Interactive Effects of Different Field Capacity and Nitrogen Levels on Soil Fertility and Microbial Community Structure in the Root Zone of Jujube (Ziziphus jujuba Mill.) Seedlings in an Arid Region of Southern Xinjiang, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Field and Experiment
- Urea (N ≥ 46%), meeting the Chinese national standard GB/T 2440-2017 [39], was used as the N fertilizer source and was produced by Aksu Huajin Chemical Fertilizer Co., Ltd., Aksu, China.
- Urease inhibitor N fertilizer: Puzhilan (N ≥ 45%), to which the dual urease inhibitors NBTP and NTTP were added. Puzhilan is produced by Proswin (Hanhe Bio-Technology Co., Ltd., Nanning, China).
- Nitrification inhibitor-type N fertilizer: Euro N (N ≥ 20.5%), to which the nitrification inhibitor DMPP was added. Euro N is produced by Eurosin Nanning Hanhe Bio-Technology Co., Ltd., Nanning, China.
- Microbial inoculant: ≥1.0 × 109 CFU/mL (Bacillussubtilis, Bacillus, amyloliquefaciens, Bacillus licheniformis, Bacillus pumilus) (produced by BioWish Inc., Cincinnati, OH, USA). According to the manufacturer’s instructions, it was applied in combination with conventional urea at an optimal ratio of 2 mL per kg of urea.
2.2. Field-Capacity Control
2.3. Soil Sample Collection and Analysis of Soil Properties
2.4. Soil DNA Extraction, PCR Amplification and Illumina MiSeq Sequencing
2.5. Bioinformatic Processing and Statistical Analysis
- (1)
- Chao1—bias-corrected species richness;
- (2)
- Faith’s PD—total phylogenetic branch length;
- (3)
- Goods_coverage—proportion of the total species represented;
- (4)
- Pielou’s evenness—Shannon-based evenness (0 = dominance, 1 = perfect evenness);
- (5)
- Shannon—composite diversity integrating richness and evenness;
- (6)
- Simpson—evenness-weighted diversity, less sensitive to rare taxa.
3. Results
3.1. Effects on Soil Physicochemical Properties Induced by Water–N Management with EENFs
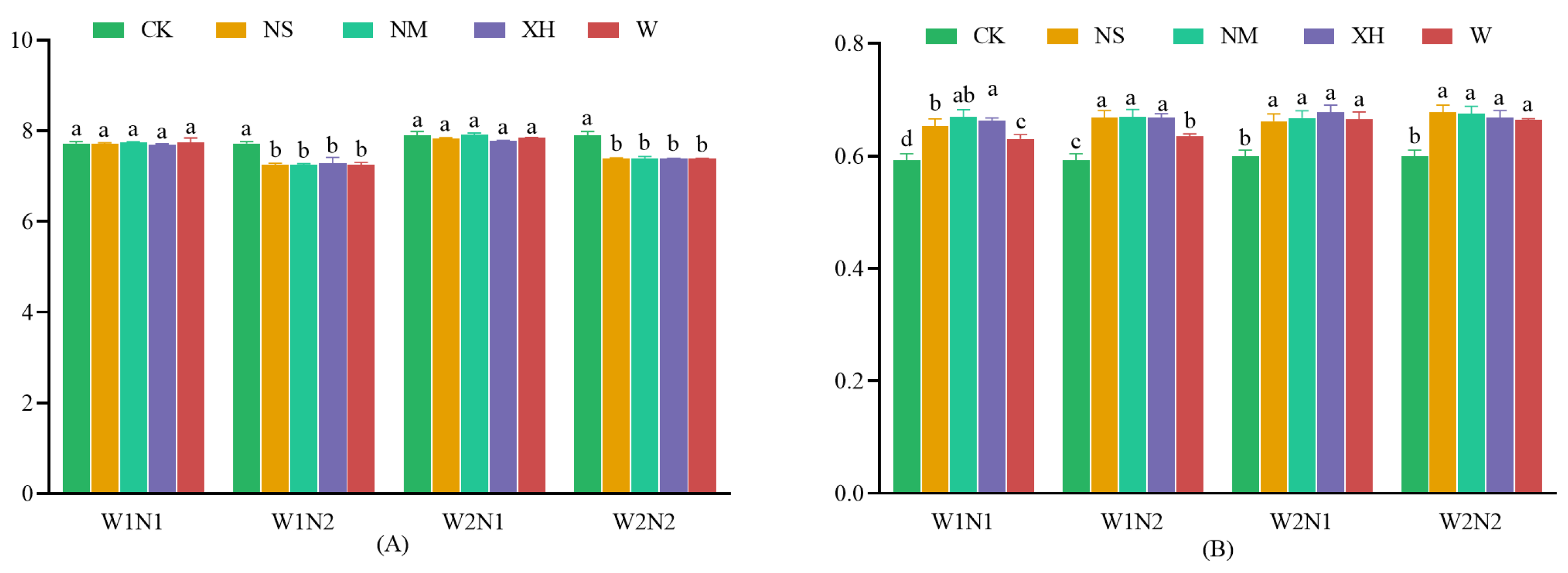
3.1.1. Response of Soil pH and Electrical Conductivity
3.1.2. Response of Soil Available Nutrients
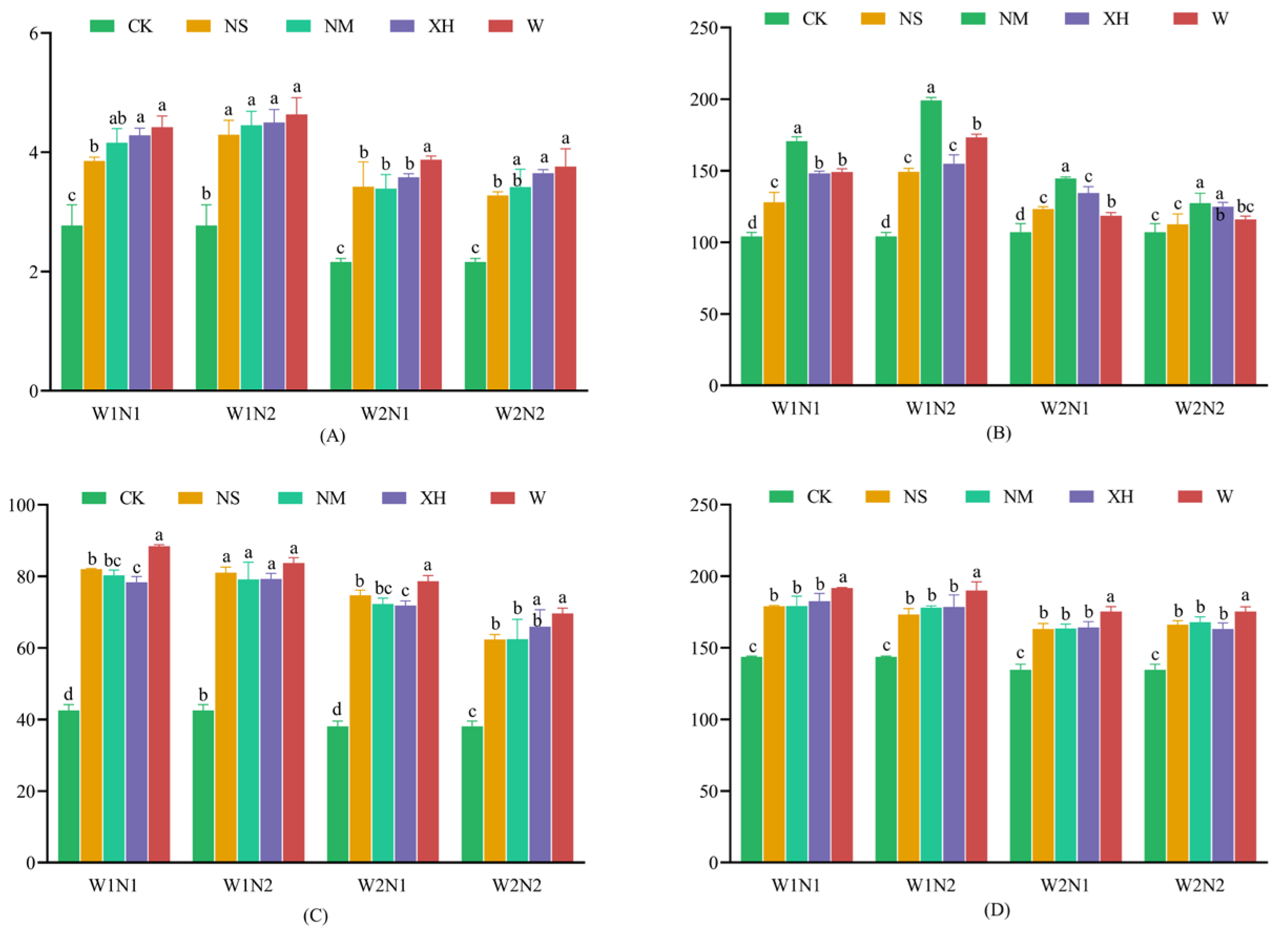
3.1.3. Response of Soil Macroelement and Microelement Concentrations
3.2. Effects of Water–N Coupling Combined with EENFs on Soil N Forms and Losses
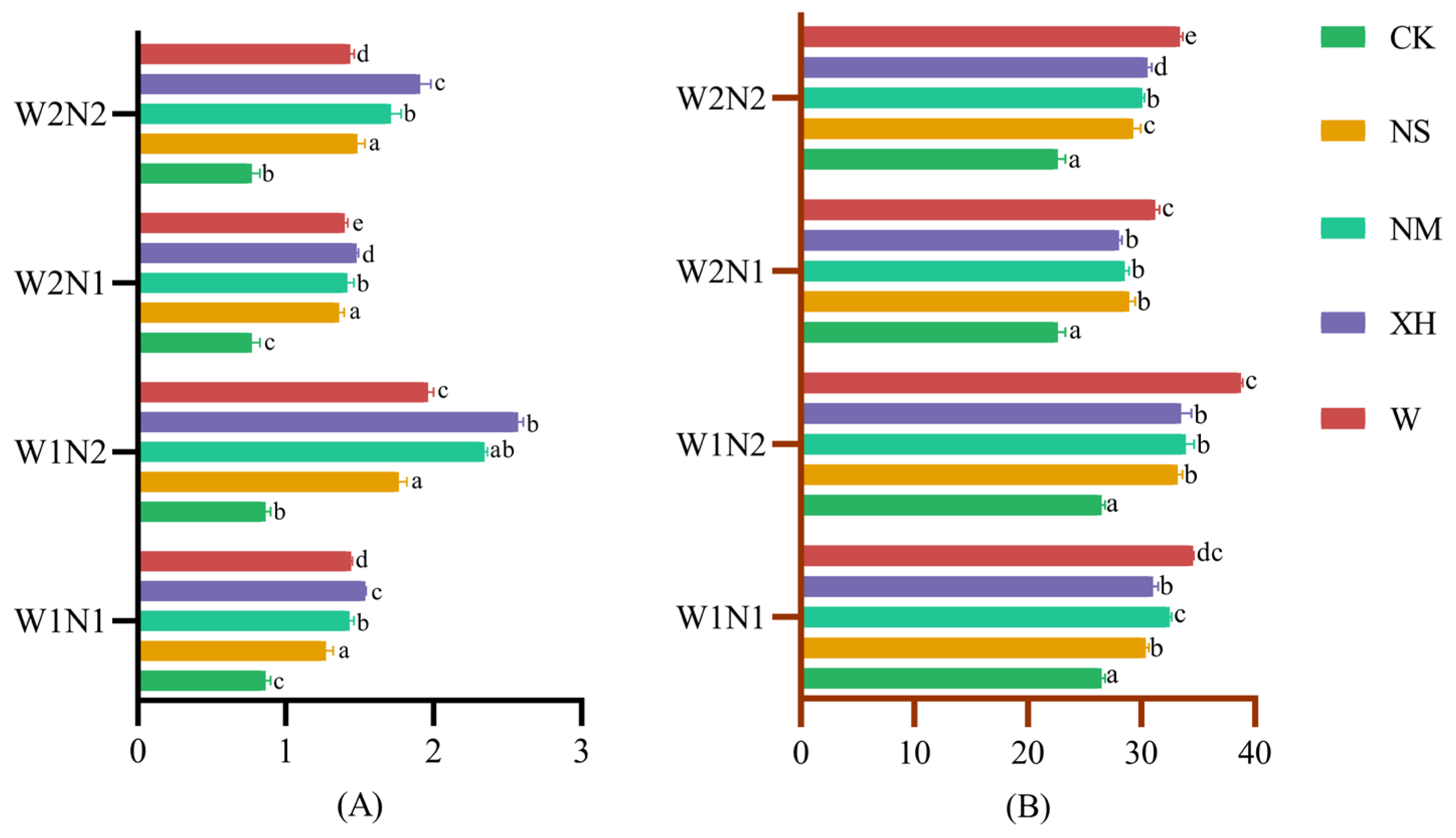
3.2.1. Effects in Soil NO3−-N and NH4+-N
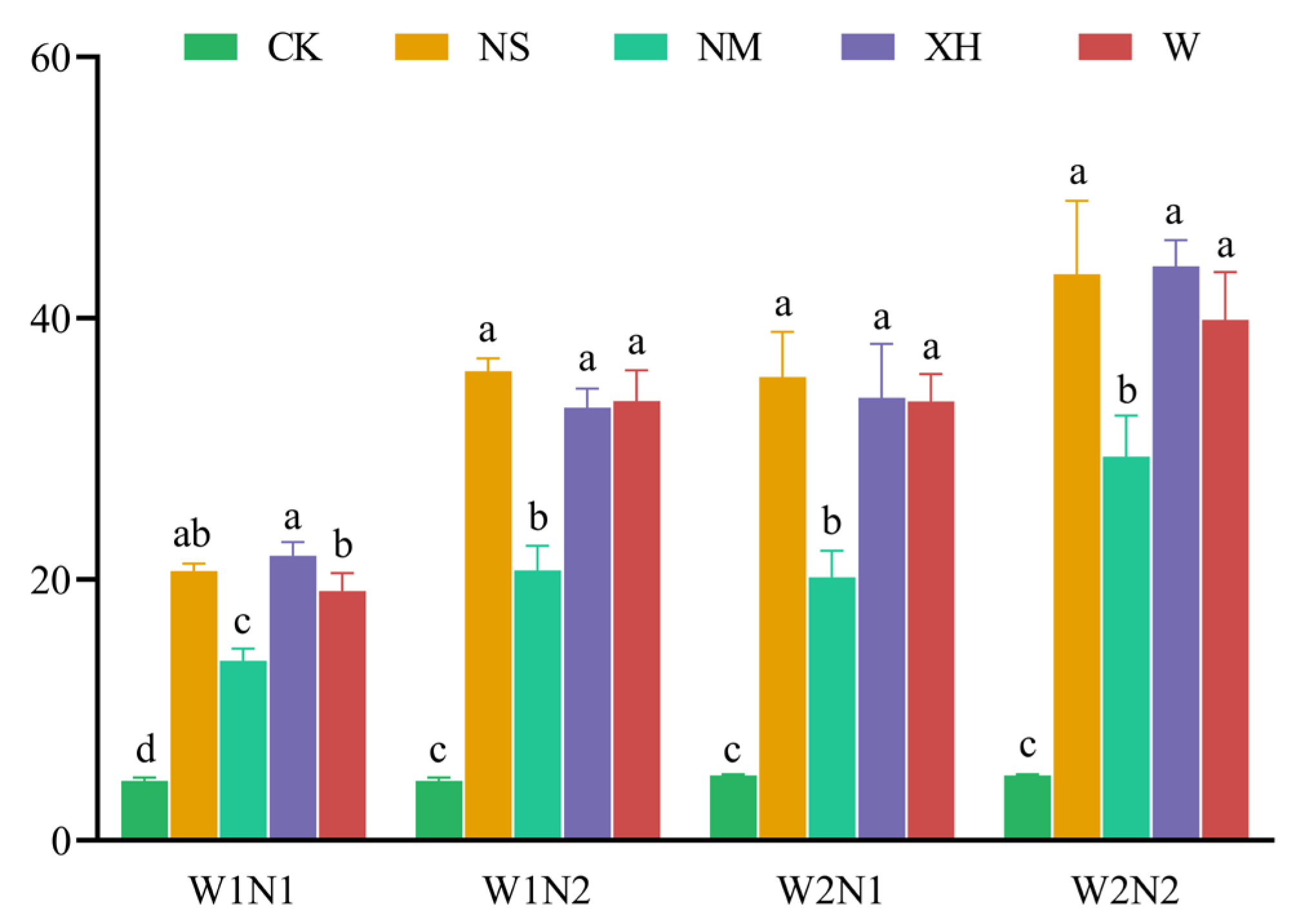
3.2.2. Response of Cumulative AV
3.3. Effects of Water–N Coupling Combined with EENFs on Soil Microbial Community Structure
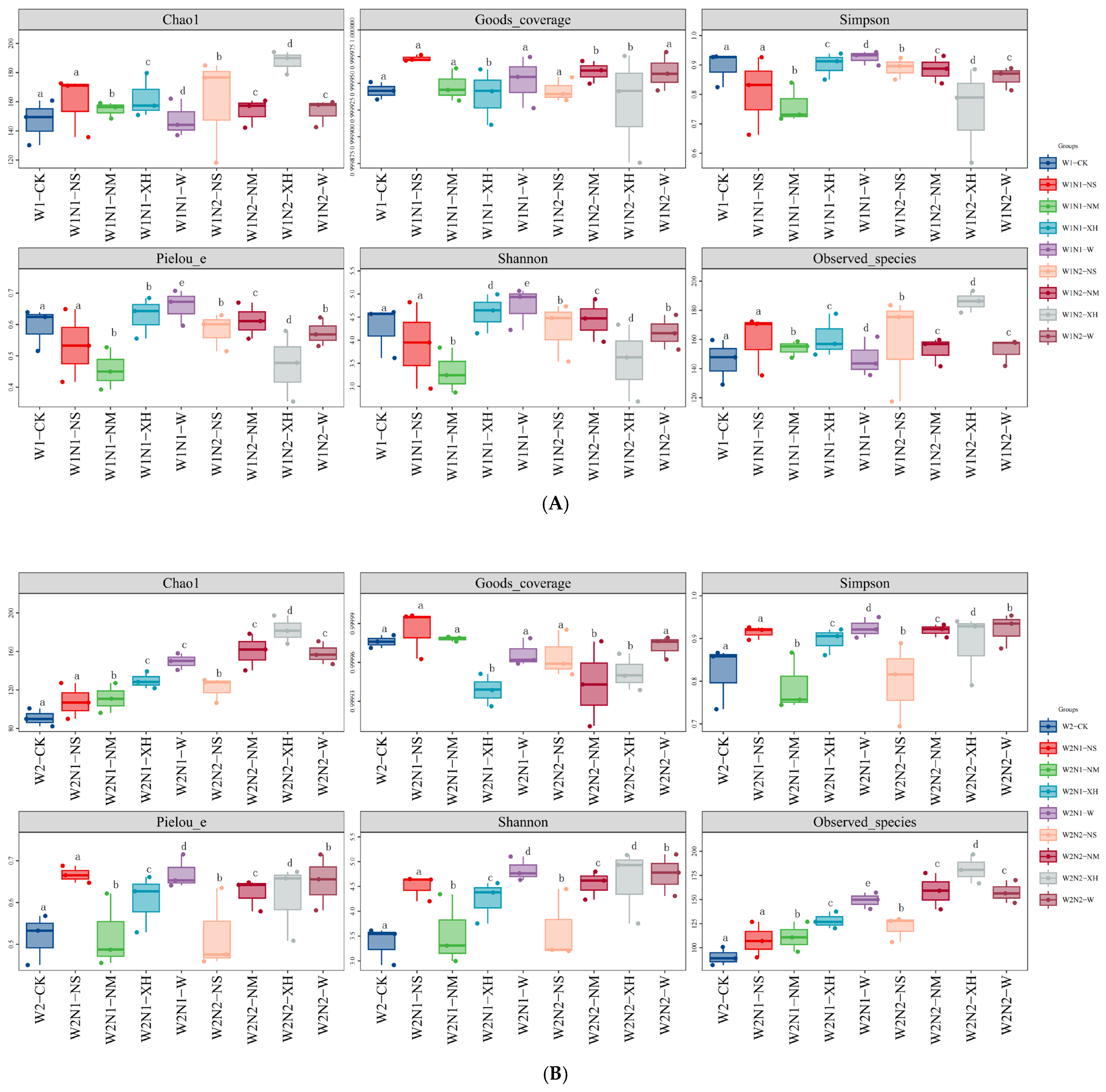
3.3.1. Effects of Water–N Coupling Combined with EENFS on Microbial α-Diversity
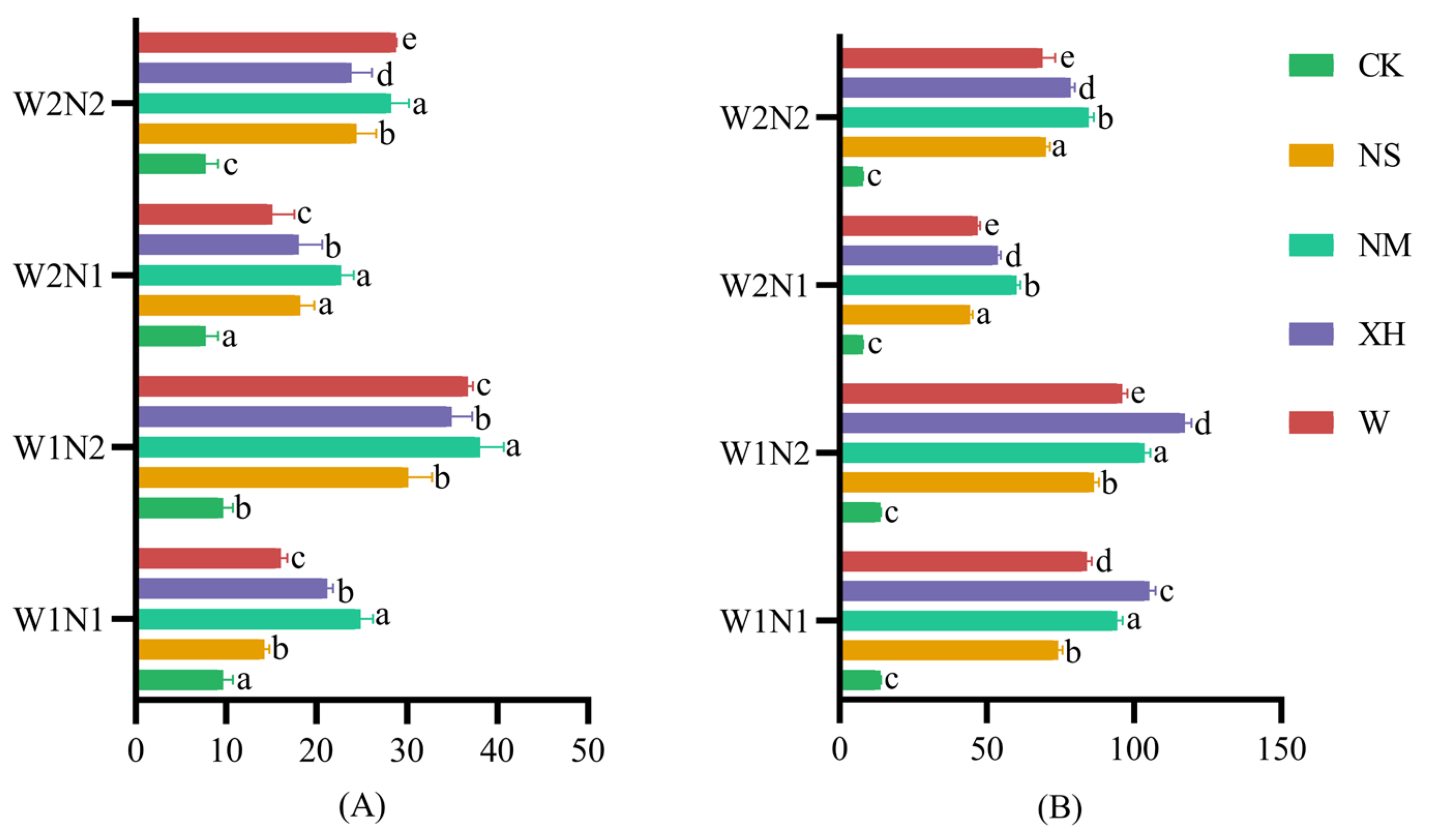
3.3.2. Effects of Water–N Coupling Combined with EENFs on Abundance of Soil Bacterial and Fungal Microbial Communities
3.3.3. Effects of Water–N Coupling Combined with EENFs on Microbial β-Diversity
3.3.4. Effects of Water–N Coupling Combined with EENFs on the Community Structure at the Phylum Level of Soil Microorganisms
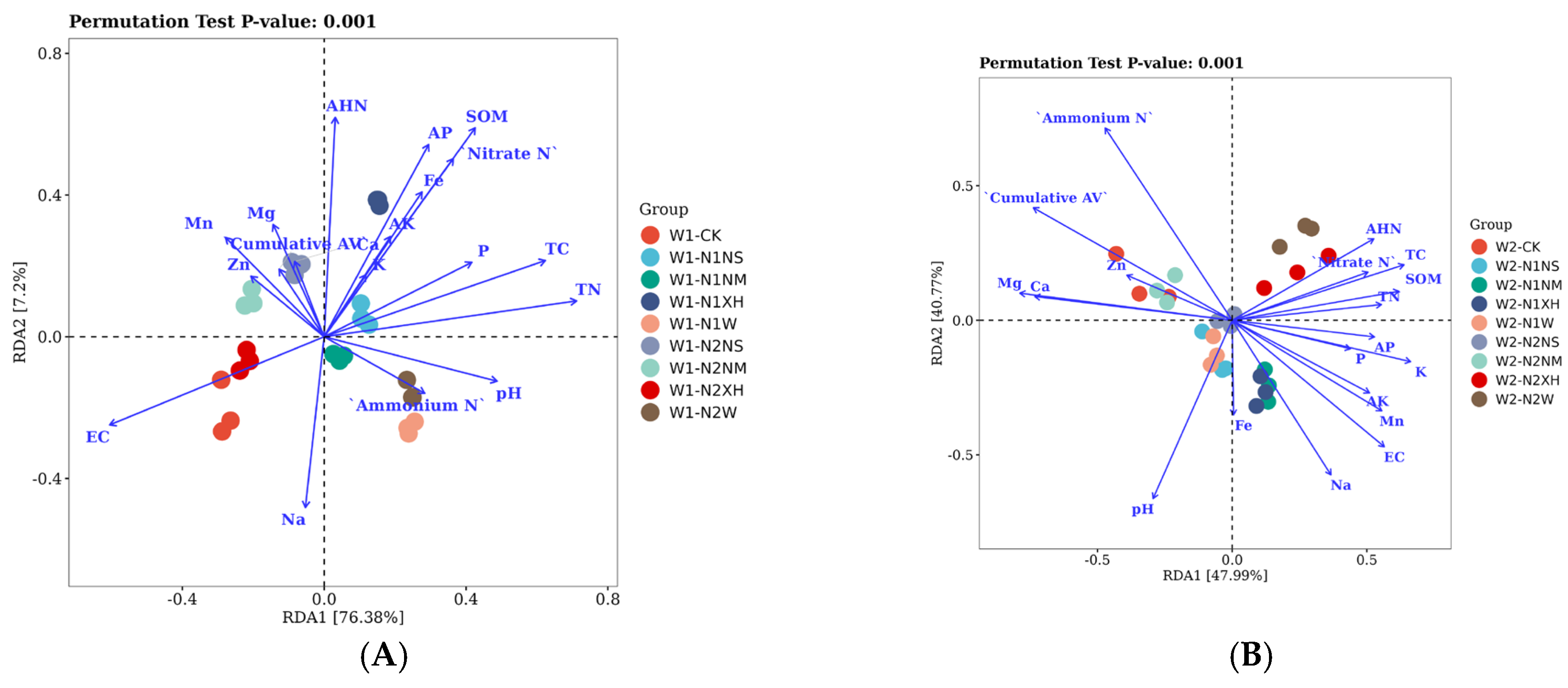
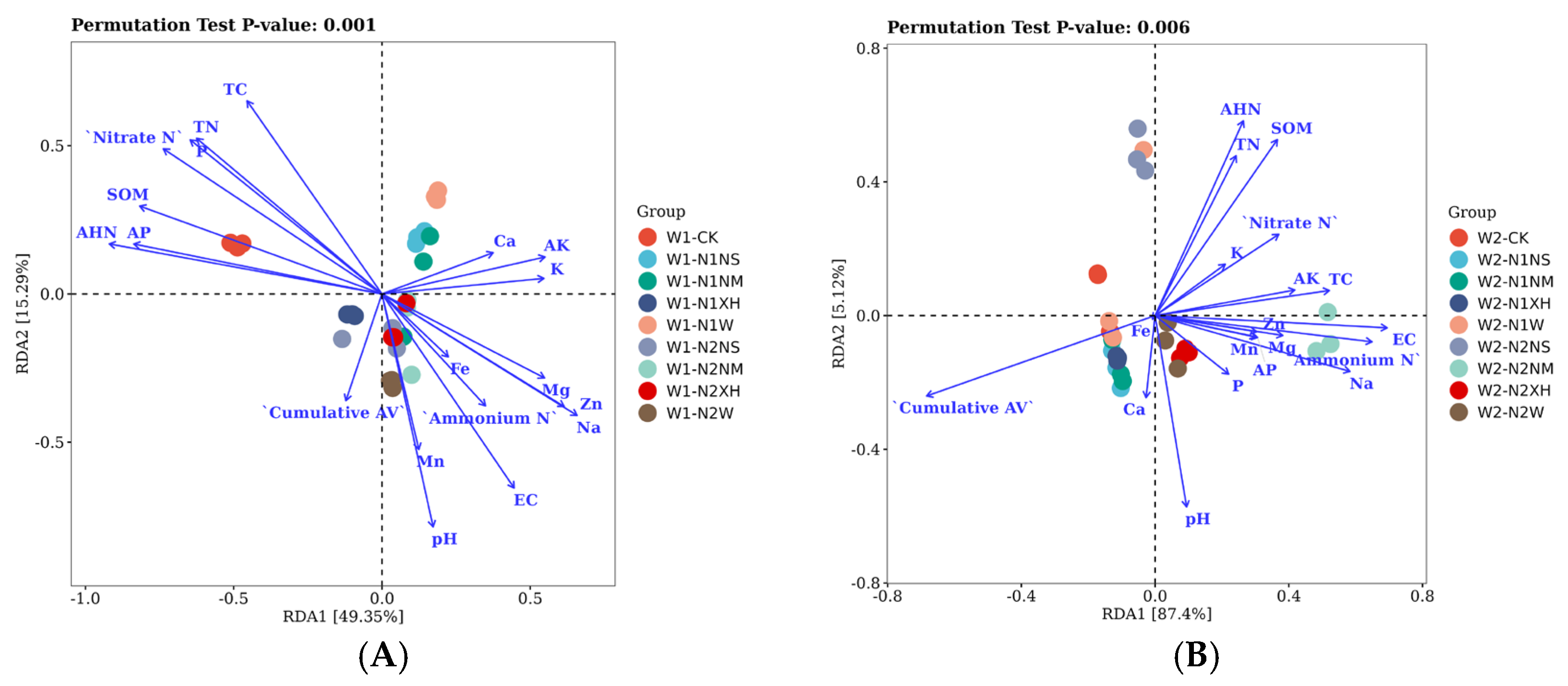
3.4. RDA-Based Dissection of Environmental Drivers Shaping Soil Microbial Communities Under Differential Water–N Management Strategies
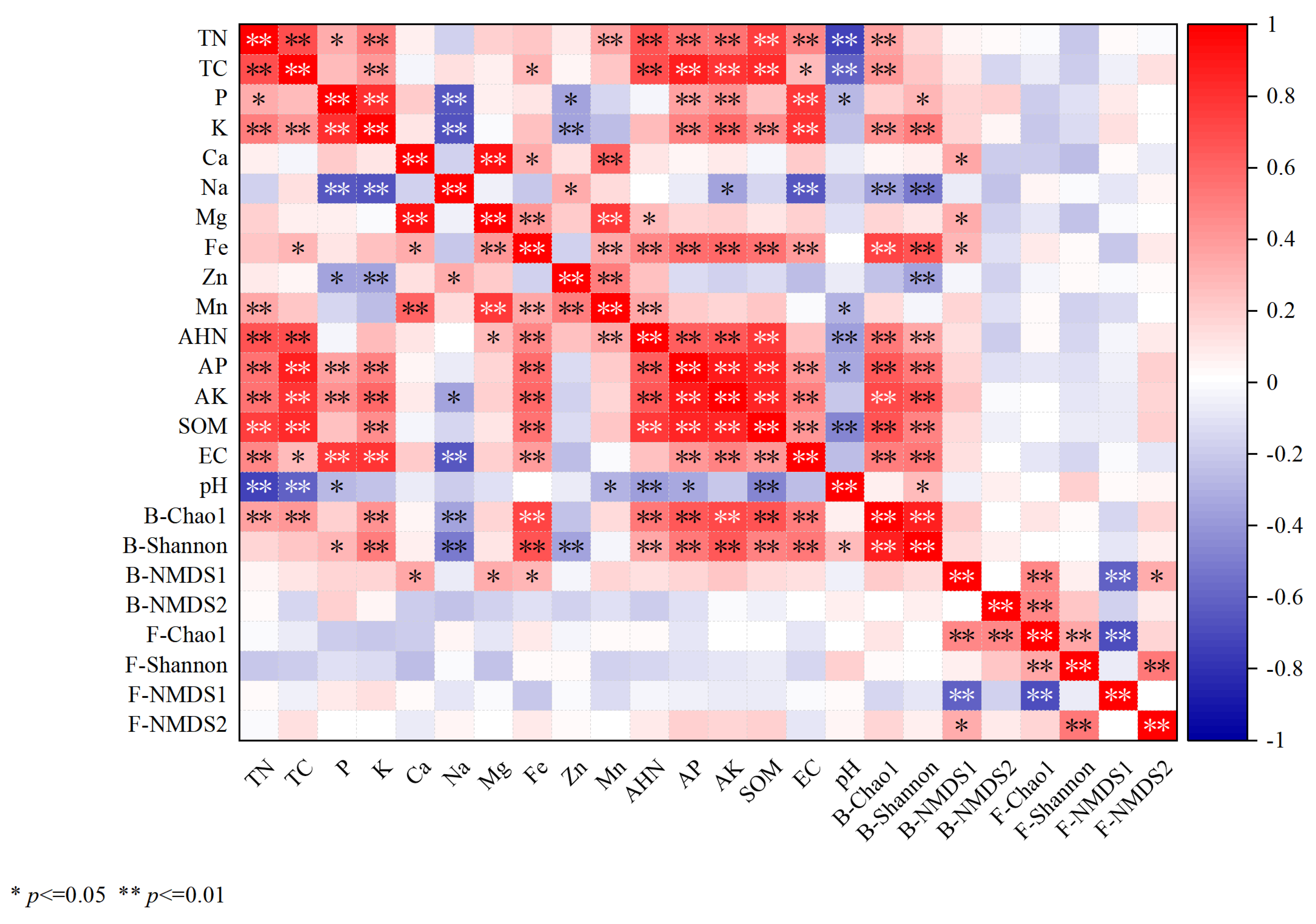
3.5. Correlation Analyses Between Soil Physicochemical Properties and the Community Structures of Bacterial and Fungal Assemblages
4. Discussion
4.1. The Interactive Effects of Moisture and N on Soil Physicochemical Properties
4.2. The Interactive Effects of Moisture and N on Microbial Community Structure
4.3. Key Roles of Environmental Factors in Water–N–Microorganism Interactions Within Arid-Zone Z. jujuba Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, A.K.; Gupta, K.J.; Singla-Pareek, S.L.; Foyer, C.H.; Pareek, A. Raising crops for dry and saline lands: Challenges and the way forward. Physiol. Plant. 2022, 174, e13730. [Google Scholar] [CrossRef]
- Sparks, D.L. A golden period for environmental soil chemistry. Geochem. Trans. 2020, 21, 5. [Google Scholar] [CrossRef]
- Lyu, R.; Wang, R.; Wu, C.; Bao, Y.; Guo, P. Comparative transcriptome analysis of leaves of sour jujube seedlings under salt stress. Acta Physiol. Plant. 2022, 44, 119. [Google Scholar] [CrossRef]
- Feng, J.; Li, F.; Deng, A.; Feng, X.; Fang, F.; Zhang, W. Integrated assessment of the impact of enhanced-efficiency nitrogen fertilizer on N2O emission and crop yield. Agric. Ecosyst. Environ. 2016, 231, 218–228. [Google Scholar] [CrossRef]
- Luhe, Z.; Tong, Z.; Huaili, H.; Bo, W.; De, Z.; Fang, W.; Duofeng, W.; Yi, L. The physiological responses of ‘Zhanhuang Jujube’ and ‘Winter Jujube’ to drought stress. Agric. Res. Arid Reg. 2023, 41, 104–113. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, C. Discussion on the Drought Resistance of Jujube Trees and Water-Saving Cultivation Techniques. Res. Econ. For. 2000, 1, 53–54. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, S.; Li, Z.; Yuan, H.; Jin, J.; Xiao, C.; Cui, Y. Quantification Assessment of Winter Wheat Sensitivity under Different Drought Scenarios during Growth. Water 2024, 16, 2048. [Google Scholar] [CrossRef]
- Liu, M.; Yang, C.; Mu, R. Effect of soil water–phosphorus coupling on the photosynthetic capacity of Robinia pseudoacacia L. seedlings in semi-arid areas of the Loess Plateau, China. Environ. Monit. Assess. 2023, 195, 932. [Google Scholar] [CrossRef]
- Ahmed Mohammed, M.E.; Refdan Alhajhoj, M.; Ali-Dinar, H.M.; Munir, M. Impact of a Novel Water-Saving Subsurface Irrigation System on Water Productivity, Photosynthetic Characteristics, Yield, and Fruit Quality of Date Palm under Arid Conditions. Agronomy 2020, 10, 1265. [Google Scholar] [CrossRef]
- Dehghanisanij, H.; Salamati, N.; Emami, S.; Emami, H.; Fujimaki, H. An intelligent approach to improve date palm crop yield and water productivity under different irrigation and climate scenarios. Appl. Water Sci. 2023, 13, 56. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, L.; Han, Y.; Cao, B.; Song, L. Effects of elevated temperature and drought stress on fruit coloration in the jujube variety ‘Lingwuchangzao’ (Ziziphus jujube cv. Lingwuchangzao). Sci. Hortic. 2020, 274, 109667. [Google Scholar] [CrossRef]
- Wang, R.; Xia, J.; Yang, J.; Liu, J.; Zhao, Y.; Sun, J. The water response characteristics of photosynthetic physiological parameters of acerola leaves in the shell sand habitat. Acta Ecol. Sin. 2013, 33, 6088–6096. (In Chinese) [Google Scholar] [CrossRef]
- Maraghni, M.; Gorai, M.; Steppe, K.; Neffati, M.; Van Labeke, M.C. Coordinated changes in photosynthetic machinery performance and water relations of the xerophytic shrub Ziziphus lASVs (L.) Lam. (Rhamnaceae) following soil drying. Photosynthetica 2019, 57, 113–120. [Google Scholar] [CrossRef]
- Xia, J.B.; Zhang, G.C.; Wang, R.R.; Zhang, S.Y. Effect of soil water availability on photosynthesis in Ziziphus jujuba var. spinosus in a sand habitat formed from seashells: Comparison of four models. Photosynthetica 2014, 52, 253–261. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Fugice, J.; Singh, U.; Lewis, T.D. Development of fertilizers for enhanced nitrogen use efficiency—Trends and perspectives. Sci. Total Environ. 2020, 731, 139113. [Google Scholar] [CrossRef]
- Cantarella, H.; Trivelin, P.C.O.; Contin, T.L.M.; Dias, F.L.F.; Rossetto, R.; Marcelino, R.; Coimbra, R.B.; Quaggio, J.A. Ammonia volatilisation from urease inhibitor-treated urea applied to sugarcane trash blankets. Sci. Agric. 2008, 65, 397–401. [Google Scholar] [CrossRef]
- Soares, J.R.; Cantarella, H.; Menegale, M.L.d.C. Ammonia volatilization losses from surface-applied urea with urease and nitrification inhibitors. Soil Biol. Biochem. 2012, 52, 82–89. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Zheng, X. Effects of nitrification inhibitors (DCD and DMPP) on nitrous oxide emission, crop yield and nitrogen uptake in a wheat–maize cropping system. Biogeosciences 2013, 10, 2427–2437. [Google Scholar] [CrossRef]
- Menéndez, S.; Barrena, I.; Setien, I.; González-Murua, C.; Estavillo, J.M. Efficiency of nitrification inhibitor DMPP to reduce nitrous oxide emissions under different temperature and moisture conditions. Soil Biol. Biochem. 2012, 53, 82–89. [Google Scholar] [CrossRef]
- Fan, X.; Yin, C.; Chen, H.; Ye, M.; Zhao, Y.; Li, T.; Wakelin, S.A.; Liang, Y. The efficacy of 3,4-dimethylpyrazole phosphate on N2O emissions is linked to niche differentiation of ammonia oxidizing archaea and bacteria across four arable soils. Soil Biol. Biochem. 2018, 130, 82–93. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Factories 2014, 13, 66. [Google Scholar] [CrossRef]
- Singh, J.S.; Pandey, V.C.; Singh, D.P. Efficient soil microorganisms: A new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 2011, 140, 339–353. [Google Scholar] [CrossRef]
- Burgess, C.J.; Myrold, D.D.; Mueller, R.S.; Wanzek, T.; Moore, J.M.; Kasschau, K.D.; Kleber, M. Drainage gradient versus seasonal cycles: Differential response of microbial community composition to variations in soil moisture. Soil Sci. Soc. Am. J. 2024, 88, 2123–2134. [Google Scholar] [CrossRef]
- Lei, L.; Marc, E.; Per, B.; Jian, L.; Dolores, A.; Häkan, W.; Josep, P. Drought legacies on soil respiration and microbial community in a Mediterranean forest soil under different soil moisture and carbon inputs. Geoderma 2021, 405, 115425. [Google Scholar] [CrossRef]
- Chen, M.M.; Zhu, Y.G.; Su, Y.H.; Chen, B.D.; Fu, B.J.; Marschner, P. Effects of soil moisture and plant interactions on the soil microbial community structure. Eur. J. Soil Biol. 2007, 43, 31–38. [Google Scholar] [CrossRef]
- Li, J.; Meng, B.; Yang, X.; Cui, N.; Zhao, T.; Chai, H.; Zhang, T.; Sun, W. Suppression of AMF accelerates N2O emission by altering soil bacterial community and genes abundance under varied precipitation conditions in a semiarid grassland. Front. Microbiol. 2022, 13, 961969. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Hu, H.-W.; Zhu-Barker, X.; Hayden, H.; Wang, J.; Suter, H.; Chen, D.; He, J.-Z. Nitrifier-induced denitrification is an important source of soil nitrous oxide and can be inhibited by a nitrification inhibitor 3,4-dimethylpyrazole phosphate. Environ. Microbiol. 2017, 19, 4851–4865. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Guerra, C.A.; Cano-Díaz, C.; Egidi, E.; Wang, J.-T.; Eisenhauer, N.; Singh, B.K.; Maestre, F.T. The proportion of soil-borne pathogens increases with warming at the global scale. Nat. Clim. Change 2020, 10, 550–554. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Pablo, C.H.D.; Mavrodi, O.V.; Weller, D.M.; Thomashow, L.S.; Mavrodi, D.V. Rhizosphere plant-microbe interactions under water stress. Adv. Appl. Microbiol. 2021, 115, 65–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-L.; Ding, J.; Zhu, D.; Hu, H.-W.; Delgado-Baquerizo, M.; Ma, Y.-B.; He, J.-Z.; Zhu, Y.-G. Rare microbial taxa as the major drivers of ecosystem multifunctionality in long-term fertilized soils. Soil Biol. Biochem. 2019, 141, 107686. [Google Scholar] [CrossRef]
- Liu, B.; Mørkved, P.T.; Frostegård, A.; Bakken, L.R. Denitrification gene pools, transcription and kinetics of NO, N2O and N2 production as affected by soil pH. FEMS Microbiol. Ecol. 2010, 72, 407–417. [Google Scholar] [CrossRef]
- Hu, H.-W.; Chen, D.; He, J.-Z. Microbial regulation of terrestrial nitrous oxide formation: Understanding the biological pathways for prediction of emission rates. FEMS Microbiol. Rev. 2015, 39, 729–749. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2004, 166, 525–530. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, L.; Xu, P.; Liu, D.; Zhang, L.; Hao, Y.; Wang, K.; Fan, H. Nitrogen use efficiency of drip irrigated sugar beet as affected by sub-optimal levels of nitrogen and irrigation. Agric. Water Manag. 2024, 298, 108849. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Xia, G.; Wu, Q.; Chi, D. Continuous regulated deficit irrigation enhances peanut water use efficiency and drought resistance. Agric. Water Manag. 2021, 255, 106997. [Google Scholar] [CrossRef]
- Chen, Q.; Qu, Z.; Ma, G.; Wang, W.; Dai, J.; Zhang, M.; Wei, Z.; Liu, Z. Humic acid modulates growth, photosynthesis, hormone and osmolytes system of maize under drought conditions. Agric. Water Manag. 2022, 263, 107447. [Google Scholar] [CrossRef]
- GB/T 2440–2017; Urea. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China; Standardization Administration of China: Beijing, China, 2017.
- Yang, W.; Diao, L.; Wang, Y.; Yang, X.; Zhang, H.; Wang, J.; Luo, Y.; An, S.; Cheng, X. Responses of soil fungal communities and functional guilds to ~160 years of natural revegetation in the Loess Plateau of China. Front. Microbiol. 2022, 13, 967565. [Google Scholar] [CrossRef]
- Brewer, K.M.; Gaudin, A.C.M. Potential of crop-livestock integration to enhance carbon sequestration and agroecosystem functioning in semi-arid croplands. Soil Biol. Biochem. 2020, 149, 107936. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar] [CrossRef]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Ågren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, R.P.; Santangeli, M.; Würsig, H.; Martín Roldán, M.; Yim, B.; Lippold, E.; Tasca, A.; Oburger, E.; Tarkka, M.; Vetterlein, D.; et al. Drought response of the maize plant-soil-microbiome system is influenced by plant size and presence of root hairs. Ann. Bot. 2025, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Sha, Z.; Zhang, K.; Liu, X. Are dual inhibitors superior to urease or nitrification inhibitors for mitigating environmental risk and enhancing agronomic efficiency? Agric. Ecosyst. Environ. 2025, 392, 109752. [Google Scholar] [CrossRef]
- Lv, J.; Gui, D.; Zhang, Y.; Li, R.; Chen, X.; Sha, Z. Field application of microbial inoculants improved crop foliar morphology and physiology performance: A global meta-analysis. Sci. Hortic. 2023, 326, 112769. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Yuan, Z.; Feng, J.; Chen, S.; Sun, G.; Wei, Z.; Hu, T. Effects of microbial fertilizer and irrigation amount on growth, physiology and water use efficiency of tomato in greenhouse. Sci. Hortic. 2023, 323, 112553. [Google Scholar] [CrossRef]
- Li, L.; Zhao, C.; Wang, X.; Tan, Y.; Wang, X.; Liu, X.; Guo, B. Effects of nitrification and urease inhibitors on ammonia-oxidizing microorganisms, denitrifying bacteria, and greenhouse gas emissions in greenhouse vegetable fields. Environ. Res. 2023, 237, 116781. [Google Scholar] [CrossRef]
- Pan, J.; Liu, Y.; Zhong, X.; Lampayan, R.M.; Singleton, G.R.; Huang, N.; Liang, K.; Peng, B.; Tian, K. Grain yield, water productivity and nitrogen use efficiency of rice under different water management and fertilizer-N inputs in South China. Agric. Water Manag. 2017, 184, 191–200. [Google Scholar] [CrossRef]
- Atwill, R.L.; Krutz, L.J.; Bond, J.A.; Reddy, K.R.; Gore, J.; Walker, T.W.; Harrell, D.L. Water management strategies and their effects on rice grain yield and nitrogen use efficiency. J. Soil Water Conserv. 2018, 73, 257–264. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an Ecological Classification of Soil Bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, D. Nitrogen and phosphorus losses by surface runoff and soil microbial communities in a paddy field with different irrigation and fertilization managements. PLoS ONE 2021, 16, e0254227. [Google Scholar] [CrossRef] [PubMed]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; van Veen, J.A.; Kuramae, E.E. The Ecology of Acidobacteria: Moving beyond Genes and Genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef]
- Fierer, N.; Leff, J.W.; Adams, B.J.; Nielsen, U.N.; Bates, S.T.; Lauber, C.L.; Owens, S.; Gilbert, J.A.; Wall, D.H.; Caporaso, J.G. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. USA 2012, 109, 21390–21395. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Shen, L.; Wen, C.; Yan, Q.; Ning, D.; Qin, Y.; Xue, K.; Wu, L.; He, Z.; et al. Temperature mediates continental-scale diversity of microbes in forest soils. Nat. Commun. 2016, 7, 12083. [Google Scholar] [CrossRef]
- Tan, W.; Wang, J.; Bai, W.; Qi, J.; Chen, W. Soil bacterial diversity correlates with precipitation and soil pH in long-term maize cropping systems. Sci. Rep. 2020, 10, 6012. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Gerard, E.M.; Carter, P.E.; Lardner, R.; Sarathchandra, U.; Burch, G.; Ghani, A.; Bell, N. Effect of the nitrification inhibitor dicyandiamide (DCD) on microbial communities in a pasture soil amended with bovine urine. Soil Biol. Biochem. 2010, 42, 1425–1436. [Google Scholar] [CrossRef]
- Chang, D.; Lu, X.; Sun, Y.; Fan, H.; Wang, K. Responses of rhizosphere microbial communities and resource competition to soil amendment in saline and alkaline soils. Plant Soil 2025. [Google Scholar] [CrossRef]
- Weng, X.; Li, J.; Sui, X.; Li, M.; Yin, W.; Ma, W.; Yang, L.; Mu, L. Soil microbial functional diversity responses to different vegetation types in the Heilongjiang Zhongyangzhan Black-billed Capercaillie Nature Reserve. Ann. Microbiol. 2021, 71, 26. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, R.; Li, X.; Hu, G.; Dong, Y.; Liu, Z. Long-term adoption of plow tillage and green manure improves soil physicochemical properties and optimizes microbial communities under a continuous peanut monoculture system. Front. Microbiol. 2025, 15, 1513528. [Google Scholar] [CrossRef] [PubMed]
- Maron, P.-A.; Sarr, A.; Kaisermann, A.; Lévêque, J.; Mathieu, O.; Guigue, J.; Karimi, B.; Bernard, L.; Dequiedt, S.; Terrat, S.; et al. High Microbial Diversity Promotes Soil Ecosystem Functioning. Appl. Environ. Microbiol. 2018, 84, e02738-17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Peng, Y.; Lin, J.; Wang, L.; Jia, Z.; Zhang, R. Optimal nitrogen management to achieve high wheat grain yield, grain protein content, and water productivity: A meta-analysis. Agric. Water Manag. 2023, 290, 108587. [Google Scholar] [CrossRef]
- Liu, L.; Chen, T.; Wang, Z.; Zhang, H.; Yang, J.; Zhang, J. Combination of site-specific nitrogen management and alternate wetting and drying irrigation increases grain yield and nitrogen and water use efficiency in super rice. Field Crops Res. 2013, 154, 226–235. [Google Scholar] [CrossRef]

| Treatment | Field Capacity (FC) | Fertilizer Application Rates for Each Treatment (kg N ha−1) | |||
|---|---|---|---|---|---|
| Urease Inhibitor-Type N Fertilizer | Nitrification Inhibitor-Type N Fertilizer | Microbial Inoculant | Urea Fertilizer | ||
| CK | 75% | 0 | 0 | 0 | 0 |
| W1N1-NS | 75% | 0 | 0 | 0 | 100 |
| W1N1-NM | 75% | 100 | 0 | 0 | 0 |
| W1N1-XH | 75% | 0 | 100 | 0 | 0 |
| W1N1-W | 75% | 0 | 0 | 100 | 0 |
| W1N2-NS | 75% | 0 | 0 | 0 | 100 |
| W1N2-NM | 75% | 100 | 0 | 0 | 0 |
| W1N2-XH | 75% | 0 | 100 | 0 | 0 |
| W1N2-W | 75% | 0 | 0 | 100 | 0 |
| CK | 45% | 0 | 0 | 0 | 0 |
| W2N1-NS | 45% | 0 | 0 | 0 | 300 |
| W2N1-NM | 45% | 300 | 0 | 0 | 0 |
| W2N1-XH | 45% | 0 | 300 | 0 | 0 |
| W2N1-W | 45% | 0 | 0 | 300 | 0 |
| W2N2-NS | 45% | 0 | 0 | 0 | 300 |
| W2N2-NM | 45% | 300 | 0 | 0 | 0 |
| W2N2-XH | 45% | 0 | 300 | 0 | 0 |
| W2N2-W | 45% | 0 | 0 | 300 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Liu, H.; Sun, J.; Wu, C.; Zhang, Y. Interactive Effects of Different Field Capacity and Nitrogen Levels on Soil Fertility and Microbial Community Structure in the Root Zone of Jujube (Ziziphus jujuba Mill.) Seedlings in an Arid Region of Southern Xinjiang, China. Agronomy 2025, 15, 2191. https://doi.org/10.3390/agronomy15092191
Ma Y, Liu H, Sun J, Wu C, Zhang Y. Interactive Effects of Different Field Capacity and Nitrogen Levels on Soil Fertility and Microbial Community Structure in the Root Zone of Jujube (Ziziphus jujuba Mill.) Seedlings in an Arid Region of Southern Xinjiang, China. Agronomy. 2025; 15(9):2191. https://doi.org/10.3390/agronomy15092191
Chicago/Turabian StyleMa, Yunqi, Haoyang Liu, Junpan Sun, Cuiyun Wu, and Yuyang Zhang. 2025. "Interactive Effects of Different Field Capacity and Nitrogen Levels on Soil Fertility and Microbial Community Structure in the Root Zone of Jujube (Ziziphus jujuba Mill.) Seedlings in an Arid Region of Southern Xinjiang, China" Agronomy 15, no. 9: 2191. https://doi.org/10.3390/agronomy15092191
APA StyleMa, Y., Liu, H., Sun, J., Wu, C., & Zhang, Y. (2025). Interactive Effects of Different Field Capacity and Nitrogen Levels on Soil Fertility and Microbial Community Structure in the Root Zone of Jujube (Ziziphus jujuba Mill.) Seedlings in an Arid Region of Southern Xinjiang, China. Agronomy, 15(9), 2191. https://doi.org/10.3390/agronomy15092191






