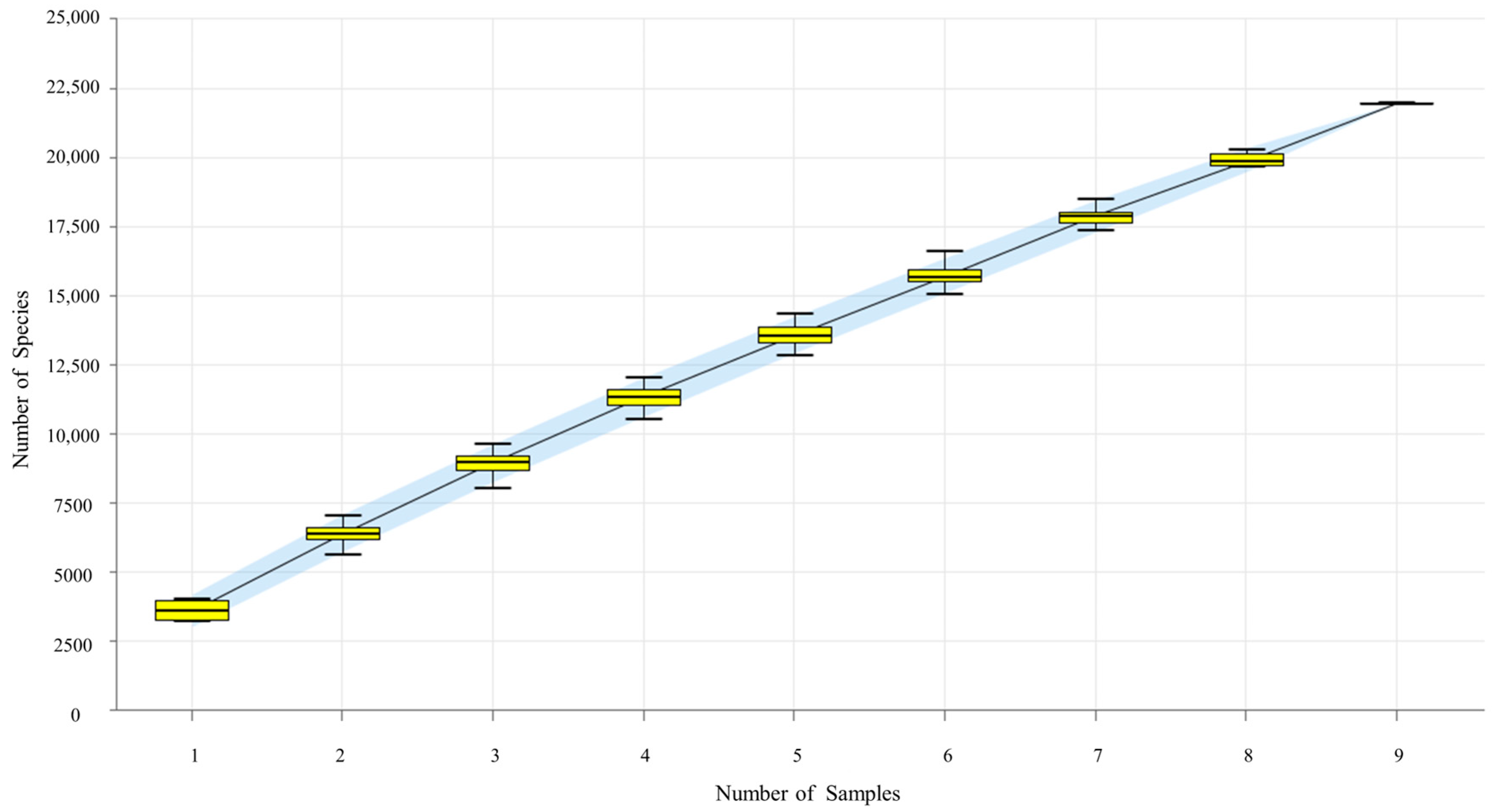
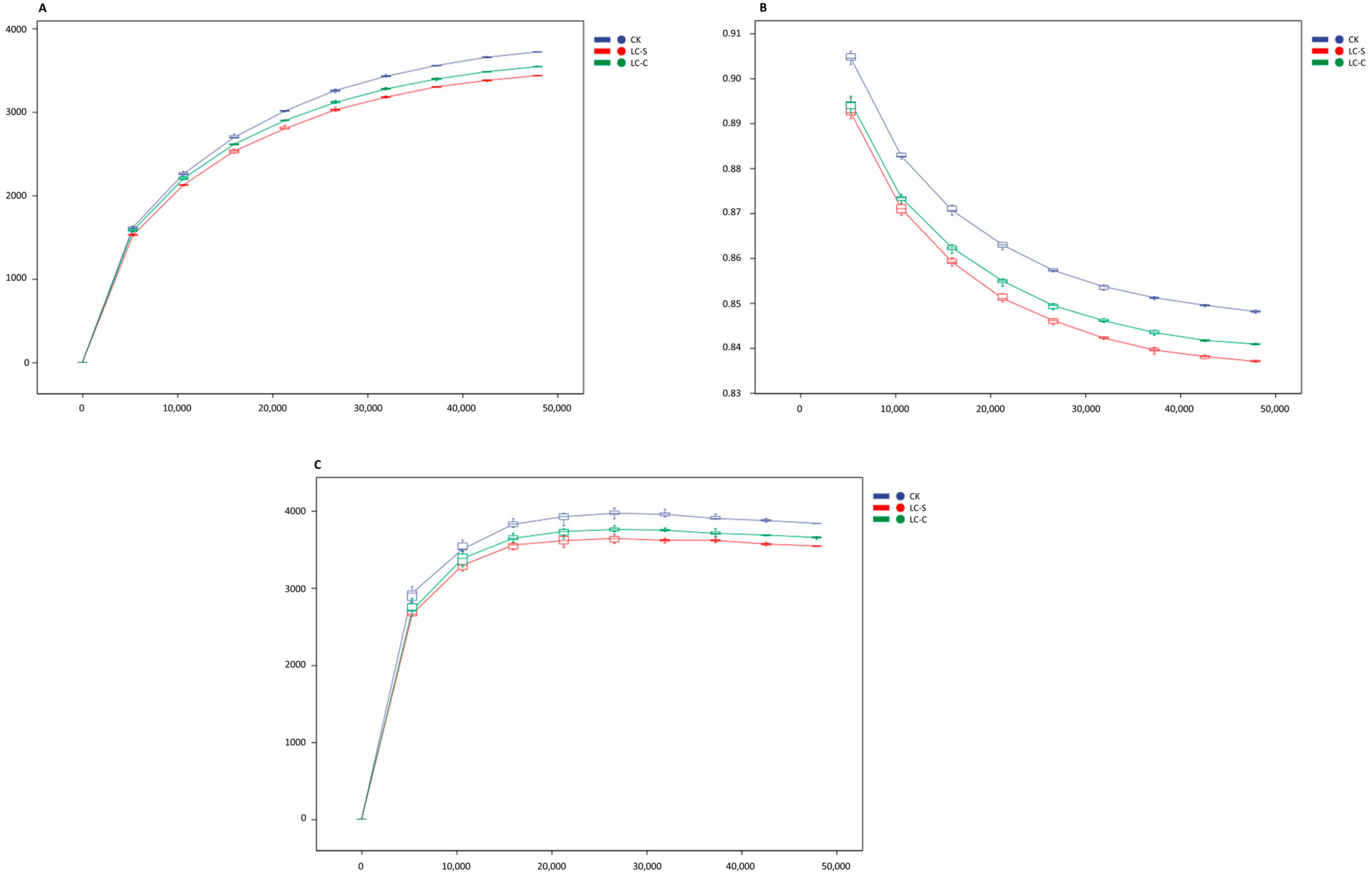
3.3.1. Species Accumulation Curve
3.3.2. Rarefaction Curves
3.4. Beta Diversity Analysis
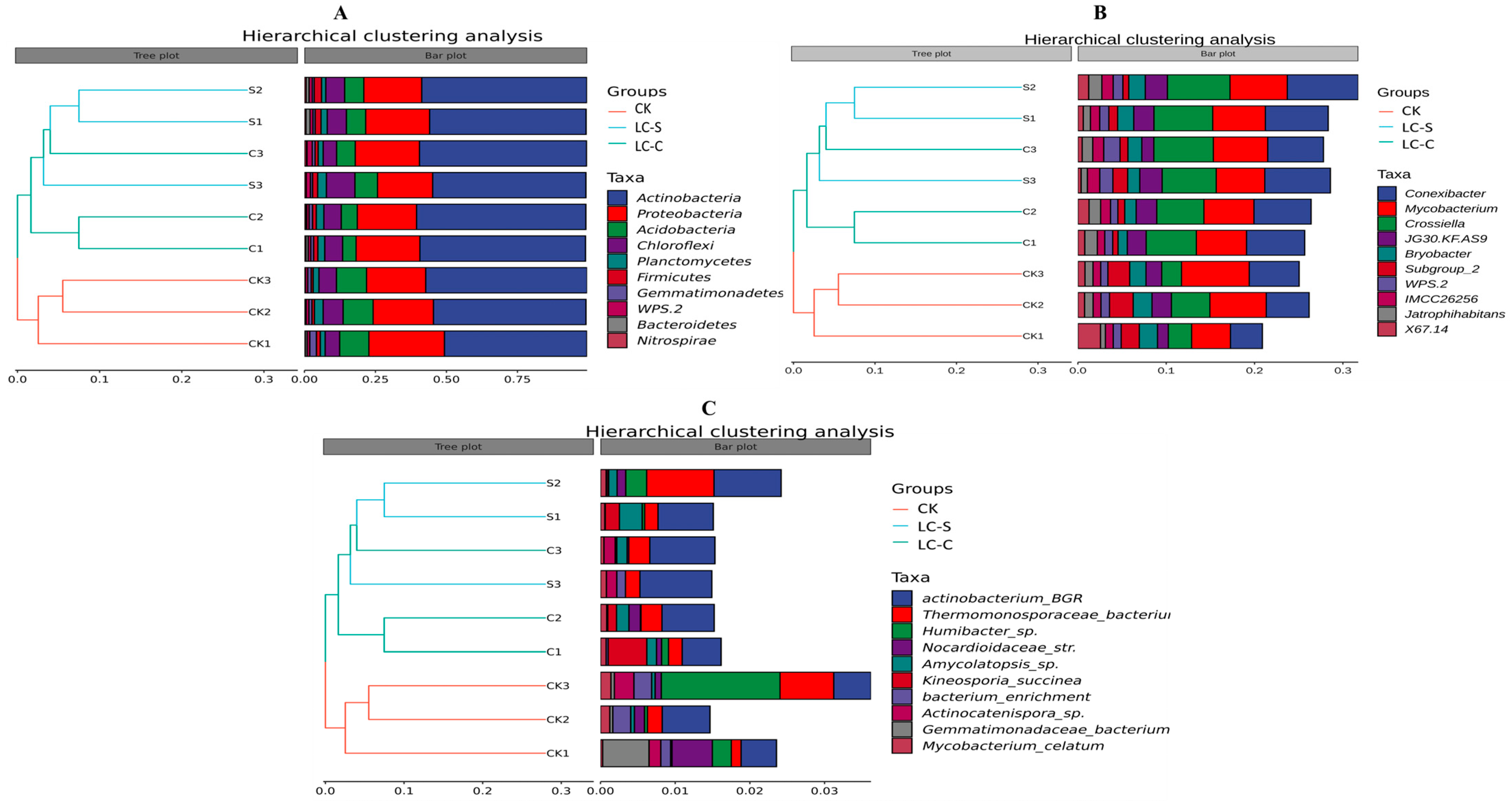
3.4.1. Hierarchical Cluster Analysis
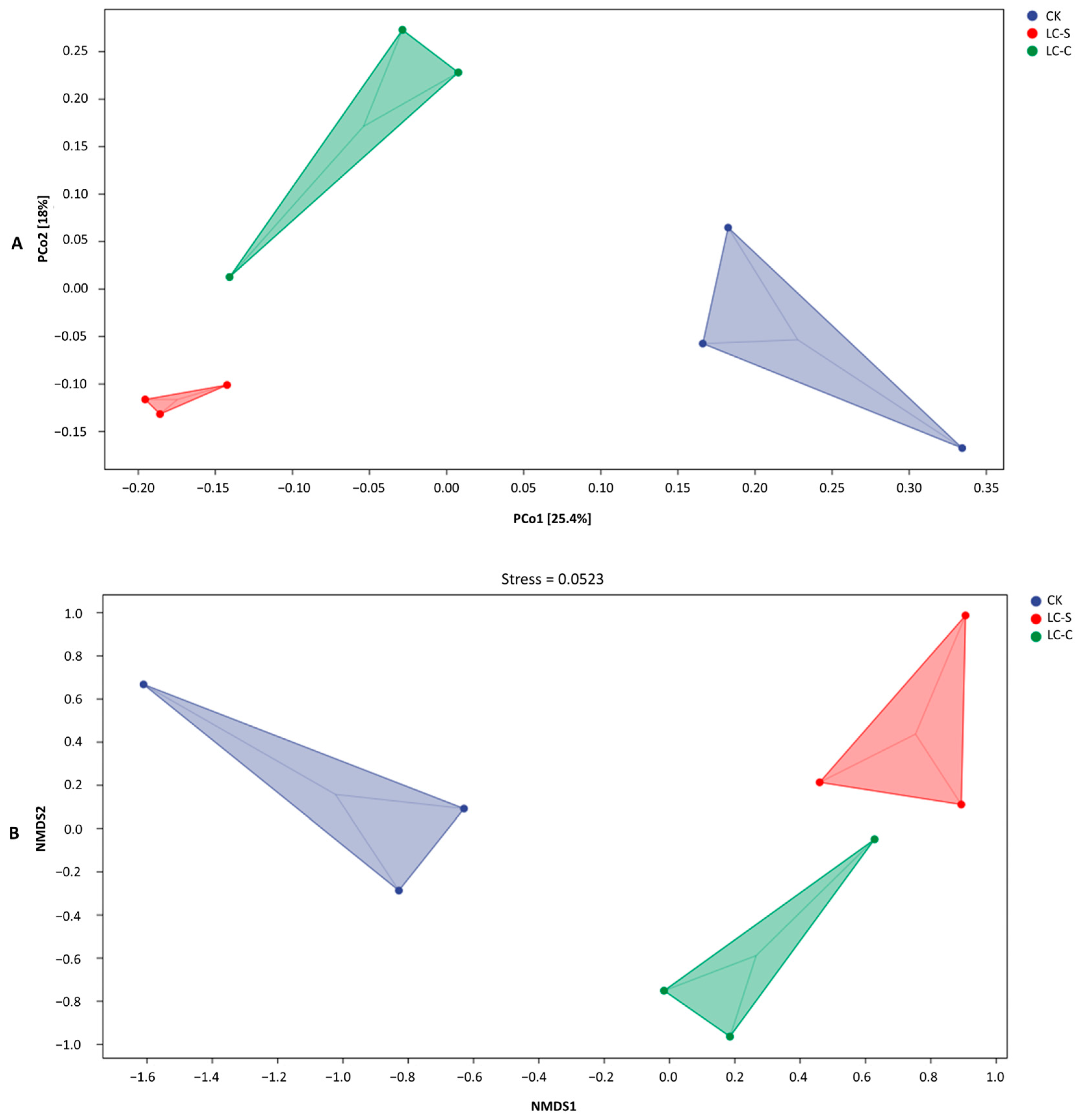
3.4.2. PCoA and NMDS Analysis
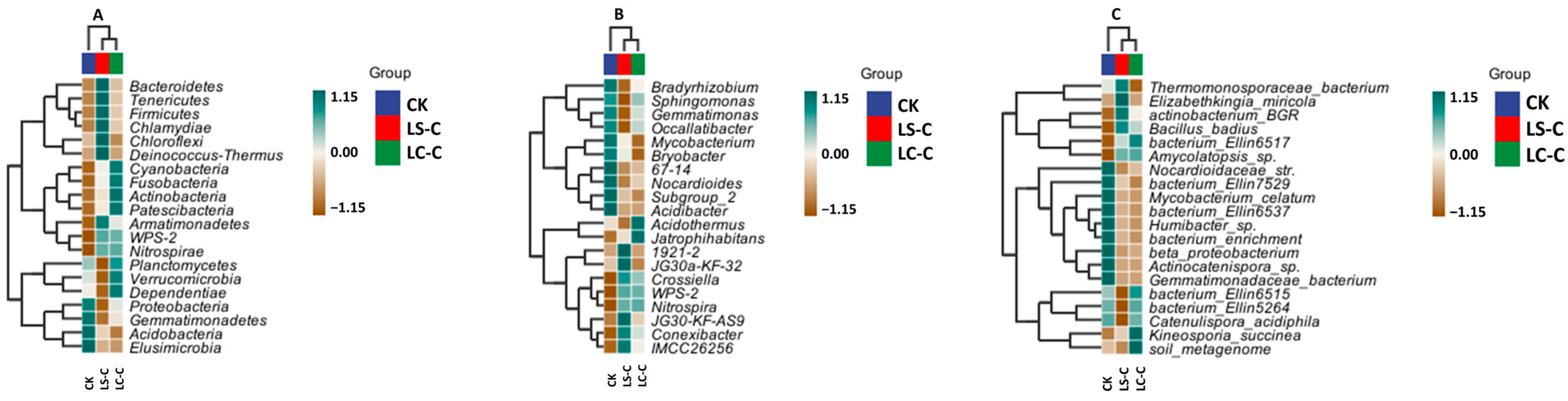
3.4.3. Cluster Heat Map Analysis
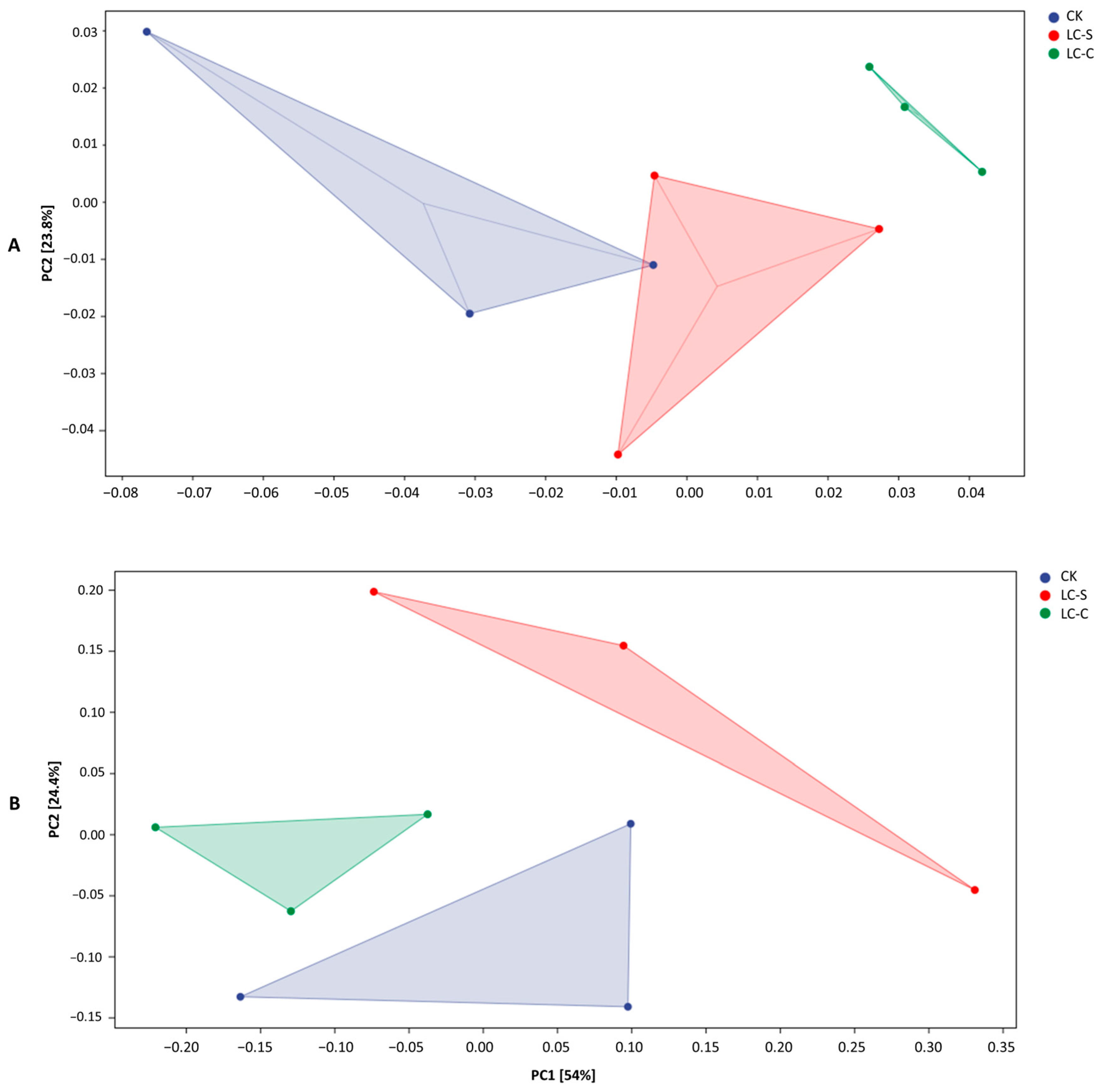
3.4.4. PCA and OPLS-DA Analysis
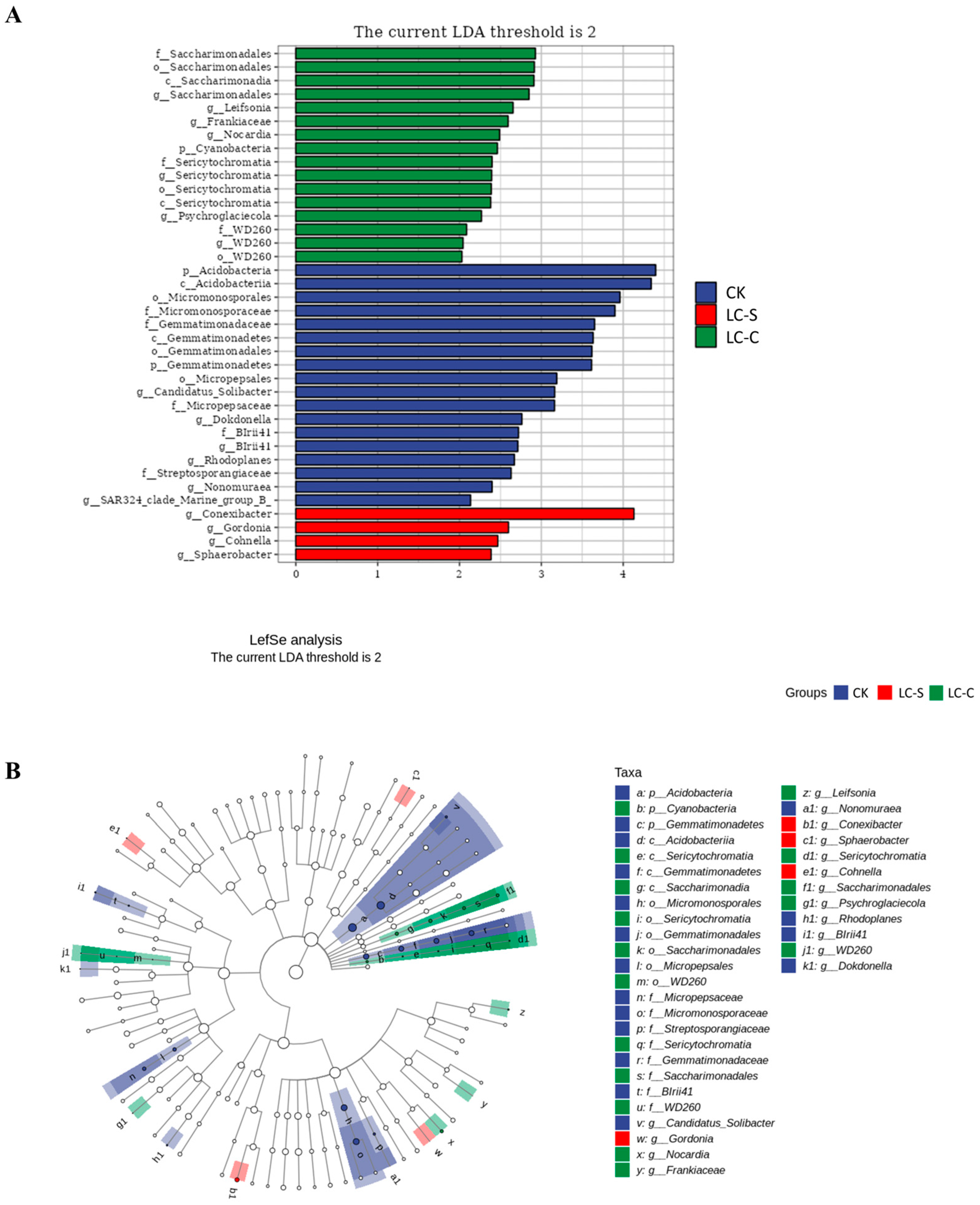
3.4.5. LEfSe Cladogram
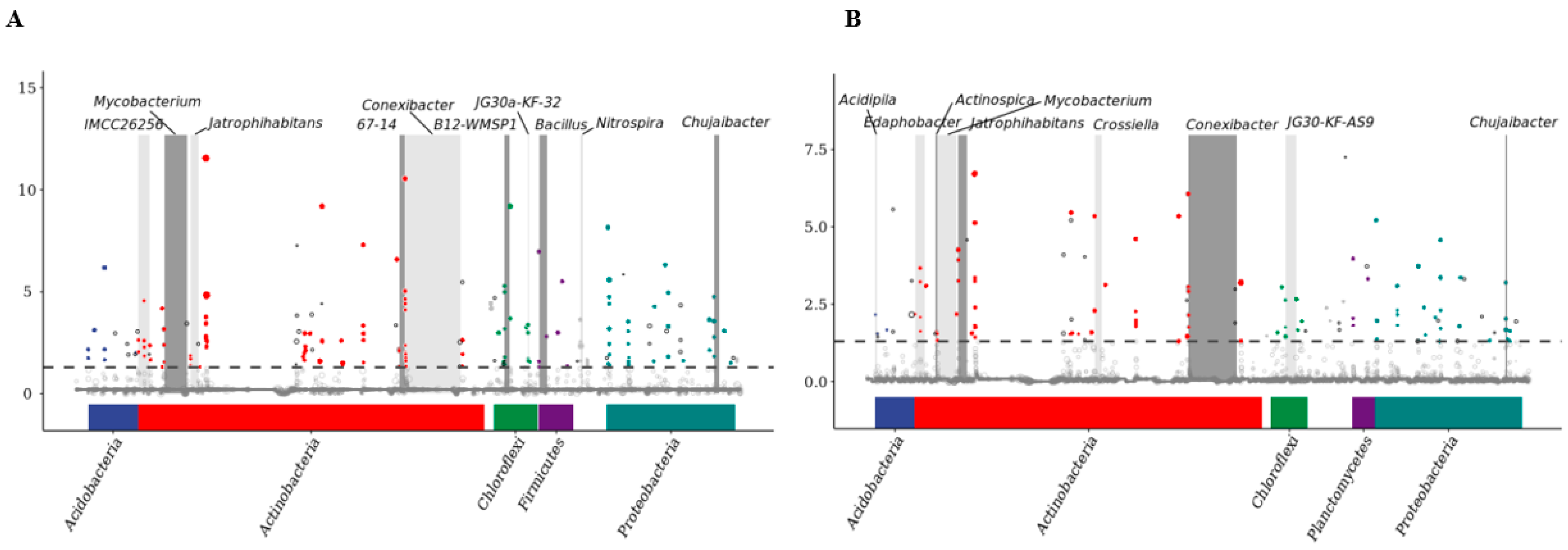
3.4.6. Metagenome Sequence Analysis
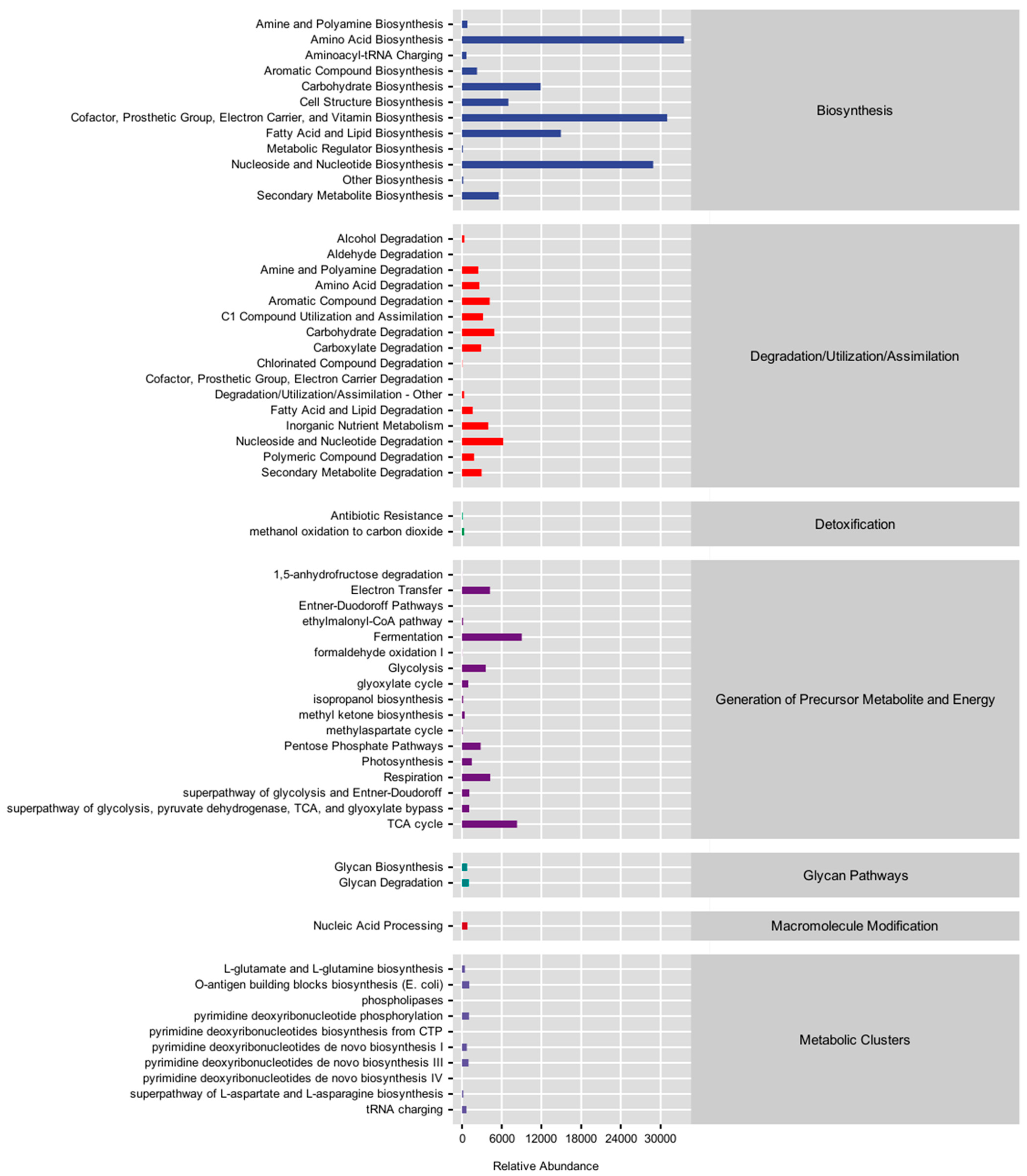
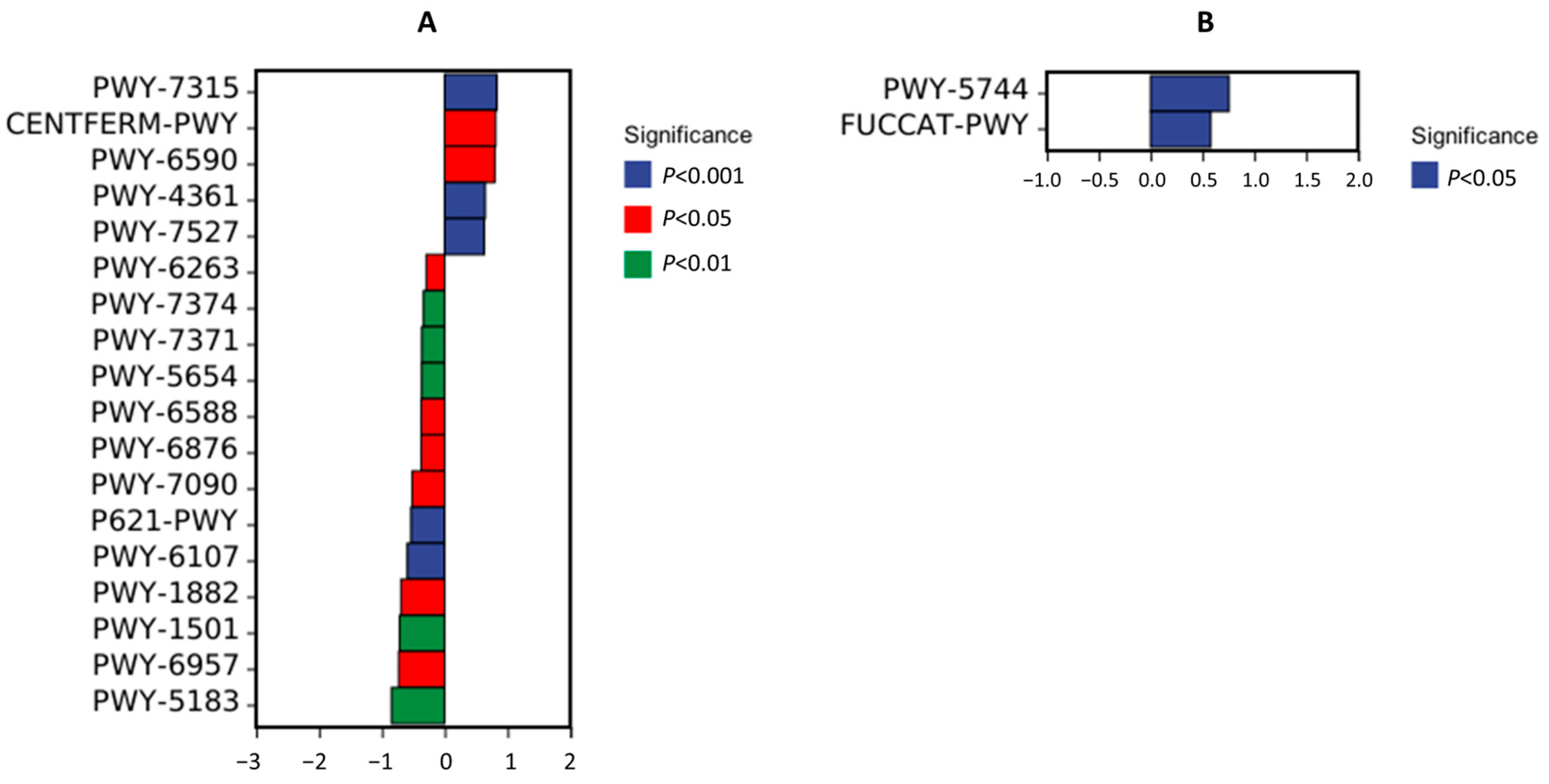
3.5. Metabolic Pathway Statistics
3.6. Metabolic Pathway Difference Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krahe, J.C.; Krahe, M.A.; Roach, P.D. Development of an Objective Measure of Quality and Commercial Value of Japanese-Styled Green Tea (Camellia L. sinensis): The Quality Index Tool. J. Food Sci. Technol. 2018, 55, 2926–2934. [Google Scholar] [CrossRef]
- Pokharel, S.S.; Shen, F.; Parajulee, M.N.; Wang, Y.; Chen, F. Effects of Elevated Atmospheric CO2 Concentration on Tea Quality and Insect Pests’ Occurrences: A Review. Glob. Ecol. Conserv. 2021, 27, e01553. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Ma, D.; Wang, L.; Zhang, X.; Ding, Y.; Fan, K.; Xu, Z.; Yuan, C.; Jia, H.; et al. Differential Responses of the Rhizosphere Microbiome Structure and Soil Metabolites in Tea (Camellia sinensis) Upon Application of Cow Manure. BMC Microbiol. 2022, 22, 55. [Google Scholar] [CrossRef]
- Badri, D.V.; Chaparro, J.M.; Zhang, R.; Shen, Q.; Vivanco, J.M. Application of Natural Blends of Phytochemicals Derived from the Root Exudates of Arabidopsis to the Soil Reveal That Phenolic-Related Compounds Predominantly Modulate the Soil Microbiome. J. Biol. Chem. 2013, 288, 4502–4512. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Shao, S.; Li, D.; Liu, H.; Xie, W.; Huang, W.; Li, J.; Nie, C.; Zhang, J.; Hong, Y.; et al. Exploring the Impact of Tea (Camellia sinensis (L.) O. Ktze.)/Trachelospermum jasminoides (Lindl.) Lem. Intercropping on Soil Health and Microbial Communities. Agronomy 2024, 14, 1261. [Google Scholar] [CrossRef]
- Scholes, M.C.; Scholes, R.J. Dust Unto Dust. Science 2013, 342, 565–566. [Google Scholar] [CrossRef]
- Basu, S.; Kumar, G.; Chhabra, S.; Prasad, R. Chapter 13—Role of Soil Microbes in Biogeochemical Cycle for Enhancing Soil Fertility. In New and Future Developments in Microbial Biotechnology and Bioengineering; Verma, J.P., Macdonald, C.A., Gupta, V.K., Podile, A.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 149–157. ISBN 978-0-444-64325-4. [Google Scholar]
- Debreczeni, K.; Kismányoky, T. Acidification of Soils in Long-Term Field Experiments. Commun. Soil Sci. Plant Anal. 2005, 36, 321–329. [Google Scholar] [CrossRef]
- Fierer, N.; Ladau, J.; Clemente, J.C.; Leff, J.W.; Owens, S.M.; Pollard, K.S.; Knight, R.; Gilbert, J.A.; McCulley, R.L. Reconstructing the Microbial Diversity and Function of Pre-Agricultural Tallgrass Prairie Soils in the United States. Science 2013, 342, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Liang, B.; Li, J. Changes in the Abundance and Structure of Bacterial Communities in the Greenhouse Tomato Cultivation System Under Long-Term Fertilization Treatments. Appl. Soil Ecol. 2017, 121, 82–89. [Google Scholar] [CrossRef]
- Qiao, C.; Xu, B.; Han, Y.; Wang, J.; Wang, X.; Liu, L.; Liu, W.; Wan, S.; Tan, H.; Liu, Y.; et al. Synthetic Nitrogen Fertilizers Alter the Soil Chemistry, Production and Quality of Tea. A Meta-Analysis. Agron. Sustain. Dev. 2018, 38, 10. [Google Scholar] [CrossRef]
- Ji, L.; Wu, Z.; You, Z.; Yi, X.; Ni, K.; Guo, S.; Ruan, J. Effects of Organic Substitution for Synthetic N Fertilizer on Soil Bacterial Diversity and Community Composition: A 10-Year Field Trial in a Tea Plantation. Agric. Ecosyst. Environ. 2018, 268, 124–132. [Google Scholar] [CrossRef]
- Pokharel, S.S.; Yu, H.; Fang, W.; Parajulee, M.N.; Chen, F. Intercropping Cover Crops for a Vital Ecosystem Service: A Review of the Biocontrol of Insect Pests in Tea Agroecosystems. Plants 2023, 12, 2361. [Google Scholar] [CrossRef]
- Shen, F.-T.; Lin, S.-H. Priming Effects of Cover Cropping on Bacterial Community in a Tea Plantation. Sustainability 2021, 13, 4345. [Google Scholar] [CrossRef]
- Sun, L.; Dong, X.; Wang, Y.; Maker, G.; Agarwal, M.; Ding, Z. Tea-Soybean Intercropping Improves Tea Quality and Nutrition Uptake by Inducing Changes of Rhizosphere Bacterial Communities. Microorganisms 2022, 10, 2149. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B.; Chakraborty, U.; De, U.; Chakraborty, A. Biological Control of Sclerotial Blight of Tea Using Arbuscular Mycorrhizal Fungus and Plant Growth Promoting Rhizobacterium. J. Tea Sci. 2012, 8, 27–35. [Google Scholar]
- Morang, P.; Dutta, B. Growth Promotion and Bi-Control Approaches of Brown Root Rot Disease of Tea by Pseudomonas Aeruginosa (PM 105). J. Plant Pathol. Microbiol. 2012, 5, 1–4. [Google Scholar] [CrossRef]
- Caballero-Mellado, J.; Onofre-Lemus, J.; Estrada-de Los Santos, P.; Martínez-Aguilar, L. The Tomato Rhizosphere, an Environment Rich in Nitrogen-Fixing Burkholderia Species with Capabilities of Interest for Agriculture and Bioremediation. Appl. Environ. Microbiol. 2007, 73, 5308–5319. [Google Scholar] [CrossRef]
- Guo, M.; Gong, Z.; Miao, R.; Jia, C.; Rookes, J.; Cahill, D.; Zhuang, J. Enhanced Polycyclic Aromatic Hydrocarbons Degradation in Rhizosphere Soil Planted with Tall Fescue: Bacterial Community and Functional Gene Expression Mechanisms. Chemosphere 2018, 212, 15–23. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, T.; Lei, X.; Cao, Y.; Liu, L.; Zou, Z.; Ma, Y.; Zhu, X.; Fang, W. Leguminous Green Manure Intercropping Changes the Soil Microbial Community and Increases Soil Nutrients and Key Quality Components of Tea Leaves. Hortic. Res. 2024, 11, uhae018. [Google Scholar] [CrossRef]
- Fagervold, S.K.; Rohée, C.; Rodrigues, A.M.S.; Stien, D.; Lebaron, P. Efficient Degradation of the Organic UV Filter Benzophenone-3 by Sphingomonas Wittichii Strain BP14P Isolated from WWTP Sludge. Sci. Total Environ. 2021, 758, 143674. [Google Scholar] [CrossRef]
- Ma, Q.; Song, L.; Niu, Z.; Qiu, Z.; Sun, H.; Ren, Z.; Wu, H.; Wang, Y.; Mei, H.; Li, X.; et al. Pea-Tea Intercropping Improves Tea Quality through Regulating Amino Acid Metabolism and Flavonoid Biosynthesis. Foods 2022, 11, 3746. [Google Scholar] [CrossRef]
- Cuartero, J.; Pascual, J.A.; Vivo, J.-M.; Özbolat, O.; Sánchez-Navarro, V.; Egea-Cortines, M.; Zornoza, R.; Mena, M.M.; Garcia, E.; Ros, M. A First-Year Melon/Cowpea Intercropping System Improves Soil Nutrients and Changes the Soil Microbial Community. Agric. Ecosyst. Environ. 2022, 328, 107856. [Google Scholar] [CrossRef]
- Barillot, C.D.C.; Sarde, C.-O.; Bert, V.; Tarnaud, E.; Cochet, N. A Standardized Method for the Sampling of Rhizosphere and Rhizoplan Soil Bacteria Associated to a Herbaceous Root System. Ann. Microbiol. 2013, 63, 471–476. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Ver Loren van Themaat, E.; Schulze-Lefert, P. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Bai, Y.; Müller, D.B.; Srinivas, G.; Garrido-Oter, R.; Potthoff, E.; Rott, M.; Dombrowski, N.; Münch, P.C.; Spaepen, S.; Remus-Emsermann, M.; et al. Functional Overlap of the Arabidopsis Leaf and Root Microbiota. Nature 2015, 528, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Heck, K.L.; van Belle, G.; Simberloff, D. Explicit Calculation of the Rarefaction Diversity Measurement and the Determination of Sufficient Sample Size. Ecology 1975, 56, 1459–1461. [Google Scholar] [CrossRef]
- Kemp, P.F.; Aller, J.Y. Bacterial Diversity in Aquatic and Other Environments: What 16s Rdna Libraries Can Tell Us. FEMS Microbiol. Ecol. 2004, 47, 161–177. [Google Scholar] [CrossRef]
- Carrion, B.; Onorati, T.; Díaz, P.; Triga, V. A Taxonomy Generation Tool for Semantic Visual Analysis of Large Corpus of Documents. Multimed. Tools Appl. 2019, 78, 32919–32937. [Google Scholar] [CrossRef]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive Metagenomic Visualization in a Web Browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. MEGAHIT: An Ultra-Fast Single-Node Solution for Large and Complex Metagenomics Assembly via Succinct De Bruijn Graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.-L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic Gene Recognition and Translation Initiation Site Identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for Clustering the Next-Generation Sequencing Data. Bioinform. Oxf. Engl. 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Wang, G.; Jin, Z.; Wang, X.; George, T.S.; Feng, G.; Zhang, L. Simulated Root Exudates Stimulate the Abundance of Saccharimonadales to Improve the Alkaline Phosphatase Activity in Maize Rhizosphere. Appl. Soil Ecol. 2022, 170, 104274. [Google Scholar] [CrossRef]
- Lei, Z.; Ding, Y.; Xu, W.; Zhang, Y. Microbial Community Structure in Rice Rhizosheaths Under Drought Stress. J. Plant Ecol. 2023, 16, 1–14. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, P.; He, Y.; Zeng, F.; Xu, J.; He, L. Enantioselective Effects of Cyflumetofen on Microbial Community and Related Nitrogen Cycle Gene Function in Acid-Soil. Sci. Total Environ. 2021, 771, 144831. [Google Scholar] [CrossRef] [PubMed]
- Egidi, E.; Wood, J.L.; Fox, E.M.; Liu, W.; Franks, A.E. Draft Genome Sequence of Leifsonia sp. Strain NCR5, a Rhizobacterium Isolated from Cadmium-Contaminated Soil. Genome Announc. 2017, 5, e00520-17. [Google Scholar] [CrossRef]
- Nordstedt, N.P.; Roman-Reyna, V.; Jacobs, J.M.; Jones, M.L. Comparative Genomic Understanding of Gram-Positive Plant Growth-Promoting Leifsonia. Phytobiomes J. 2021, 5, 263–274. [Google Scholar] [CrossRef]
- Normand, P.; Lapierre, P.; Tisa, L.S.; Gogarten, J.P.; Alloisio, N.; Bagnarol, E.; Bassi, C.A.; Berry, A.M.; Bickhart, D.M.; Choisne, N.; et al. Genome Characteristics of Facultatively Symbiotic Frankia sp. Strains Reflect Host Range and Host Plant Biogeography. Genome Res. 2007, 17, 7–15. [Google Scholar] [CrossRef]
- Thompson, R.M.; George, D.; del Carmen Montero-Calasanz, M. Actinorhizal Plants and Frankiaceae: The Overlooked Future of Phytoremediation. Environ. Microbiol. Rep. 2024, 16, e70033. [Google Scholar] [CrossRef]
- Fuentes, M.S.; Benimeli, C.S.; Cuozzo, S.A.; Amoroso, M.J. Isolation of Pesticide-Degrading Actinomycetes from a Contaminated Site: Bacterial Growth, Removal and Dechlorination of Organochlorine Pesticides. Int. Biodeterior. Biodegrad. 2010, 64, 434–441. [Google Scholar] [CrossRef]
- Das, R.; Bharadwaj, P.; Thakur, D. Insights into the Functional Role of Actinomycetia in Promoting Plant Growth and Biocontrol in Tea (Camellia sinensis) Plants. Arch. Microbiol. 2024, 206, 65. [Google Scholar] [CrossRef] [PubMed]
- Bennur, T.; Kumar, A.R.; Zinjarde, S.; Javdekar, V. Nocardiopsis Species: Incidence, Ecological Roles and Adaptations. Microbiol. Res. 2015, 174, 33–47. [Google Scholar] [CrossRef]
- Drzyzga, O. The Strengths and Weaknesses of Gordonia: A Review of an Emerging Genus with Increasing Biotechnological Potential. Crit. Rev. Microbiol. 2012, 38, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Jien, S.H.; Kuo, Y.L.; Liao, C.S.; Wu, Y.T.; Igalavithana, A.D.; Tsang, D.C.W.; Ok, Y.S. Effects of Field Scale in Situ Biochar Incorporation on Soil Environment in a Tropical Highly Weathered Soil. Environ. Pollut. 2021, 272, 116009. [Google Scholar] [CrossRef]
- Wu, S.; Wang, W.; Zhu, W.; Chen, W.; Xu, W.; Sui, M.; Jiang, G.; Xiao, J.; Ning, Y.; Ma, C.; et al. Microbial Community Succession in the Fermentation of Qingzhuan Tea at Various Temperatures and Their Correlations with the Quality Formation. Int. J. Food Microbiol. 2022, 382, 109937. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Zhou, L.; Chen, P.; Du, Q.; Pang, T.; Song, C.; Wang, X.; Liu, W.; Yang, W.; Yong, T. Effects of Maize-Soybean Relay Intercropping on Crop Nutrient Uptake and Soil Bacterial Community. J. Integr. Agric. 2019, 18, 2006–2018. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, L.; Ruineng, X.U.; Hanyu, X.U.; Sun, L.; Liao, H. Intercropping Tea Plantations with Soybean and Rapeseed Enhances Nitrogen Fixation Through Shifts in Soil Microbial Communities. Front. Agric. Sci. Eng. 2022, 9, 344–355. [Google Scholar] [CrossRef]
- Esperschütz, J.; Gattinger, A.; Mäder, P.; Schloter, M.; Fließbach, A. Response of Soil Microbial Biomass and Community Structures to Conventional and Organic Farming Systems Under Identical Crop Rotations. FEMS Microbiol. Ecol. 2007, 61, 26–37. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative Metagenomic, Phylogenetic and Physiological Analyses of Soil Microbial Communities Across Nitrogen Gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef]
- Kielak, A.M.; Cipriano, M.A.P.; Kuramae, E.E. Acidobacteria Strains from Subdivision 1 Act as Plant Growth-Promoting Bacteria. Arch. Microbiol. 2016, 198, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Lidbury, I.D.E.A.; Borsetto, C.; Murphy, A.R.J.; Bottrill, A.; Jones, A.M.E.; Bending, G.D.; Hammond, J.P.; Chen, Y.; Wellington, E.M.H.; Scanlan, D.J. Niche-Adaptation in Plant-Associated Bacteroidetes Favours Specialisation in Organic Phosphorus Mineralisation. ISME J. 2021, 15, 1040–1055. [Google Scholar] [CrossRef] [PubMed]
- Stolz, A. Degradative Plasmids from Sphingomonads. FEMS Microbiol. Lett. 2014, 350, 9–19. [Google Scholar] [CrossRef]
- Golubev, S.N.; Muratova, A.Y.; Panchenko, L.V.; Shchyogolev, S.Y.; Turkovskaya, O.V. Mycolicibacterium Sp. Strain Pam1, an Alfalfa Rhizosphere Dweller, Catabolizes Pahs and Promotes Partner-Plant Growth. Microbiol. Res. 2021, 253, 126885. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, J.; Li, Z.; Li, X.; Korpelainen, H.; Li, C. Circadian Rhythms of Microbial Communities and Their Role in Regulating Nitrogen and Phosphorus Cycling in the Rhizosphere of Tea Plants. Hortic. Res. 2025, 12, uhae267. [Google Scholar] [CrossRef]
- Yang, S.; Xiao, J.; Liang, T.; He, W.; Tan, H. Response of Soil Biological Properties and Bacterial Diversity to Different Levels of Nitrogen Application in Sugarcane Fields. AMB Expr. 2021, 11, 172. [Google Scholar] [CrossRef]
- Suh, M.K.; Kim, J.-S.; Eom, M.K.; Kim, H.S.; Do, H.E.; Shin, Y.K.; Lee, J.-S. Jatrophihabitans Cynanchi sp. nov., Isolated from Rhizosphere Soil of Cynanchum Wilfordii. Antonie Van Leeuwenhoek 2024, 117, 19. [Google Scholar] [CrossRef]
- Hu, F.; An, J.; Su, A.; Wang, B.; Ding, Z.; Yan, X.; Wei, S.; Xu, M.; Zhang, H. Green Manure Plant and Exogenous Degrading Bacteria Synergistically Promote Atrazine Removal by Enriching Indigenous Rhizosphere Degraders. J. Environ. Sci. 2025, 159, 694–704. [Google Scholar] [CrossRef]
- Arunrat, N.; Uttarotai, T.; Kongsurakan, P.; Sereenonchai, S.; Hatano, R. Bacterial Community Structure in Soils with Fire-Deposited Charcoal Under Rotational Shifting Cultivation of Upland Rice in Northern Thailand. Ecol. Evol. 2025, 15, e70851. [Google Scholar] [CrossRef]
- Li, J.; Huang, W.; Lin, X.; Khan, D.; Zhao, H.; Sun, B.; Liu, S.; Zheng, P. Study on Soil Nutrients and Microbial Community Diversity in Ancient Tea Plantations of China. Agronomy 2025, 15, 1608. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, X.; Nie, C.; Wu, T.; Dai, W.; Liu, H.; Yang, R. Analysis of Unculturable Bacterial Communities in Tea Orchard Soils Based on Nested Pcr-Dgge. World J. Microbiol. Biotechnol. 2012, 28, 1967–1979. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Kong, H.G.; Song, G.C.; Ryu, C.-M. Disruption of Firmicutes and Actinobacteria Abundance in Tomato Rhizosphere Causes the Incidence of Bacterial Wilt Disease. ISME J. 2021, 15, 330–347. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-C.; Li, B.-X.; Xu, C.-Y.; Raza, M.; Wang, Q.; Wang, Q.-Z.; Fu, Y.-N.; Hu, J.-Y.; Imoulan, A.; Hussain, M.; et al. Intercropping Walnut and Tea: Effects on Soil Nutrients, Enzyme Activity, and Microbial Communities. Front. Microbiol. 2022, 13, 852342. [Google Scholar] [CrossRef]
- Walsh, C.M.; Gebert, M.J.; Delgado-Baquerizo, M.; Maestre, F.T.; Fierer, N. A Global Survey of Mycobacterial Diversity in Soil. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef]
- Kui, L.; Xiang, G.; Wang, Y.; Wang, Z.; Li, G.; Li, D.; Yan, J.; Ye, S.; Wang, C.; Yang, L.; et al. Large-Scale Characterization of the Soil Microbiome in Ancient Tea Plantations Using High-Throughput 16S rRNA and Internal Transcribed Spacer Amplicon Sequencing. Front. Microbiol. 2021, 12, 745225. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, C.; Cao, Y.; Dai, J.; Cheng, X.; Hua, S.; Wang, W.; Duan, Y.; Petropoulos, E.; Wang, H.; et al. Tea Plant-Legume Intercropping Simultaneously Improves Soil Fertility and Tea Quality by Changing Bacillus Species Composition. Hortic. Res. 2022, 9, uhac046. [Google Scholar] [CrossRef]
- Do, Q.T. The Endophytic Bacterium Bacillus subtilis R8 as a Prospective Biocontrol Agent for Managing Tea Blister Blight and Enhancing Tea Yield. BioControl 2025, 70, 379–390. [Google Scholar] [CrossRef]
- Wen, X.; Wu, D.; Chen, D.; Xu, P.; Tang, Y. Abundance, Diversity, and Community Composition of Comammox nitrospira in Tea (Camellia sinensis) Plantation Soils. J. Soil Sci. Plant Nutr. 2024, 24, 7901–7918. [Google Scholar] [CrossRef]
- Wen, Z.; Yang, M.; Lu, G.; Han, M.; Song, Y.; Sun, Y.; Tu, Q.; Yin, T.; Niu, K.; Sun, S.; et al. Microbial Alliances: Unveiling the Effects of a Bacterial and Fungal Cross-Kingdom SynCom on Bacterial Dynamics, Rhizosphere Metabolites, and Soybean Resilience in Acidic Soils. J. Agric. Food Chem. 2025, 73, 18013–18031. [Google Scholar] [CrossRef]
- Rahav, E.; Giannetto, M.J.; Bar-Zeev, E. Contribution of Mono and Polysaccharides to Heterotrophic N2 Fixation at the Eastern Mediterranean Coastline. Sci. Rep. 2016, 6, 27858. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, M.; Yuan, M.M.; Wang, E.; Bai, Y.; Crowther, T.W.; Zhou, J.; Ma, Z.; Zhang, L.; Wang, Y.; et al. Root Microbiota Confers Rice Resistance to Aluminium Toxicity and Phosphorus Deficiency in Acidic Soils. Nat. Food 2023, 4, 912–924. [Google Scholar] [CrossRef]
- Singh, R.; Dubey, A.K. Diversity and Applications of Endophytic Actinobacteria of Plants in Special and Other Ecological Niches. Front. Microbiol. 2018, 9, 1767. [Google Scholar] [CrossRef]
- Qin, S.; Li, W.-J.; Klenk, H.-P.; Hozzein, W.N.; Ahmed, I. Editorial: Actinobacteria in Special and Extreme Habitats: Diversity, Function Roles and Environmental Adaptations, Second Edition. Front. Microbiol. 2019, 10, 944. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Shang, X.; Liu, G.; Zou, Z.; Zhu, X.; Ma, Y.; Li, F.; Fang, W. The Effects of Tea Plants-Soybean Intercropping on the Secondary Metabolites of Tea Plants by Metabolomics Analysis. BMC Plant Biol. 2021, 21, 482. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef]
- Laichao, S.; Zhanhai, N.; Shiliang, C.; Shilei, Z.; Ziyuan, Q.; Yu, W.; Xuewen, H.; Zhaotang, D.; Qingping, M. Effects of Pea-Tea Intercropping on Rhizosphere Soil Microbial Communities. Plant Soil 2025, 506, 125–135. [Google Scholar] [CrossRef]
- Wang, S.; Tang, L.; Ye, S. Dynamics of Soil Bacterial Community Diversity and Composition at Aggregate Scales in a Chronosequence of Tea Gardens. CATENA 2021, 206, 105486. [Google Scholar] [CrossRef]
- Jibola-Shittu, M.Y.; Heng, Z.; Keyhani, N.O.; Dang, Y.; Chen, R.; Liu, S.; Lin, Y.; Lai, P.; Chen, J.; Yang, C.; et al. Understanding and Exploring the Diversity of Soil Microorganisms in Tea (Camellia sinensis) Gardens: Toward Sustainable Tea Production. Front. Microbiol. 2024, 15, 1379879. [Google Scholar] [CrossRef]
- Ahmed, S.; Griffin, T.S.; Kraner, D.; Schaffner, M.K.; Sharma, D.; Hazel, M.; Leitch, A.R.; Orians, C.M.; Han, W.; Stepp, J.R.; et al. Environmental Factors Variably Impact Tea Secondary Metabolites in the Context of Climate Change. Front. Plant Sci. 2019, 10, 939. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chen, Z.; Feng, X.; Wang, M.; Luo, Y.; Wang, Y.; Xu, P. L-Theanine Exuded from Camellia Sinensis Roots Regulates Element Cycling in Soil by Shaping the Rhizosphere Microbiome Assembly. Sci. Total Environ. 2022, 837, 155801. [Google Scholar] [CrossRef] [PubMed]
- Rehan, M. Biosynthesis of Diverse Class Flavonoids via Shikimate and Phenylpropanoid Pathway. In Bioactive Compounds-Biosynthesis, Characterization and Applications; IntechOpen: London, UK, 2021; ISBN 978-1-83969-270-3. [Google Scholar] [CrossRef]
- Paul, A.; Acharya, K.; Chakraborty, N. Involvement of Phenylpropanoid Pathway and Shikimic Acid Pathway in Environmental Stress Response. In Biology and Biotechnology of Environmental Stress Tolerance in Plants; Apple Academic Press: Palm Bay, FL, USA, 2023; pp. 27–66. ISBN 978-1-00-334617-3. [Google Scholar]
- Sun, L. Exploration of Factors to Enhance Aromatic Quality in Green Tea. Ph.D. Thesis, Murdoch University, Perth, Australia, 2022. Available online: https://researchportal.murdoch.edu.au/esploro/fulltext/doctoral/Exploration-of-factors-to-enhance-aromatic/991005542568407891?repId=12135762170007891&mId=13136870480007891&institution=61MUN_INST (accessed on 18 September 2025).
- Xie, Y.; Cao, C.; Huang, D.; Gong, Y.; Wang, B. Effects of Microbial Biocontrol Agents on Tea Plantation Microecology and Tea Plant Metabolism: A Review. Front. Plant Sci. 2025, 15, 1492424. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Song, Y.; Wang, S.; Fan, K.; Wang, H.; Mao, Y.; Zhang, J.; Xu, Y.; Yin, X.; Wang, Y.; et al. Improved Phyllosphere Microbiome Composition of Tea Plant with the Application of Small Peptides in Combination with Rhamnolipid. BMC Microbiol. 2023, 23, 302. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, W.; Xiao, H.; Zhai, Y.; Luo, Y.; Wang, Y.; Liu, Z.; Li, Q.; Huang, J. A Review on Rhizosphere Microbiota of Tea Plant (Camellia sinensis L.): Recent Insights and Future Perspectives. J. Agric. Food Chem. 2023, 71, 19165–19188. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokharel, S.S.; Ali, Z.; Wang, C.; Jiang, X.; Chen, F. Leguminous Cover Crops Promote Microbial Community Diversity in the Rhizosphere Soil of Tea Plants: Insights from 16S rRNA Microbiome Analysis. Agronomy 2025, 15, 2217. https://doi.org/10.3390/agronomy15092217
Pokharel SS, Ali Z, Wang C, Jiang X, Chen F. Leguminous Cover Crops Promote Microbial Community Diversity in the Rhizosphere Soil of Tea Plants: Insights from 16S rRNA Microbiome Analysis. Agronomy. 2025; 15(9):2217. https://doi.org/10.3390/agronomy15092217
Chicago/Turabian StylePokharel, Sabin Saurav, Zahid Ali, Changyu Wang, Xingfu Jiang, and Fajun Chen. 2025. "Leguminous Cover Crops Promote Microbial Community Diversity in the Rhizosphere Soil of Tea Plants: Insights from 16S rRNA Microbiome Analysis" Agronomy 15, no. 9: 2217. https://doi.org/10.3390/agronomy15092217
APA StylePokharel, S. S., Ali, Z., Wang, C., Jiang, X., & Chen, F. (2025). Leguminous Cover Crops Promote Microbial Community Diversity in the Rhizosphere Soil of Tea Plants: Insights from 16S rRNA Microbiome Analysis. Agronomy, 15(9), 2217. https://doi.org/10.3390/agronomy15092217








