1. Introduction
Lilium brownii F. E. Brown var.
viridulum Baker, known locally as the Longya lily, belongs to the genus
Lilium of the family Liliaceae. Its bulbs are rich in sugars, proteins, minerals, and active ingredients (such as steroidal saponins, phenolic acids, polysaccharides, alkaloids, etc.), making it a popular natural health product that combines medicine and food, with economic benefits that are three to ten times greater than those of other crops [
1,
2,
3]. In production, the Longya lily is mainly propagated asexually through scale cutting, bulb division, and stem bulblets. However, these propagation methods not only have a long cycle and low propagation coefficient, but also cause issues, especially the accumulation of viruses and the deterioration of species, which ultimately results in a decrease in yield and quality [
4]. High-quality and efficient bulb propagation is the only way to solve the development problems of the Longya lily industry.
Plant tissue culture technology is an effective technique to obtain a large number of identical plants in a short span of time and produce disease-free planting material [
5,
6]. In the early 1950s, Robb first achieved success in tissue culture using the bulb scales of
Lilium speciosum [
7]. Since then, the tissue culture of lilies has gradually gained popularity and is currently in lilies for successful applications, such as
Lilium leucanthum [
8],
Lilium longiflorum [
9],
Lilium monodelphum var.
Armenum [
10],
Lilium regale [
11],
Lilium fargesii [
12]. Although many parts may be used in the in vitro propagation of
Lilium, scales are most frequently used [
13,
14]. At present, the tissue culture technology system of lily is mainly completed through two processes: the differentiation and proliferation of adventitious buds from scales and the formation and enlargement of bulblets [
15]. The successful culture of explants requires complex nutritional and hormonal requirements. In the in vitro culture of lily, commonly used media include Murashige and Skoog (MS), N6, B5, White, etc. Among them, the MS medium is the most frequently used in lily tissue culture and can also achieve excellent results [
16]. Han’s research also indicated that MS was the optimal medium, as it could efficiently induce bulb formation and result in the largest number of bulblets produced [
17]. Plant hormones or plant growth regulators (PGRs) and sucrose play important roles in the induction and development of bulblets. During the bulblet induction stage, the sucrose concentration is typically 30 g·L
−1 [
16,
18]. A relatively higher sucrose concentration is typically used for bulblet enlargement or growth. In the study on the growth of the
Lilium oriental hybrid ’Casablanca’, Lian et al. found that larger bulblets formed on media containing higher concentrations (90 g·L
−1) of sucrose [
19]. The contribution of PGRs on the induction, proliferation, and development of bulblets has been studied in various lily plants. Prior research findings have suggested that auxins 1-Naphthaleneacetic acid (NAA), and cytokinins 6-benzylaminopurine (6-BA) and Thidiazuron (TDZ) are predominantly used in the bulblet induction of lilies, such as
L. longiflorum [
20],
Lilium orientalis [
21], and Oriental
Lilium Hybrid Cv. ‘Ravenna’ [
18]. During the proliferation stage, plant hormones can increase the number of bulblets, enabling propagation. It has been reported that the combined application of 1.5 mg·L
−1 NAA and 2 mg·L
−1 BA significantly induces bulblet regeneration in Oriental hybrid lilies [
22]. In
Lilium rosthornii, the greatest proliferation ratio was from the combination of 0.1 mg·L
−1 NAA and 0.5 mg·L
−1 TDZ [
23]. In the stage of bulblet enlargement, studies have reported that low concentrations of jasmonic acid (JA) can promote the growth and development of bulblets [
12]. The application of a certain concentration of N-(2-Chloro-4-pyridyl)-N’-phenylurea (CPPU) in Asiatic hybrid lily ‘Matrix’ can promote bulblet initiation and development [
24]. However, the impact of PGRs on the enlargement of
L. brownii var.
viridulum remains to be further studied.
The establishment of a tissue culture technology system is one of the key links for the efficient and high-quality propagation of lilies. At present, most of the tissue culture studies on
L. brownii var.
viridulum focus on the differentiation research using scales as explants, and there is a lack of systematic studies on its sterilization, browning, differentiation, proliferation, and enlargement [
25]. Moreover, the research mainly focuses on 6-BA and NAA, with a lack of application of other growth regulators, which leads to problems such as a long differentiation cycle, a low differentiation coefficient, and the non-enlargement of bulbs [
26].
Therefore, this study explored the effects of different types and concentrations of sterilizing agents as well as hormone treatment combinations on scale differentiation and optimized the differentiation and proliferation system of L. brownii var. viridulum. It also investigated the impact of exogenously added substances on bulb enlargement and studied the bulb enlargement system. The aim is to establish an efficient propagation system for L. brownii var. viridulum and provide technical support for its industrial production.
4. Discussion
The surface disinfection process of explants is the first step in the tissue culture, and its purpose is to eliminate the microorganisms carried by the explants [
27,
28]. The selection and optimization of the disinfection process are conducive to reducing the contamination rate of explants and improving the survival rate. HgCl
2 and NaClO are the most widely used in lilies [
10,
29,
30]. However, HgCl
2 is toxic to both explants and the human body. As a disinfectant, NaClO is effective against many bacteria and viruses. In tissue culture, it is commonly used for the surface sterilization of explants in lilies [
10,
30], peonies [
31], sugarcanes [
32], etc. Different explants require different concentrations of NaClO and time, which is approximately 0.1% to 2% for 10–30 min, but higher concentrations are also used. In the in vitro culture of
Lilium monodelphum var.
Armenum, a 25% NaClO solution was used for 10 min for surface sterilization [
10]. In our study, 10% was the recommended sterilization concentration. We found that 20% NaClO was also highly effective, but led to aggravated browning of scales, which also increased the mortality rate of scales. This is consistent with the research results of water lilies [
33].
In plant tissue culture, the addition of PGRs plays a crucial role in plant morphology cultivation and development [
34,
35]. Auxins are mainly used to induce cell division and promote root differentiation. Cytokinins can promote cell division and shoot regeneration from calli or organs [
36]. Auxins and cytokinins are often used in combination to exert their effects on organ differentiation [
37]. Selecting PGR concentrations and the proper ratios of different PGRs are critical for tissue culture research [
38]. BA is considered the main factor causing excessive water accumulation, and this issue can be avoided by reducing the concentration of BA or replacing it with other cytokinins such as KIN and TDZ [
31]. TDZ is a proven effective and potent synthetic PGR, which can be used in organogenesis, regeneration, and development pathways, including axillary bud and adventitious bud proliferation, somatic embryogenesis, and in vitro flowering [
10,
39,
40]. However, high concentrations of TDZ inhibit the development of explants. TDZ (>2.0 μM) induced undesirable changes in plant morphology, such as abnormal leaf morphology, fasciated shoots, and swollen shoot bases [
40]. Abnormal bulblets with small bulb scales and swollen basal plates from bulb scales of the
Lilium oriental hybrid ‘Casablanca’ formed in media containing 4.5 µM TDZ [
17], which is consistent with the results of this study. In addition, our results showed that low concentrations of TDZ combined with certain concentrations of NAA and KIN can improve the differentiation rate of
L. brownii var.
viridulum scales, which has not been reported in previous studies on lily.
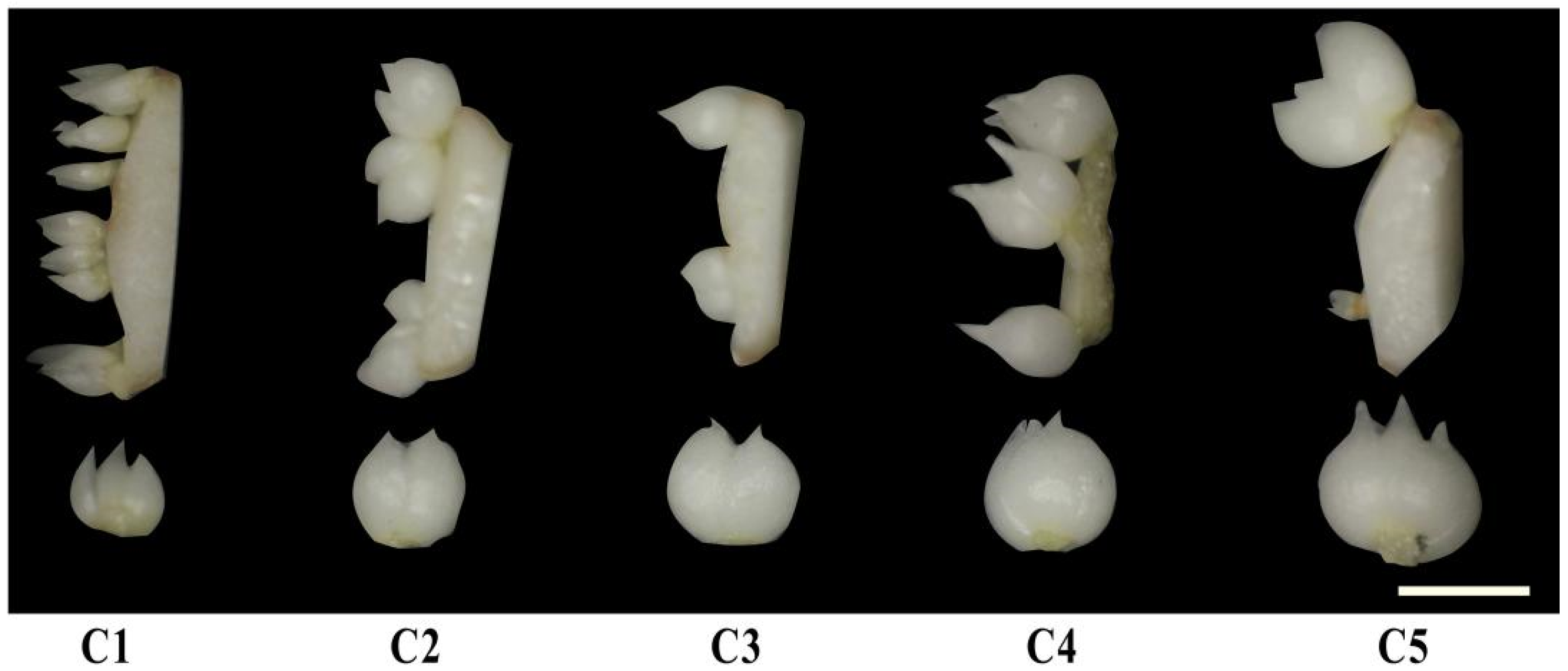
Browning was observed at the cut surface during the differentiation of
L. brownii var.
viridulum, which seriously affected the differentiation of scales. AC is considered to be able to adsorb inhibitory substances derived from the explants themselves that may exist in the culture medium, as well as inhibitory substances such as phenolics and other organic compounds contained in the culture medium [
41,
42]. AC can enhance the establishment of protoplast cultures, preventing the development of abnormal seedlings and bud formation [
42]. It was found that when 200 mg·L
−1 AC was added to the culture medium, the bud formation of lettuce could be effectively induced, with an induction rate of 90.8 ± 7.9% [
43]. Steinitz and Yahel and Peck and Cumming found that adding AC during the bulb culture of
Narcissus and
Pleurotus eryngii could effectively reduce browning and promote bulb formation [
44,
45]. This study has obtained similar results that adding AC can not only effectively improve browning but also promote the formation of bulblets in
L. brownii var.
viridulum.
Proliferation culture is a key step in realizing the industrialization of plant tissue culture. An appropriate combination of growth regulators, usually auxins and cytokinins, can effectively improve the proliferation coefficient. In the proliferation culture of Acacia arabica, no proliferation occurred in media without growth regulators, and the highest average numbers of somatic embryos was 72.6 after 8 weeks of culture on medium containing 6.66 μM of BA and 6.78 μM of 2,4-D [
46]. In the shoot regeneration of Sinningia Hybrida ‘Isa’s Murmur’, the combination of TDZ and NAA was more favorable for shoot proliferation. The highest shoot proliferation coefficient (24.5) was reached in 0.1 mg·L
−1 of NAA and 2.0 mg·L
−1 of TDZ [
47]. Fu et al. found that MS medium with combinations of 0.5 mg·L
−1 of 6-BA and 1.0 mg·L
−1 of NAA was predominant for the callus growth of
L.
brownii var.
viridulum, and the frequency of callus proliferation was 56.30% [
4]. In our study, the highest proliferation rate was the combination of 6-BA (1.50 mg·L
−1) and NAA (0.50 mg·L
−1). However, our study found that 6-BA promotes proliferation within a certain concentration range, but when its concentration exceeds a specific threshold, the proliferation of bulblets is inhibited, which was consistent with the results obtained in
Aeschynanthus pulcher [
48],
Cnidoscolus aconitifolius [
49]. In addition, this study found that an excessively high sucrose concentration was not conducive to the proliferation of bulblets and would affect the normal development of bulblet roots. The same result was observed in the study on Moringa oleifera [
50]. When the sucrose concentration was 30 g·L
−1, the shoot proliferation coefficient was 4.13. As the sucrose concentration increased, the proliferation coefficient decreased, which was significantly lower than that of the treatment with 30 g·L
−1 of sucrose, being 2.50 and 2.41, respectively.
At present, the tissue culture technology system of the lily is mainly completed through two processes: the differentiation and proliferation of adventitious buds from scales, and the formation and enlargement of bulblets [
15]. Among them, the formation and enlargement of bulblets are the key to accelerating the production and localization of lily bulbs. However, slow development and the failure to enlarge bulblets are common in lily tissue culture. Studies have shown that macronutrients, such as N, P, and K, promote the growth of lily plants. Potassium is an essential nutrient for plant living cells and is crucial for plant osmotic regulation and phloem transport [
51,
52]. A single application of nitrogen and phosphorus fertilizer can promote the growth of lily plants, but its increase is lower than that of potassium fertilizer. Sha et al. conducted a study on the effects of potassium application on
Lilium davidii var.
unicolor growth, and the results showed that adding an appropriate K concentration could increase the bulb weight and bulb circumference [
53], which is consistent with the results of this study. But the bulb weight and circumference decreased significantly with excessive K fertilizer application [
54]. This is possibly because the application of an excessive amount of K hinders the absorption of other nutrients such as N and P, thereby negating the positive effects of K fertilization [
55]. In addition, the application of PGRs, such as JA, salicylic acids, forchlorfenuron, and humic acids, can also promote the formation and expansion of bulbs [
56,
57]. Studies have shown that exogenous application of 5 µM or 7.5 µM of Me-JA can promote the enlargement of garlic bulbs [
58]. However, it has been found that medium with high concentrations (300–1000 µL·L
−1) of Me-JA significantly inhibit the regeneration rate and fresh weight of bulbs in Lilium speciosum [
30]. This is consistent with the result of the present study that excessively high concentrations of Me-JA have an inhibitory effect on the growth and development of bulblets. CPPU is known to be effective for enhancing fruit enlargement by stimulating cell division and/or cell expansion in many kinds of fruits and bulbous plants [
56,
59]. A total of 10 mg·L
−1 of CPPU promoted the increase in fruit size and weight of red-fleshed kiwifruit [
60]. There is a significant positive correlation between bulb growth and starch accumulation. Spray treatment with an appropriate concentration of CPPU (1.0 mg·L
−1) could promote the activity of starch synthase and accelerate the accumulation of starch in bulblets, which was beneficial for the enlargement of
Lycoris aurea bulblets [
56], which was consistent with our results of CPPU promoting the size and fresh weight of
Lilium brownii var.
viridulum bulblets. BRs are a group of steroid hormones that play an essential role in promoting plant growth and rooting, enhancing stress resistance, increasing plant yield, and improving plant quality [
61]. In the study of
Bupleurum chinense, BR treatments increased fresh root weight, dry root weight, taproot length, and taproot diameter of
B. chinense compared with the CK group [
62]. In the study of bulbil formation and development in
Pinellia ternata, the tuber and bulbil yield, starch, and soluble sugar content were significantly enhanced by BR treatment [
63,
64]. In our study, BRs at different concentrations could promote the enlargement of bulblets, with the concentration of 0.5 mg·L
−1 being recommended. Beyond this concentration, an inhibitory effect was observed as the BR concentration increased. This is consistent with the role of BR in increasing the flower diameter of
Freesia hybrida ‘Hongshi’ that, within a certain concentration range, the promoting effect strengthens as the BR concentration increases, while inhibition occurs when the concentration exceeds a certain threshold [
65].