Transcriptome-Based Analysis of Mitochondrial Influence on Key Agronomic Traits and Nutritional Components in Auricularia heimuer
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Strain
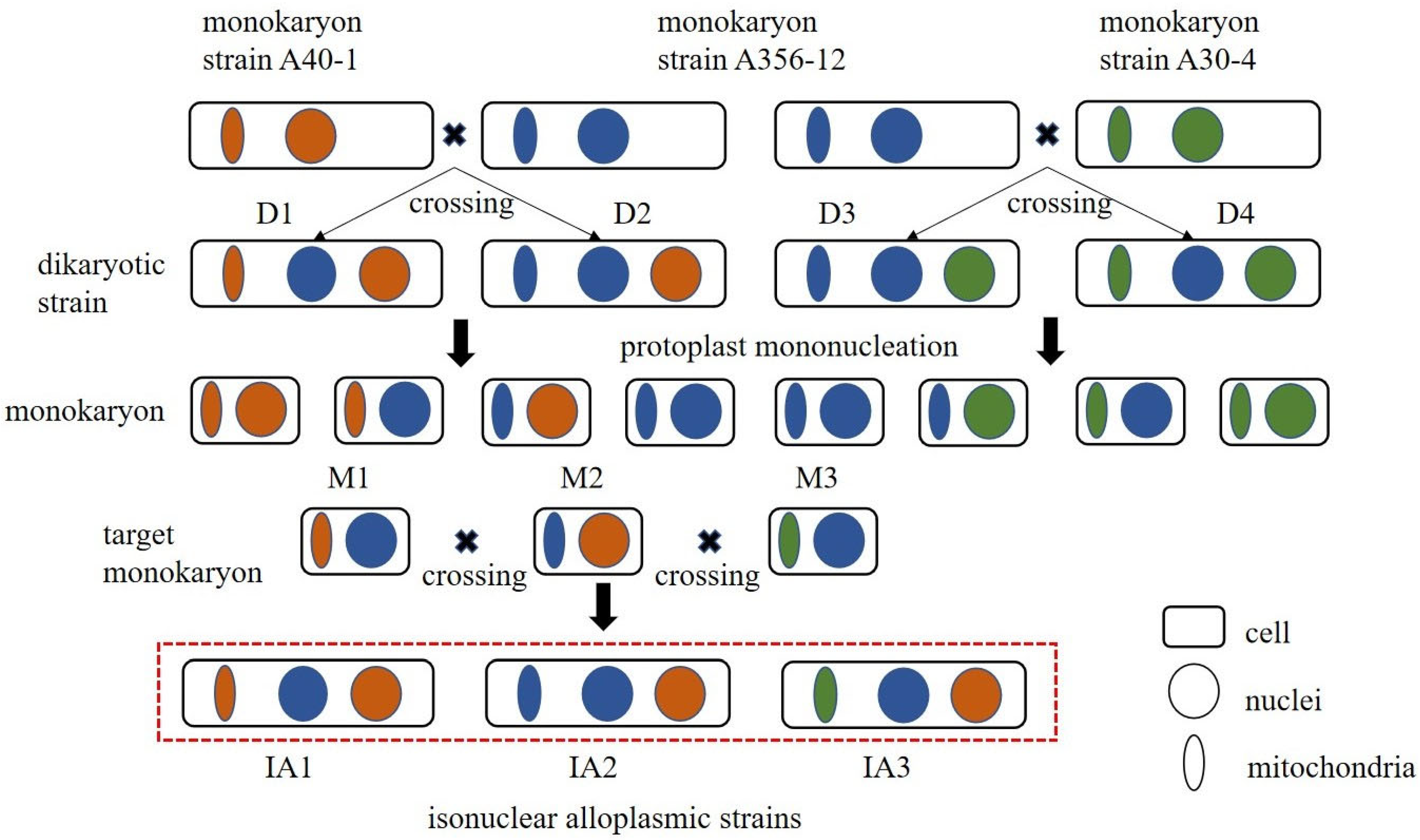
2.2. Construction of Isonuclear Alloplasmic Strains
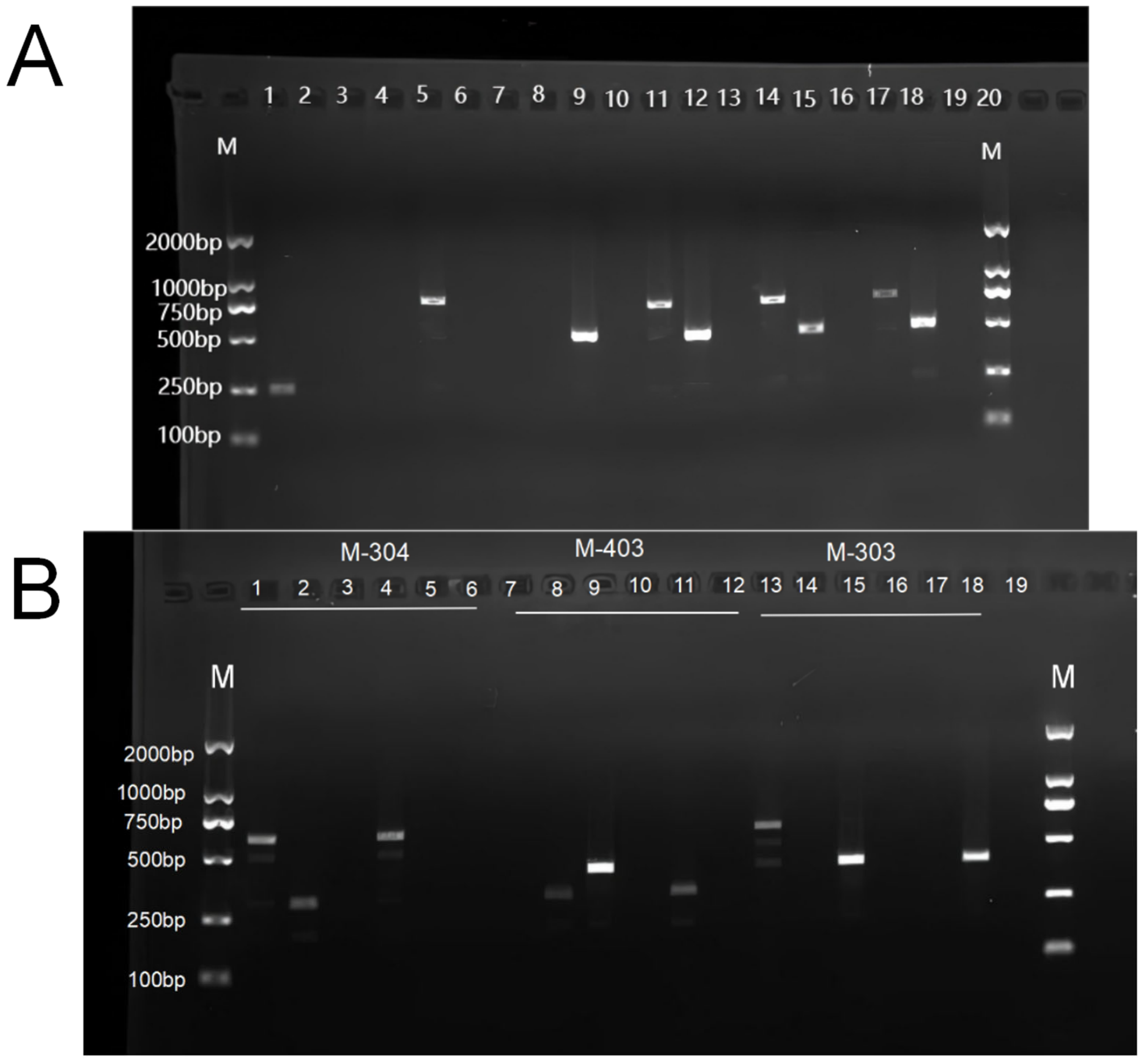
2.3. Verification of Isonuclear Alloplasmic Strains
2.4. Measurement of Phenotypic Traits
2.5. Determination of Bioactive Components
2.6. RNA Extraction and Transcriptome Sequencing Analysis
3. Results
3.1. Verification of Nuclear and Cytoplasmic Backgrounds of Isonuclear Alloplasmic Strains
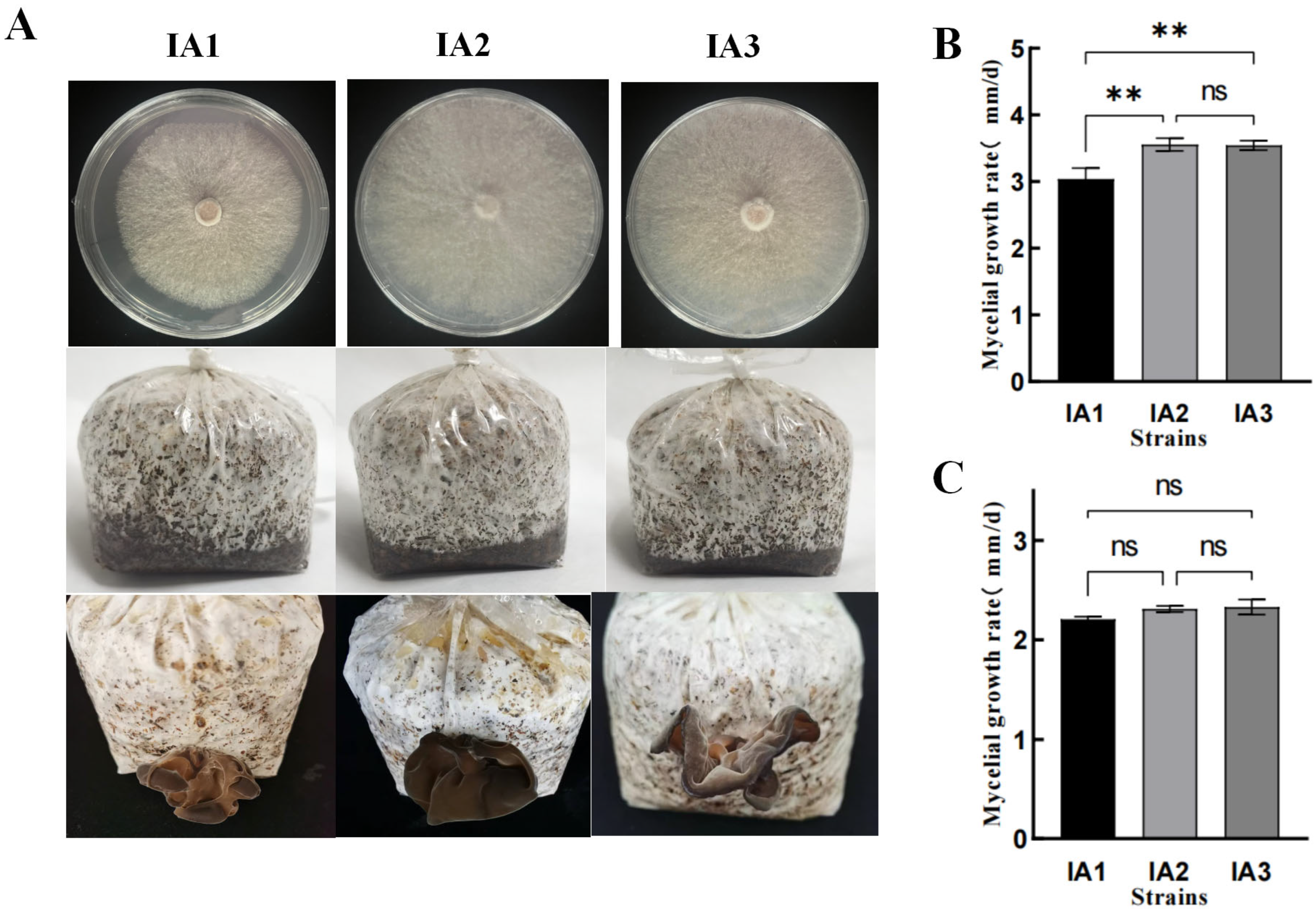
3.2. Morphological Observation of Mycelia and Fruiting Bodies of Isonuclear Alloplasmic Strains
3.3. Comparative Analysis of Bioactive Component Contents in Isonuclear Alloplasmic Strains
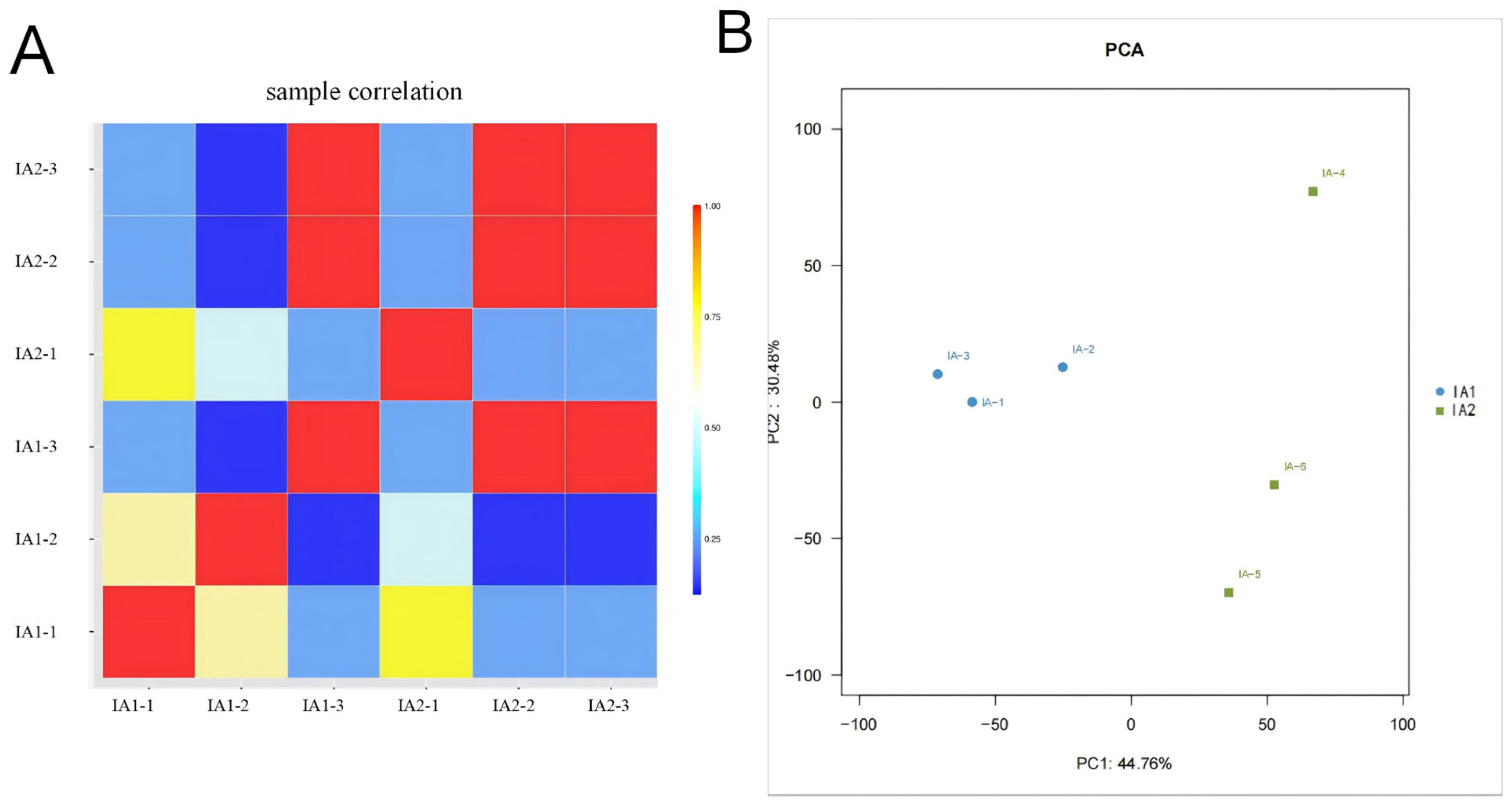
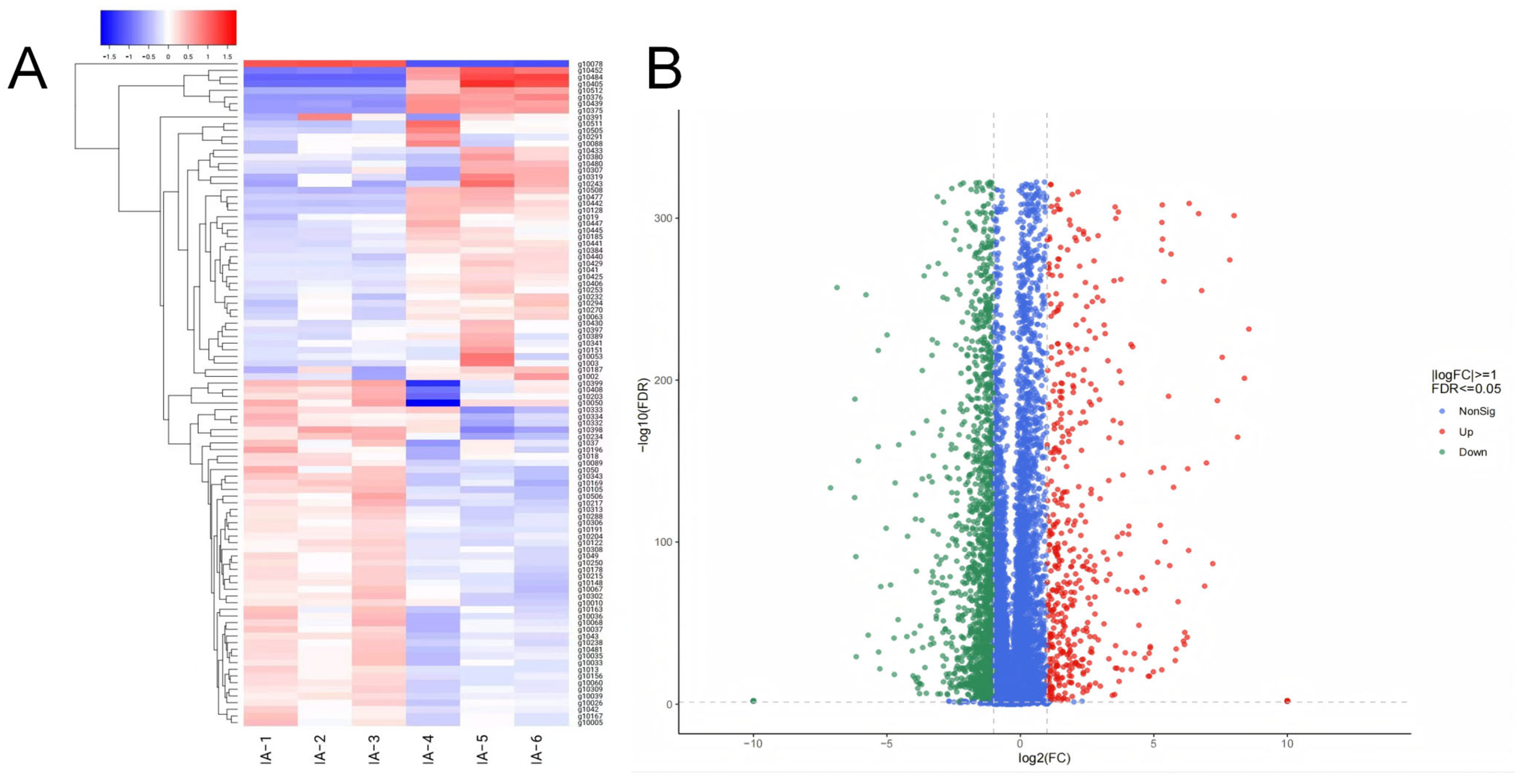
3.4. Transcriptome Analysis of Isonuclear Alloplasmic Strains
3.5. Differential Expression Analysis Between Isonuclear Alloplasmic Strains
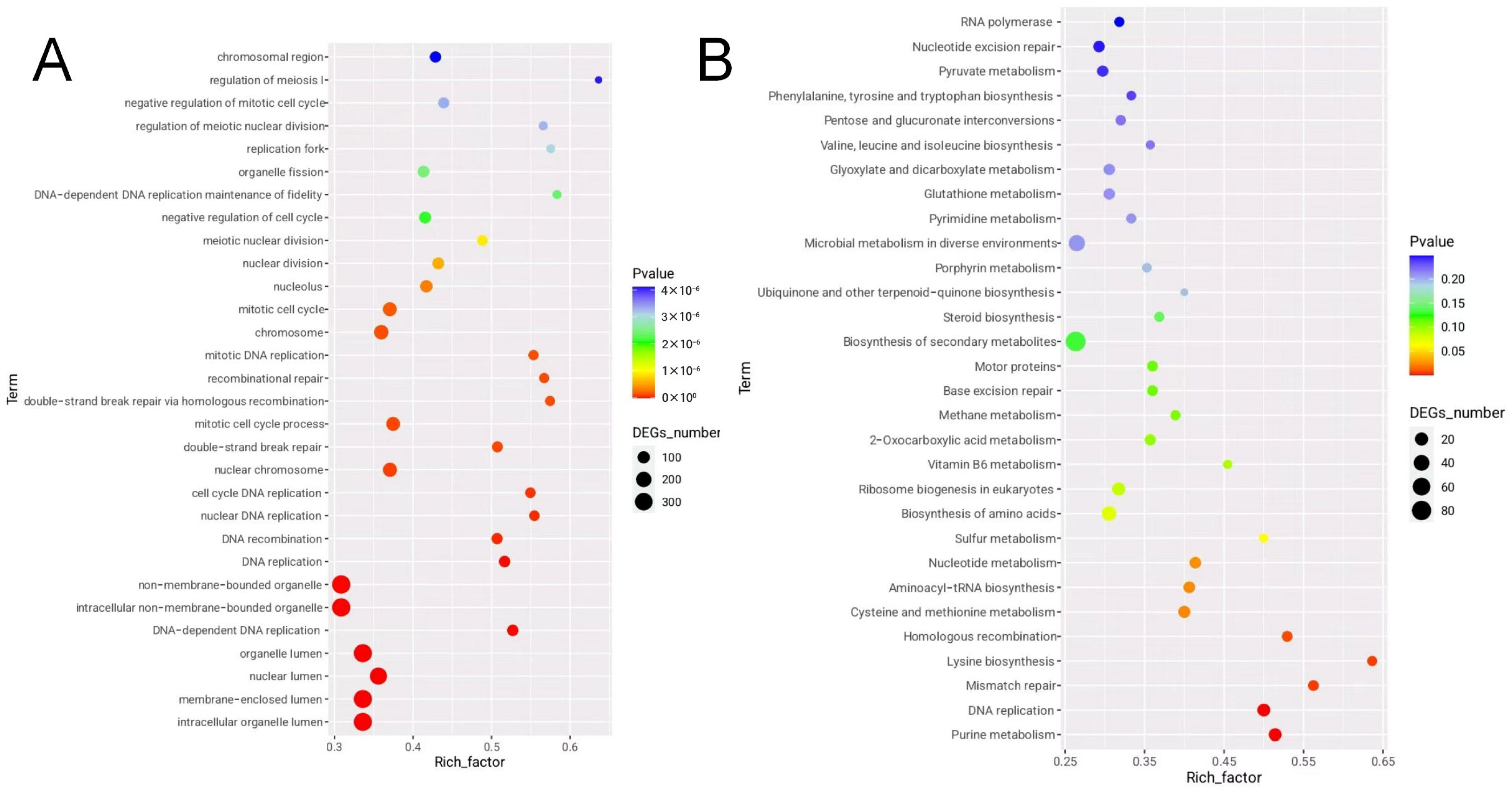
3.6. Enrichment Analysis of DEGs Between Isonuclear Alloplasmic Strains
3.7. Lysine Biosynthesis Pathway Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Primer Name | Differential Parenting | Primer Sequence | Production Size (bp) |
|---|---|---|---|
| Primer1 | A30 |
F: GTATCCTACTTTGTTAGGAC R: GATATTGTCCAACTCTACTC | 254 |
| Primer2 | A40 |
F: GTATCCTACTTTGTTAGGAC R: GATATTGTCCAACTCTACTC | 749 |
| Primer3 | A356 |
F: GTATCCTACTTTGTTAGGAC R: GATATTGTCCAACTCTACTC | 520 |
| PrimerM-304 | A356-12, A40-1 |
F: GTATCCTACTTTGTTAGGAC R: GATATTGTCCAACTCTACTC | 600 |
| PrimerM-403 | A30-4, A356-12 |
F: GTATCCTACTTTGTTAGGAC R: GATATTGTCCAACTCTACTC | 302 |
| PrimerM-303 | A30-4, A40-1 |
F: GTATCCTACTTTGTTAGGAC R: GATATTGTCCAACTCTACTC | 414 |
References
- Wu, F.; Tohtirjap, A.; Fan, L.-F.; Zhou, L.-W.; Alvarenga, R.L.M.; Gibertoni, T.B.; Dai, Y.-C. Global Diversity and Updated Phylogeny of Auricularia (Auriculariales, Basidiomycota). J. Fungi 2021, 7, 933. [Google Scholar] [CrossRef]
- Hermawan, N.; Romulo, A.; Wardana, A.A. Development and texture profile of wood-ear mushroom (Auricularia auricula) sausage formulated with carrageenan. IOP Conf. Ser. Earth Environ. Sci. 2020, 426, 012182. [Google Scholar] [CrossRef]
- Khan, A.A.; Lu, L.-X.; Yao, F.-J.; Fang, M.; Wang, P.; Zhang, Y.-M.; Meng, J.-J.; Ma, X.-X.; He, Q.; Shao, K.-S.; et al. Characterization, antioxidant activity, and mineral profiling of Auricularia cornea mushroom strains. Front. Nutr. 2023, 10, 1167805. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Yao, F.; Kang, W.; Lu, L.; Xu, B. A systematic study on mycochemical profiles, antioxidant, and anti-inflammatory activities of 30 varieties of Jew’s ear (Auricularia auricula-judae). Food Sci. Hum. Wellness 2022, 11, 781–794. [Google Scholar] [CrossRef]
- Pak, S.; Chen, F.; Ma, L.; Hu, X.; Ji, J. Functional perspective of black fungi (Auricularia auricula): Major bioactive components, health benefits and potential mechanisms. Trends Food Sci. Technol. 2021, 114, 245–261. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, X.; Chen, W.; Zhang, L.; Zhu, H. Production of natural edible melanin by Auricularia auricula and its physicochemical properties. Food Chem. 2016, 196, 486–492. [Google Scholar] [CrossRef]
- Yao, H.; Liu, Y.; Ma, Z.F.; Zhang, H.; Fu, T.; Li, Z.; Li, Y.; Hu, W.; Han, S.; Zhao, F. Analysis of nutritional quality of black fungus cultivated with corn stalks. J. Food Qual. 2019, 2019, 9590251. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Jiang, K. Antioxidant activity in vitro and in vivo of the polysaccharides from different varieties of Auricularia auricula. Food Funct. 2016, 7, 3868–3879. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Xu, B. Phenolic profiles, antioxidant capacities and metal chelating ability of edible mushrooms commonly consumed in China. LWT-Food Sci. Technol. 2016, 72, 423–431. [Google Scholar] [CrossRef]
- Lu, A.; Shen, M.; Fang, Z.; Xu, Y.; Yu, M.; Wang, S.; Zhang, Y.; Wang, W. Antidiabetic effects of the Auricularia auricular polysaccharides simulated hydrolysates in experimental type-2 diabetic rats. Nat. Prod. Commun. 2018, 13, 195–200. [Google Scholar] [CrossRef]
- Yoon, S.-J.; Yu, M.-A.; Pyun, Y.-R.; Hwang, J.-K.; Chu, D.-C.; Juneja, L.R.; Mourão, P.A.S. The nontoxic mushroom Auricularia auricula contains a polysaccharide with anticoagulant activity mediated by antithrombin. Thromb. Res. 2003, 112, 151–158. [Google Scholar] [CrossRef]
- Huang, D.; Liu, Z.; Zhang, Y. Review on anti-tumor mechanism of Auricularia auricular Polysaccharide. J. Plateau Agric. 2019, 3, 694–699. [Google Scholar]
- Hu, X.; Liu, C.; Wang, X.; Jia, D.; Lu, W.; Sun, X.; Liu, Y.; Yuan, L. Hpyerglycemic and anti-diabetic nephritis activities of polysaccharides separated from Auricularia auricular in diet-streptozotocin-induced diabetic rats. Exp. Ther. Med. 2017, 13, 352–358. [Google Scholar] [CrossRef]
- Zou, Y.; Xie, C.; Fan, G.; Gu, Z.; Han, Y. Optimization of ultrasound-assisted extraction of melanin from Auricularia auricula fruit bodies. Innov. Food Sci. Emerg. Technol. 2010, 11, 611–615. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, P.; Dai, X.; Yao, X.; Zhou, S.; Ma, Q.; Liu, J.; Tian, S.; Zhu, J.; Zhang, J.; et al. Extraction, physicochemical properties, and antioxidant activity of natural melanin from Auricularia heimuer fermentation. Front. Nutr. 2023, 10, 1131542. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Liu, X.; Fan, H.; Wei, C. Aqueous extracts of se-enriched Auricularia auricular attenuates D-galactose-induced cognitive deficits, oxidative stress and neuroinflammation via suppressing RAGE/MAPK/NF-κB pathway. Neurosci. Lett. 2019, 704, 106–111. [Google Scholar] [CrossRef]
- Joardar, V.; Abrams, N.F.; Hostetler, J.; Paukstelis, P.J.; Pakala, S.; Pakala, S.B.; Zafar, N.; Abolude, O.O.; Payne, G.; Andrianopoulos, A.; et al. Sequencing of mitochondrial genomes of nine Aspergillus and Penicillium species identifies mobile introns and accessory genes as main sources of genome size variability. BMC Genom. 2012, 13, 698. [Google Scholar] [CrossRef]
- Ballard, J.W.O.; Whitlock, M.C. The incomplete natural history of mitochondria. Mol. Ecol. 2004, 13, 729–744. [Google Scholar] [CrossRef] [PubMed]
- Kouvelis, V.N.; Hausner, G. Editorial: Mitochondrial Genomes and Mitochondrion Related Gene Insights to Fungal Evolution. Front. Microbiol. 2022, 13, 897981. [Google Scholar] [CrossRef]
- Chatre, L.; Ricchetti, M. Are mitochondria the Achilles’ heel of the Kingdom Fungi? Curr. Opin. Microbiol. 2014, 20, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Bernt, M.; Braband, A.; Schierwater, B.; Stadler, P.F. Genetic aspects of mitochondrial genome evolution. Mol. Phylogenetics Evol. 2013, 69, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Gallone, B.; Verstrepen, K.J. Interspecific hybridization as a driver of fungal evolution and adaptation. Nat. Rev. Microbiol. 2021, 19, 485–500. [Google Scholar] [CrossRef]
- Burger, G.; Gray, M.W.; Lang, B.F. Mitochondrial genomes: Anything goes. Trends Genet. 2003, 19, 709–716. [Google Scholar] [CrossRef]
- Soledad, R.B.; Charles, S.; Samarjit, D. The secret messages between mitochondria and nucleus in muscle cell biology. Arch. Biochem. Biophys. 2019, 666, 52–62. [Google Scholar] [CrossRef]
- Ng, S.; De Clercq, I.; Van Aken, O.; Law, S.R.; Ivanova, A.; Willems, P.; Giraud, E.; Van Breusegem, F.; Whelan, J. Anterograde and Retrograde Regulation of Nuclear Genes Encoding Mitochondrial Proteins during Growth, Development, and Stress. Mol. Plant 2014, 7, 1075–1093. [Google Scholar] [CrossRef]
- Ye, L.; He, X.; Su, C.; Feng, H.; Meng, G.; Chen, B.; Wu, X. The Effect of Mitochondria on Ganoderma lucidum Growth and Bioactive Components Based on Transcriptomics. J. Fungi 2022, 8, 1182. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, T.; Song, L.; Kong, J.; Niu, Q.; Wang, Y.; Wu, C.; Deng, B.; Wang, H.; Gai, Y. Comparative Transcriptome of Isonuclear Alloplasmic Strain Revealed the Important Role of Mitochondrial Genome in Regulating Flammulina filiformis. Agronomy 2023, 13, 998. [Google Scholar] [CrossRef]
- Ito, S.; Del Bino, S.; Hirobe, T.; Wakamatsu, K. Improved HPLC Conditions to Determine Eumelanin and Pheomelanin Contents in Biological Samples Using an Ion Pair Reagent. Int. J. Mol. Sci. 2020, 21, 5134. [Google Scholar] [CrossRef]
- Koenig, S.; Rühmann, B.; Sieber, V.; Schmid, J. Quantitative assay of β-(1,3)–β-(1,6)–glucans from fermentation broth using aniline blue. Carbohydr. Polym. 2017, 174, 57–64. [Google Scholar] [CrossRef]
- Peng, B.; Shen, Y.; Gao, Z.; Zhou, M.; Ma, Y.; Zhao, S. Determination of total iron in water and foods by dispersive liquid–liquid microextraction coupled with microvolume UV–vis spectrophotometry. Food Chem. 2015, 176, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Huarachi-Olivera, R.; Teresa Mata, M.; Ardiles-Candia, A.; Escobar-Méndez, V.; Gatica-Cortes, C.; Ahumada, M.; Orrego, J.; Vidal-Veuthey, B.; Cárdenas, J.P.; González, L.; et al. Modification of the Trizol Method for the Extraction of RNA from Prorocentrum triestinum ACIZ_LEM2. Int. J. Mol. Sci. 2024, 25, 9642. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Bhattacharjee, J.K. α-Aminoadipate Pathway for the Biosynthesis of Lysine in Lower Eukaryotes. CRC Crit. Rev. Microbiol. 1985, 12, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Shaba, C.; Urban, P.L. Metabolic remodeling during fructification of enoki mushroom. Food Chem. 2025, 486, 144613. [Google Scholar] [CrossRef]

| Strain ID | Melanin Content (mg/g) | β-Glucan Content (%of Dry Weight) | Iron Content (mg/kg) | Amino Acid Content (g/100 g) |
|---|---|---|---|---|
| IA1 | 1.25 ± 0.08 b | 10.35 ± 1.69 b | 56.84 ± 5.09 b | 10.64 ± 0.06 b |
| IA2 | 1.68 ± 0.18 a | 15.68 ± 3.11 a | 65.49 ± 3.28 a | 9.45 ± 0.08 a |
| IA3 | 1.54 ± 0.17 ab | 14.33 ± 0.41 ab | 64.13 ± 1.30 a | 9.42 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, K.; Yao, F.; Fang, M.; Lu, L.; Ma, X.; Wang, W.; Meng, J.; Sun, X.; Cui, Y.; Sun, J. Transcriptome-Based Analysis of Mitochondrial Influence on Key Agronomic Traits and Nutritional Components in Auricularia heimuer. Agronomy 2025, 15, 2188. https://doi.org/10.3390/agronomy15092188
Shao K, Yao F, Fang M, Lu L, Ma X, Wang W, Meng J, Sun X, Cui Y, Sun J. Transcriptome-Based Analysis of Mitochondrial Influence on Key Agronomic Traits and Nutritional Components in Auricularia heimuer. Agronomy. 2025; 15(9):2188. https://doi.org/10.3390/agronomy15092188
Chicago/Turabian StyleShao, Kaisheng, Fangjie Yao, Ming Fang, Lixin Lu, Xiaoxu Ma, Wei Wang, Jingjing Meng, Xu Sun, Yuling Cui, and Jian Sun. 2025. "Transcriptome-Based Analysis of Mitochondrial Influence on Key Agronomic Traits and Nutritional Components in Auricularia heimuer" Agronomy 15, no. 9: 2188. https://doi.org/10.3390/agronomy15092188
APA StyleShao, K., Yao, F., Fang, M., Lu, L., Ma, X., Wang, W., Meng, J., Sun, X., Cui, Y., & Sun, J. (2025). Transcriptome-Based Analysis of Mitochondrial Influence on Key Agronomic Traits and Nutritional Components in Auricularia heimuer. Agronomy, 15(9), 2188. https://doi.org/10.3390/agronomy15092188





