Relevance of Organic Matter Compositions, Structures and Associations to Soil Aggregates and to Sustainable Productivity
Abstract
1. Introduction
2. Soil Organic Matter and Humic Substances: Basic Definitions
- (1)
- Unaltered materials including fresh organic debris and non-transformed plant or animal remnants;
- (2)
- Transformed products (or humus), which no longer exhibit morphological features of their original biological structures. These humified components comprise both humic and non-humic substances, and can be further categorized into:
- (a)
- Brown-coloured amorphous humic components, differentiated on the basis of their solubility in acid and alkali into humic acids, fulvic acids, and humins.
- (b)
- Other components belonging to recognizable classes, such as polysaccharides, polypeptides, altered lignins, etc. These can be synthesized by microorganisms or can arise from modifications of similar compounds in the original debris. Such materials, though components of humus, would not be regarded as HSs.
3. The Formation of Humic Substances and Their Recalcitrant Properties
3.1. Genesis of Products of Humification
3.2. Indications of the Origins of Components of Soil Organic Matter
4. Isolation from Soil of Humic, Hydrophilic, and Humin Components of SOM
4.1. Isolation of SOM Components in Aqueous Solvent Systems
4.1.1. Extractions in Neutral Salt Solutions
4.1.2. Extraction with Dilute Aqueous Base Solutions
4.1.3. Extractions in Base Solutions Amended with Urea
4.2. Extractions with Organic Solvents
4.2.1. Relevant Properties of Organic Solvents
4.2.2. Applications of Organic Solvents to Isolate Humic and Humin Soil Components
4.3. Summary of Solvents and of Techniques Used for the Isolation of SOM Components
5. Fractionation of Soil Humic Substances
5.1. Gel Chromatography Techniques
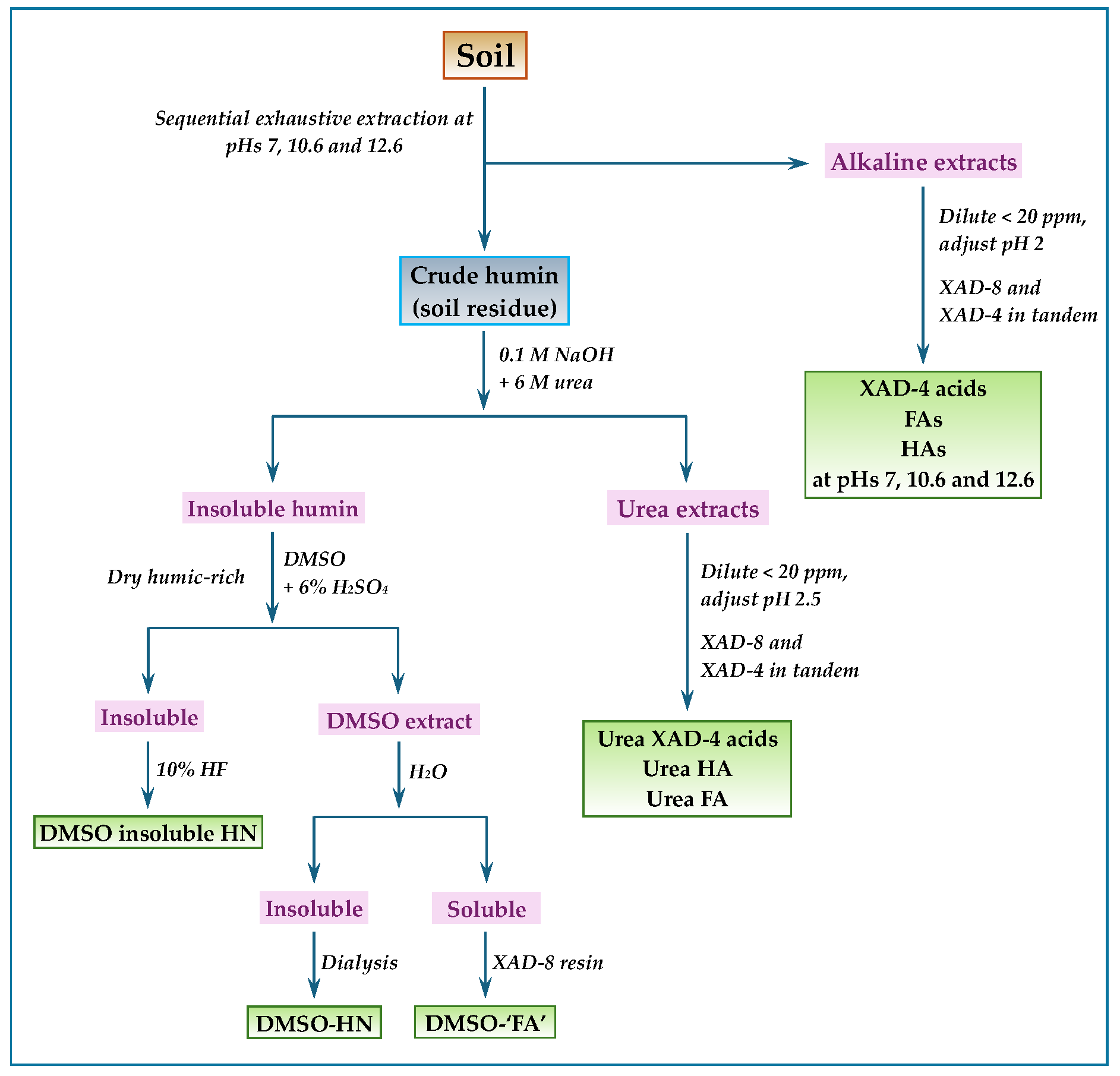
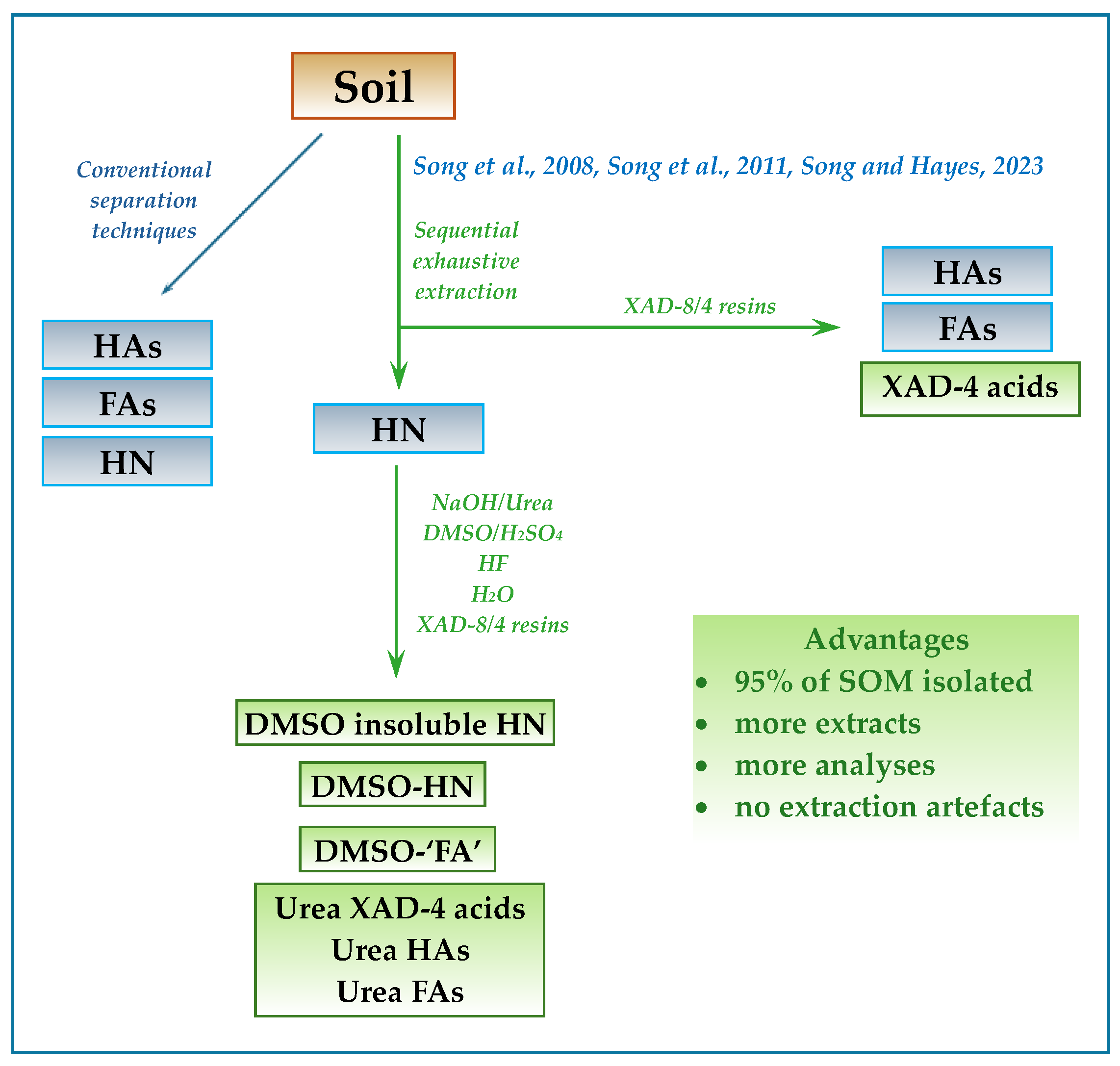
5.2. Sequential Exhaustive Extractions and XAD-8 (DAX-8) and XAD-4 Resins in Tandem
5.3. Polyvinyl Pyrrolidone Resin
5.4. High Performance Liquid Chromatography (HPLC)
5.5. Electrophoresis
5.6. DEAE Preparations
5.7. XAD-8 (DAX-8) and XAD-4 Resin Techniques
5.8. General Summary
6. Sizes, Shapes, Compositions of Humic Structures
6.1. The Classical Concepts
6.2. Molecular Associations
6.3. General Inferences
7. Soil Structure, Aggregates, Compositions, Stabilities
7.1. Soil Structure: General Considerations
7.2. The ‘Building Blocks’ of Microaggregates
7.3. Roles of Microbial Polysaccharides and Peptide Structures
7.4. Sorption of Saccharides and of Peptides by Clays
7.4.1. Interactions of Uncharged Polysaccharides with Clays
7.4.2. Adsorption by Clays of Charged Polysaccharides
7.4.3. Adsorption of Polysaccharides by (Hydr)oxides
7.4.4. Soil Polysaccharides
7.4.5. Adsorption of Soil Polysaccharides by Clays
7.5. Role of Humic Substances in Soil Aggregate Stabilization
7.6. Possible Roles of Humin
8. Overview and Suggestions for the Future
8.1. Sizes and Shapes of Humic Components
8.2. ‘Families’ of Molecules
8.3. The Composition and the Role of Humin in the Soil Environment
8.4. Soil Aggregates: Formation and Stabilization
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cárceles Rodríguez, B.; Durán-Zuazo, V.H.; Soriano Rodríguez, M.; García-Tejero, I.F.; Gálvez Ruiz, B.; Cuadros Tavira, S. Conservation agriculture as a sustainable system for soil health: A review. Soil Systems 2022, 6, 87. [Google Scholar] [CrossRef]
- Gayan, A.; Borah, P.; Nath, D.; Kataki, R. Chapter 4—Soil microbial diversity, soil health and agricultural sustainability. In Sustainable Agriculture and the Environment; Farooq, M., Gogoi, N., Pisante, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 107–126. [Google Scholar]
- Scharlemann, J.P.W.; Tanner, E.V.J.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2015, 5, 81–91. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Weng, Z.; Lehmann, J.; Van Zwieten, L.; Joseph, S.; Archanjo, B.S.; Cowie, B.; Thomsen, L.; Tobin, M.J.; Vongsvivut, J.; Klein, A.; et al. Probing the nature of soil organic matter. Crit. Rev. Env. Sci. Tec. 2022, 52, 4072–4093. [Google Scholar] [CrossRef]
- Lehmann, J.; Hansel, C.M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J.P.; Torn, M.S.; et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Swift, R.S. Vindication of Humic Substances as a Key Component of Organic Matter in Soil and Water. Adv. Agron. 2020, 163, 1–38. [Google Scholar]
- Jenkinson, D.S. The fate of plant and animal residues in soil. In The Chemistry of Soil Processes; Greenland, D.J., Hayes, M.H.B., Eds.; Wiley: Chichester, UK, 1981; pp. 505–561. [Google Scholar]
- Kononova, M.M. Soil Organic Matter: Its Nature, Its Role in Soil Formation and in Soil Fertility; Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Kononova, M.M. Humus of virgin and cultivated soils. In Soil Components; Gieseking, J.E., Ed.; Springer: Berlin, Germany, 1975; Volume 1, pp. 475–526. [Google Scholar]
- Hayes, M.H.B.; Swift, R.S. The chemistry of soil organic colloids. In The Chemistry of Soil Constituents; Greenland, D.J., Hayes, M.H.B., Eds.; Wiley: Chichester, UK, 1978; pp. 175–320. [Google Scholar]
- Wershaw, R.L. The study of humic substances-In search of a paradigm. In Humic Substances. Versatile Components of Plants, Soil and Water; Ghabbour, E.A., Davies, G., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2000; pp. 1–7. [Google Scholar]
- Burdon, J. Are the traditional concepts of the structures of humic substances realistic? Soil Sci. 2001, 166, 752–769. [Google Scholar] [CrossRef]
- Stevenson, F.J.; Cole, M. Cycles of Soil: Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2000. [Google Scholar]
- Hatcher, P.G.; Spiker, E.C. Selective degradation of plant biomolecules. In Humic Substances and Their Role in the Environment; Frimmel, F.H., Christman, R.F., Eds.; Wiley: Chichester, UK, 1988; pp. 59–74. [Google Scholar]
- Vicuna, R. Ligninolysis—A very peculiar microbial process. Mol. Biotechnol. 2000, 14, 173–176. [Google Scholar] [CrossRef]
- DiDonato, N.; Chen, H.; Waggoner, D.; Hatcher, P.G. Potential origin and formation for molecular components of humic acids in soils. Geochim. Cosmochim. Acta 2016, 178, 210–222. [Google Scholar] [CrossRef]
- Waggoner, D.C.; Chen, H.; Willoughby, A.S.; Hatcher, P.G. Formation of black carbon-like and alicyclic aliphatic compounds by hydroxyl radical initiated degradation of lignin. Org. Geochem. 2015, 82, 69–76. [Google Scholar] [CrossRef]
- Knicker, H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 2007, 85, 91–118. [Google Scholar] [CrossRef]
- Knicker, H.; Hischer, A.; Gonzales-Vila, F.J.; Almendros, G.A. A new conceptual model for the structural properties of char produced during vegetation fires. Org. Geochem. 2008, 39, 935–939. [Google Scholar] [CrossRef]
- van Krevelen, D.W. Coal; Elsevier: Amsterdam, The Netherlands, 1961. [Google Scholar]
- Nutsubidze, N.N.; Sarkanen, S.; Schmidt, E.I.; Shashikanth, S. Consecutive polymerization and depolymerization of Kraft lignin by Trametes cingulata. Phytochemistry 1998, 49, 1203–1212. [Google Scholar] [CrossRef]
- Flaig, W. Comparative chemical investigation of soil humic compounds and model substances. Sci. Proc. R. Dublin Soc. 1960, 4, 49–62. [Google Scholar]
- Flaig, W.; Beutelspacher, H. Investigations of humic acids with the analytical ultracentrifuge. In Isotopes and Radiation in Soil Organic Matter Studies, Proceedngs of the Symposium on Soil Organic Matter Studies, Vienna, Austria, 15–19 July 1968; IAEA: Vienna, Austria, 1968; pp. 23–30. [Google Scholar]
- Flaig, W.; Beutelspacher, H.; Reitz, E. Chemical composition and physical properties of humic substances. In Soil Components: Vol. 1. Organic Components; Gieseking, J.E., Ed.; Springer: New York, NY, USA, 1975; pp. 1–211. [Google Scholar]
- Ziechmann, W. Huminstoffe; Verlag Chemie: Weinheime, Germany, 1980. [Google Scholar]
- Wershaw, R.L. Model for humus in soils and sediments. Environ. Sci. Technol. 1993, 27, 814–816. [Google Scholar] [CrossRef]
- Wershaw, R.L. Membrane-Micelle Model for Humus in Soils and Sediments and its Relation to Humification; USGS Water-Supply Paper 2410; USGS: Reston, VA, USA, 1994. [Google Scholar]
- Wang, T.S.C.; Huang, P.M.; Chou, C.H.; Chen, J.-H. The role of soil minerals in the abiotic polymerization of phenolic compounds and formation of humic substances. In Interactions of Soil Minerals with Natural Organics and Microbes; Huang, P.M., Schnitzer, M., Eds.; SSSA: Madison, WI, USA, 1986; pp. 251–281. [Google Scholar]
- Shindo, H.; Huang, P.M. Catalytic effects of manganese (IV), iron (III), aluminium and silicon oxides on the formation of phenolic polymers. Soil Sci. Soc. Am. J. 1984, 48, 927–934. [Google Scholar] [CrossRef]
- Polubesova, T.; Eldad, S.; Chefetz, B. Adsorption and OxidativeTransformation of Phenolic Acids ByFe(III)-Montmorillonite. Environ. Sci. Technol. 2010, 44, 4203–4209. [Google Scholar] [CrossRef]
- Birkef, U.; Gerold, G.; Niemeyer, J. Abiotic reactions of organics on clay mineral surfaces. In Developments in Soil Science; Violante, A., Huang, P.M., Bollag, J.-M., Gianfreda, L., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2002; Volume 28A, pp. 437–447. [Google Scholar]
- Wallis, P.; Booth, K.; Patti, A.F.; Scott, J.L. Oxidative recoupling revisited. Solvent free, heterogeneous and in water. Green Chem. 2006, 8, 333–337. [Google Scholar] [CrossRef]
- Roulia, M.; Vassiliadis, A.A. Clay-catalyzed phenomena of cationic-dye aggregation and hydroxo-chromium oligomerization. Micropor. Mesopor. Mater. 2009, 122, 13–19. [Google Scholar] [CrossRef]
- Martin, J.P.; Haider, K. Phenolic polymers of Stachybotrys atra, Stachybotrys chartarum and Epicoccum nigrum in relation to humic acid formation. Soil Sci. 1969, 107, 260–270. [Google Scholar] [CrossRef]
- Martin, J.P.; Haider, K. Microbial activity in relation to soil humus formation. Soil Sci. 1971, 111, 54–63. [Google Scholar] [CrossRef]
- Martin, J.P.; Haider, K. Decomposition of specifically carbon-14-labeled ferulic acid: Free and linked into model humic-acid type polymers. Soil Sci. Soc. Am. J. 1976, 40, 377–380. [Google Scholar] [CrossRef]
- Martin, J.P.; Haider, K. Decomposition of specifically 14C labelled DHP and corn stalk lignins, model humic acid-type polymers, and coniferyl alcohols. In Proceedings of the IAEA-FAO-Agrochemica Symposium, Braunschweig, Germany, 6–10 September 1976; Volume 2, pp. 23–32. [Google Scholar]
- Martin, J.P.; Richards, S.J.; Haider, K. Properties and decomposition and binding action in soil “humic acid” synthesized by Epicoccum nigrum. Soil Sci. Soc. Am. Proc. 1967, 31, 657–662. [Google Scholar] [CrossRef]
- Martin, J.P.; Haider, K.; Wolf, D. Synthesis of phenols and phenolic polymers by Hendersonula toruloideain in relation to humic acid formation. Soil Sci. Soc. Amer. Proc. 1972, 36, 311–315. [Google Scholar] [CrossRef]
- Martin, J.P.; Haider, K.; Saiz-Jimenez, C. Sodium amalgam reductive degradation of fungal and model phenolic polymers, soil humic acids and simple phenolic compounds. Soil Sci. Soc. Amer. Proc. 1974, 38, 760–765. [Google Scholar] [CrossRef]
- Martin, J.P.; Haider, K.; Bondietti, E. Properties of model humic acids synthesized by phenoloxidase and autoxidation of phenols and other components formed by soil fungi. In Proceedings of the International Meeting Humic Substances, Nieuwersluis, The Netherlands, 29–31 May 1972; pp. 171–186. [Google Scholar]
- Haider, K.; Martin, J.P. Synthesis and transformation of phenolic compounds by Epicoccum nigrum in relation to humic acid formation. Soil Sci. Soc. Amer. Proc. 1967, 31, 766–772. [Google Scholar] [CrossRef]
- Haider, K.; Martin, J.P. Decomposition of specifically carbon-14-labeled benzoic acid and cinnamic acid derivatives in soil. Soil Sci. Soc. Am. J. 1975, 39, 657–662. [Google Scholar] [CrossRef]
- Haider, K.; Martin, J.P.; Reitz, E. Decomposition in soil of 14C-labeled coumaryl alcohols: Free and linked into dehydropolymer and plant lignins and model humic acids. Soil Sci. Soc. Am. J. 1977, 41, 556–562. [Google Scholar] [CrossRef]
- Bondietti, E.; Martin, J.P.; Haider, K. Influence of nitrogen source and clay on growth and phenolic polymer production by Stachybotrys species, Hendersonula torulidea and Aspergillus sydowi. Soil Sci. Soc. Amer. Proc. 1971, 35, 917–922. [Google Scholar] [CrossRef]
- Jong-Rok, J.; Baldrianm, P.; Murugesan, K.; Chang, Y.-S. Laccase catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar]
- Hollman, F.; Arends, I.W.C. Enzyme initiated radical polymerisations. Polymers 2012, 4, 759–793. [Google Scholar] [CrossRef]
- Richter, M.; Schulenburg, C.; Jankowska, D.; Heck, T.; Faccio, G. Novel materials through nature’s catalysts. Mater. Today 2015, 18, 459–467. [Google Scholar] [CrossRef]
- Chefetz, B.; Chen, Y.; Hadar, Y. Purification and characterization of laccase from Chaetomium thermophilium and its role in humification. Appl. Environ. Microbiol. 1998, 64, 3175–3179. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.I.; Marxsen, J.; Sinsabaugh, R.I.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biohem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Maillard, L.C. Action des acides amines sur les sucre; formation des melanoidinens par voie methodique. C.R. Acad. Sci. Paris 1912, 154, 66–68. [Google Scholar]
- Maillard, L.C. Synthese des matieres humiques par action des acides amines sur les sucres reductours. Ann. Chim. 1916, 5, 258–317. [Google Scholar]
- Maillard, L.C. Identite des matieres humiques de syntheses avec les matieres humiques naturelles. Ann. Chim. 1917, 7, 113–152. [Google Scholar]
- Benzig-Purdie, L.; Ripmeister, J. Melanoidins and soil organic matter: Evidence of strong similarities revealed by 13C CP-MAS NMR. Soil Sci. Soc. Am. J. 1983, 47, 56–61. [Google Scholar] [CrossRef]
- Knicker, H.; Lüdemann, D. N-15 and C-13 CPMAS and solution NMR studies of N-15 enriched plant material during 600 days of microbial degradation. Org. Geochem. 1995, 23, 329–341. [Google Scholar] [CrossRef]
- Knicker, H. Biogenic nitrogen in soils as revealed by solid-state carbon-13 and nitrogen-15 nuclear magnetic resonance spectroscopy. J. Environ. Qual. 2000, 29, 715–723. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. Analytical approaches for characterising soil organic matter. Org. Geochem. 2000, 31, 1023–1028. [Google Scholar] [CrossRef]
- Poirier, N.; Derenne, S.; Rouzaud, J.-N.; Largeau, C.; Mariotti, A.; Balesdent, J.; Maquet, J. Chemical structure and sources of the macromolecular, resistant, organic fraction isolated from a forest soil (Lacadée, south-west France). Org. Geochem. 2000, 31, 813–827. [Google Scholar] [CrossRef]
- Poirier, N.; Derenne, S.; Balesdent, J.; Rouzaud, N.; Mariotti, A.; Largeau, C. Abundance and composition of the refractory, organic fraction of an ancient, tropical soil (Pointe Noire, Congo). Org. Geochem. 2002, 33, 383–391. [Google Scholar] [CrossRef]
- Enders, C. Wie ensteht der Humus in der Natur. Die. Chemie. 1943, 56, 281–292. [Google Scholar]
- Enders, C. Uber den Chemismus den Huminsaurbildung unter physiologischen Bedungen. IV. Mitt. Die Role der Mikroorganismen bei den Humifizierunsvorgangen. Biochem. Z. 1943, 315, 259–292. [Google Scholar]
- Enders, C.; Fries, G. Zur Analogie von Melanoiden und Huminsauren. Kolloid Z. 1936, 76, 289–291. [Google Scholar] [CrossRef]
- Enders, C.; Sigurdsson, S. Uber den Chemismus der bildung unter physiologischen Bindungen. VII Mitt. Biochem. Z. 1948, 318, 44–46. [Google Scholar]
- Enders, C.; Tschapek, M.; Glane, R. Vergleichende Untersuchungen einer kolloider Eigenschaften von naturlichen Huminsauren und Synthetischen Melanoidenen. Kolloid Z. 1948, 110, 240–244. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Mylotte, R.; Swift, R.S. Chapter 2: Humin: Its Composition and Importance in Soil Organic Matter. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Burlington, MA, USA, 2017; Volume 143, pp. 47–138. [Google Scholar]
- Hayes, M.H.B. Subsidence and Humification in Peats. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 1960. [Google Scholar]
- Hayes, M.H.B. Evolution of concepts of environmental natural non-living organic matter. In Biophysico-Chemical Process Involving Natural Nonliving Organic Matter in Environmental Systems; Senesi, N., Xing, B., Huang., P.M., Eds.; Wiley: New York, NY, USA, 2009; Volume 2, pp. 1–39. [Google Scholar]
- Waksman, S.A.; Iyer, K.R.N. Contribution to our knowledge of the chemical nature and origin of humus: IV. Fixation of proteins by lignins and formation of complexes resistant to microbial attack. Soil Sci. 1933, 36, 69–82. [Google Scholar] [CrossRef]
- MacCarthy, P. The principles of humic substances. Soil Sci. 2001, 166, 738–751. [Google Scholar] [CrossRef]
- Wershaw, R.L.; Leenheer, J.A.; Kennedy, K.R.; Noyes, T.I. Use of 13C NMR and FTIR for elucidation of degradation pathways during natural litter decomposition and composting. I. Early stage leaf degradation. Soil Sci. 1996, 161, 667–679. [Google Scholar] [CrossRef]
- Wershaw, R.L.; Kennedy, K.R.; Henrich, J.E. Use of 13C NMR and FTIR for elucidation of degradation pathways during natural litter decomposition and composting. II. Changes in leaf composition after senescence. In Humic Substances: Structures, Properties and Uses; Davies, G., Ghabbour, E.A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1998; pp. 29–46. [Google Scholar]
- Wershaw, R.L.; Leenheer, J.A.; Kennedy, K.R. Use of 13C NMR and FTIR for elucidation of degradation pathways during natural litter decomposition and composting. III. Characterization of leachate from different types of leaves. In Humic Substances: Structures, Properties and Uses; Davies, G., Ghabbour, E.A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1998; pp. 47–68. [Google Scholar]
- Simpson, A.J.; Kingery, W.L.; Hayes, M.H.B.; Spraul, M.; Humpfer, E.; Dvortsak, P.; Kerssebaum, R.; Godejohann, M.; Hofmann, M. The structures and associations of organic molecules in the terrestrial environment. Naturwissenschaften 2002, 89, 84–88. [Google Scholar] [CrossRef]
- Senesi, N.; Miano, T.M.; Brunetti, G. Humic-like substances in organic amendments and effects on native soil humic substances. In Humic Substances in Terrestrial Ecosystems; Piccolo, A., Ed.; Elsevier: New York, NY, USA, 1996; pp. 531–593. [Google Scholar]
- Balesdent, J.; Mariotti, A.; Guillet, B. Natural 13C abundance as a tracer for studies of soil organic matter dynamics. Soil Biol. Biochem. 1987, 19, 25–30. [Google Scholar] [CrossRef]
- Balesdent, J.; Wagner, G.H.; Mariotti, A. Soil organic matter turnover in long term field experiments as revealed by carbon-13 natural abundance. Soil Sci. Soc. Am. J. 1988, 52, 118–124. [Google Scholar] [CrossRef]
- Martin, A.; Mariotti, A.; Balesdent, J.; Lavelle, P.; Vuattoux, R. Estimate of organic matter turnover rate in a savanna soil by 13C natural abundance measurements. Soil Biol. Biochem. 1989, 22, 517–523. [Google Scholar] [CrossRef]
- Deines, P. The isotopic composition of reduced organic carbon. In Handbook of Environmental Isotope Geochemistry; Fritz, P., Fontes, J.C., Eds.; Elsevier: New York, NY, USA, 1980; Volume 1, pp. 329–406. [Google Scholar]
- Clapp, C.E.; Layese, M.F.; Hayes, M.H.B.; Huggins, D.R.; Allmaras, R.R. Natural abundances of 13C in soils and waters. In Humic Substances, Peats and Sludges: Health and Environmental Aspects; Hayes, M.H.B., Wilson, W.S., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1997; pp. 158–175. [Google Scholar]
- Huggins, D.R.; Buyanovsky, G.A.; Wagner, G.H.; Brown, J.R. Soil organic C in the tallgrass prairie-derived region of the corn belt: Effects of long-term crop management. Soil Tillage Res. 1998, 47, 219–234. [Google Scholar] [CrossRef]
- Clapp, C.E.; Almiras, R.R.; Layese, M.F.; Linden, D.R.; Dowdy, R.H. Soil organic carbon and 13C abundance as related to tillage, crop residue, and nitrogen fertilization under continuous corn management in Minnesota. Soil Tillage Res. 2000, 55, 127–142. [Google Scholar] [CrossRef]
- Delwichem, C.C.; Steyn, P.L. Nitrogen isotope fractionation in soils and microbial reactions. Environ. Sci. Technol. 1970, 4, 929–935. [Google Scholar] [CrossRef]
- Sutherland, R.A.; van Kessel, C.; Pennock, D.J. Spatial variability of nitrogen-15 abundance. Soil Sci. Soc. Am. J. 1991, 55, 1339–1347. [Google Scholar] [CrossRef]
- Létolle, R. Nitrogen-15 in the natural environment. In Handbook of Environmental Isotope Geochemistry; Fritz, P., Fontes, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1980; Volume 1, pp. 407–433. [Google Scholar]
- Hayes, T.M. Study of the Humic Substances from Soils and Waters and Their Interactions with Anthropogenic Organic Chemicals. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 1996. [Google Scholar]
- Skjemstad, J.O.; Krull, E.S.; Swift, R.S.; Szarvas, S. Mechanisms of protection of soil organic matter under pasture following clearing of rainforest on an Oxisol. Geoderma 2008, 143, 231–242. [Google Scholar] [CrossRef]
- Barré, P.; Eglin, T.; Christensen, B.T.; Ciais, P.; Houot, S.; Kätterer, T.; van Oort, F.; Peylin, P.; Poulton, P.R.; Romanenkov, V.; et al. Quantifying and isolating stable soil organic carbon using long-term bare fallow experiments. Biogeosciences 2010, 7, 3839–3850. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Swift, R.S. Lumping or splitting: Holistic or fractionation approaches in studies of humic substances. In Functions of Natural Organic Matter in Changing Environment, Proceedings of the 16th Meeting of the International Humic Substances Society (IHSS 16), Hangzhou, China, 9–14 September 2012; Xu, J., Wu, J., He, Y., Eds.; Zhejiang University Press: Hangzhou, China; Springer Science+Business Media: Dordrecht, The Netherlands, 2013; pp. 31–33. [Google Scholar]
- Clapp, C.E.; Hayes, M.H.B.; Simpson, A.J.; Kingery, W.L. Chemistry of soil organic matter. In Chemical Processes in Soils; Tabatabai, M.A., Sparks, D.L., Eds.; Soil Science Society of America: Madison, WI, USA, 2005; pp. 1–150. [Google Scholar]
- Hayes, M.H.B. Extraction of humic substances from soil. In Humic Substances in Soil, Sediment and Water; Aiken, G.R., McKnight, D.M., Wershaw, R.L., MacCarthy, P., Eds.; Wiley: New York, NY, USA, 1985; pp. 329–362. [Google Scholar]
- Franks, F. Solute—Water interactions: Do polyhydroxy compounds alter the properties of water? Cryobiology 1983, 20, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Achard, F.K. Chemische Untersuchung des Torfs. Crell’s Chem. Ann. 1786, 2, 391–402. [Google Scholar]
- Sprengel, C. Über Pflanenzhumus, Humussäure and Humussaure Slaze. Kastne’s Arch. Ges. Naturlehre 1826, 8, 45–220. [Google Scholar]
- Sprengel, C. Die Bodenkunde Oder Die Lehre vom Boden; Immanuel Müller Publ. Co.: Leipzig, Germany, 1837. [Google Scholar]
- Bremner, J.M.; Lees, H. Studies on soil organic matter. II. The extraction of organic matter from soil by neutral reagents. J. Agr. Sci. 1949, 39, 274–279. [Google Scholar] [CrossRef]
- Bremner, J.M. Some observations on the oxidation of soil organic matter in the presence of alkali. J. Soil Sci. 1950, 1, 198–204. [Google Scholar] [CrossRef]
- Kleber, M.; Lehmann, J. Humic substances extracted by alkali are invalid proxies for the dynamics and functions of organic matter in terrestrial and aquatic ecosystems. J. Env. Qual. 2019, 48, 207–216. [Google Scholar] [CrossRef]
- Aiken, G.R. Isolation and concentration techniques for aquatic humic substances. In Humic Substances in Soil, Sediment, and Water; Aiken, G.R., McKnight, D.M., Wershaw, R.L., MacCarthy, P., Eds.; Wiley: New York, NY, USA, 1985; pp. 363–385. [Google Scholar]
- Green, N.W.; McInnis, D.; Hertkorn, N.; Maurice, P.A.; Perdue, E.M. Suwannee River natural organic matter: Isolation of the 2R101N reference sample by reverse osmosis. Environ. Eng. Sci. 2015, 32, 38–44. [Google Scholar] [CrossRef]
- Hayes, T.M.; Hayes, M.H.B.; Skjemstad, J.O.; Swift, R.S. Compositional relationship between organic matter in a grassland soil and its drainage waters. Eur. J. Soil Sci. 2008, 59, 603–616. [Google Scholar] [CrossRef]
- Hayes, T.M.; Hayes, M.H.B.; Swift, R.S. Detailed investigation of organic matter components in extracts and drainage waters from a soil under long term cultivation. Org. Geochem. 2012, 52, 13–22. [Google Scholar] [CrossRef]
- Malcolm, R.L.; MacCarthy, P. Quantitative evaluation of XAD-8 and XAD-4 resins used in tandem for removing organic solutes from water. Environ. Int. 1992, 18, 597–607. [Google Scholar] [CrossRef]
- Mylotte, R.T.V.; Verheyen, T.V.H.; Reynolds, A.; Dalton, C.; Patti, A.F.; Chang, R.R.; Burdon, J.; Hayes, M.H.B. Isolation and characterisation of recalcitrant organic components from an estuarine sediment core. J. Soils Sediments 2015, 15, 211–224. [Google Scholar] [CrossRef]
- Mylotte, R.; Sutrisno, A.; Farooq, H.; Masoom, H.; Soong, R.; Hayes, M.H.B.; Simpson, A.J. Insights into the composition of recalcitrant organic matter from estuarine sediments using NMR spectroscopy. Org. Geochem. 2016, 98, 155–165. [Google Scholar] [CrossRef]
- Ritchie, J.D.; Perdue, E.M. Proton-binding study of standard and reference fulvic acids, humic acids, and natural organic matter. Geochim. Cosmochim. Acta 2003, 67, 85–96. [Google Scholar] [CrossRef]
- Serkiz, M.; Perdue, E.M. Isolation of dissolved organic matter from the Suwannee river using reverse osmosis. Water Res. 1990, 24, 911–916. [Google Scholar] [CrossRef]
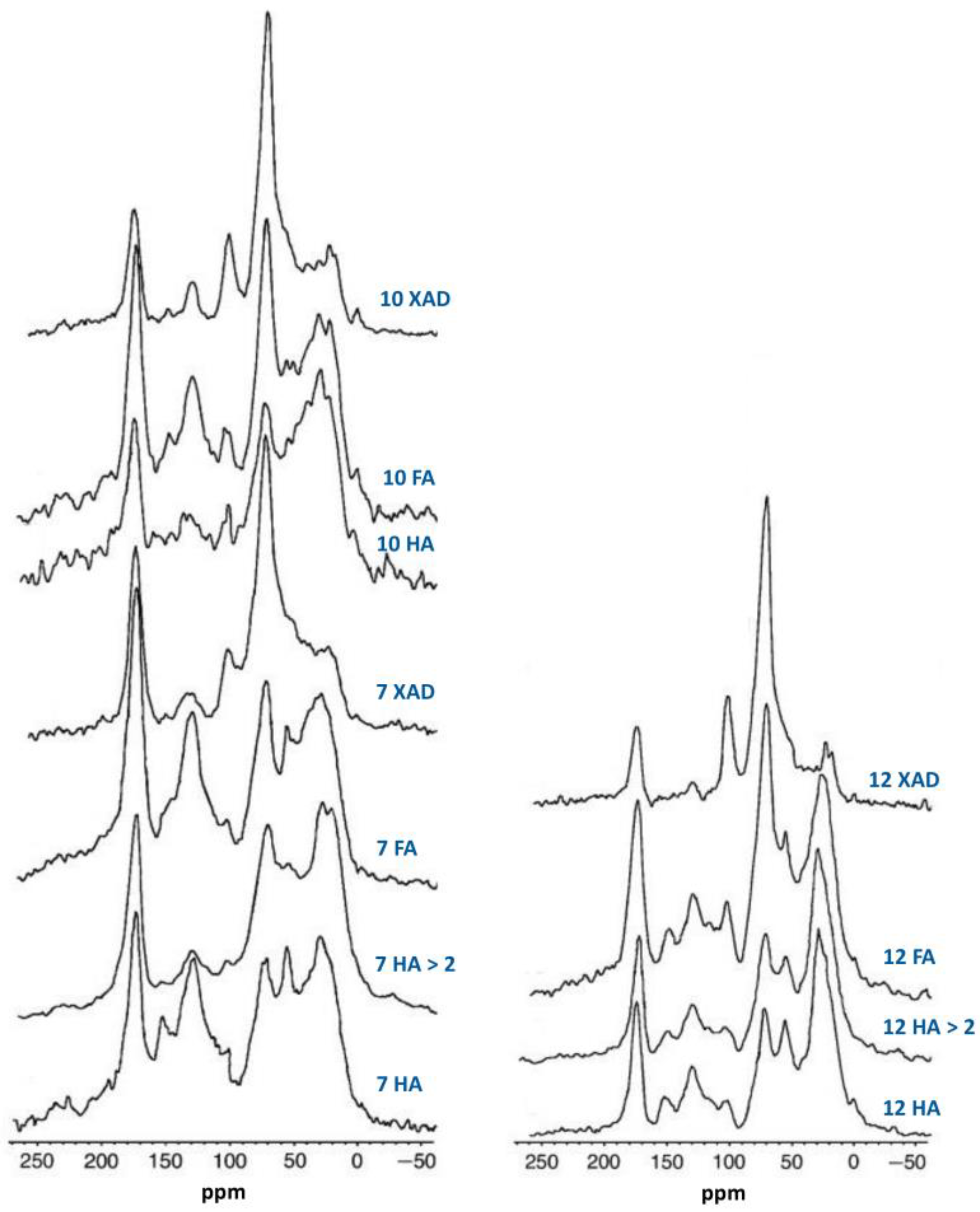
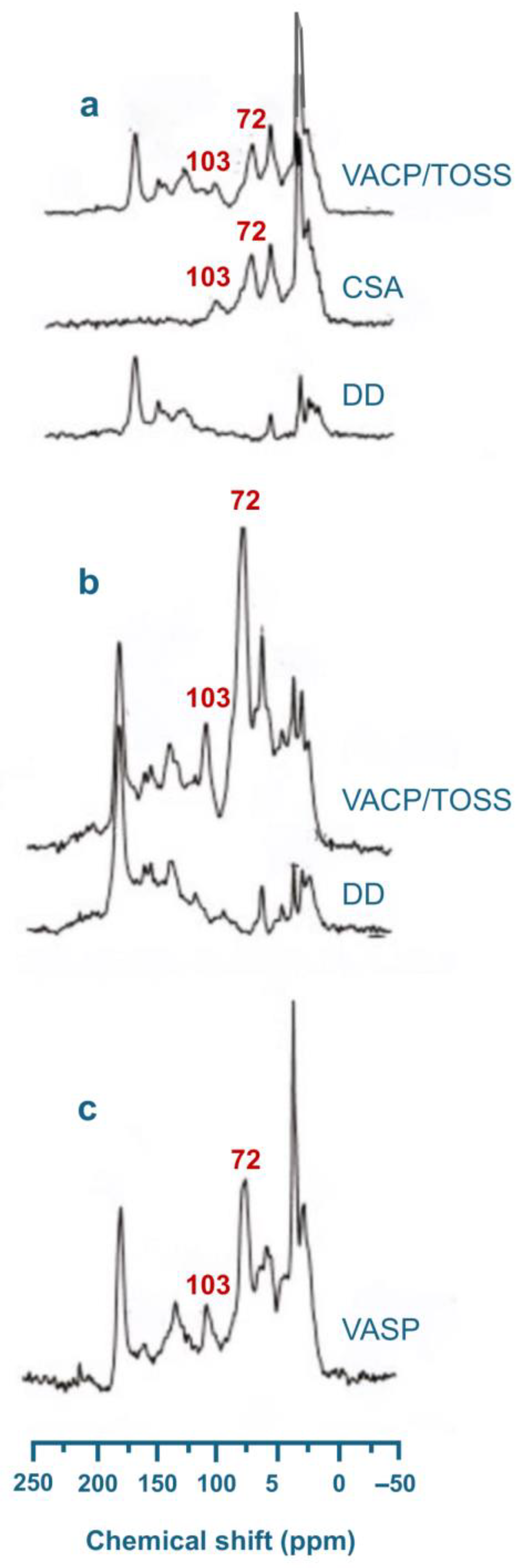
- Song, G.; Novotny, E.H.; Clapp, C.E.; Hayes, M.H.B. Sequential exhaustive extraction of a Mollisol soil, and characterizations of humic components, including humin, by solid and solution state NMR. Eur. J. Soil Sci. 2008, 59, 505–516. [Google Scholar] [CrossRef]
- Song, G.; Hayes, M.H.B.; Novotny, E.H.; Simpson, A.J. Isolation and fractionation of soil humin materials using alkaline urea and DMSO plus sulphuric acid. Naturwissenschaften 2011, 98, 5–13. [Google Scholar] [CrossRef]
- Swift, R.S. Organic matter characterization. In Methods of Soil Analysis. Part 3; Sparks, D.L., Ed.; SSSA: Madison, WI, USA, 1996; pp. 1011–1069. [Google Scholar]
- Waksman, S.A. Humus: Origin, Chemical Composition, and Importance in Nature; Williams & Wilkins: Baltimore, MD, USA, 1936. [Google Scholar]
- Swift, R.S.; Posner, A.M. Autoxidation of humic acid under alkaline conditions. J. Soil Sci. 1972, 23, 381–393. [Google Scholar] [CrossRef]
- Oh-Ishi, M.; Maeda, T. Separation techniques for high-molecular-mass proteins. J. Chromatogr. B. 2002, 771, 49–56. [Google Scholar] [CrossRef]
- Ballet, N.; Besle, J.M.; Demarquilly, C. Effect of ammonia and urea treatments on digestibility and nitrogen content of dehydrated lucerne. Anim. Feed Sci. Technol. 1997, 67, 69–82. [Google Scholar] [CrossRef]
- Fondevila, M.; Castrillo, C.; Guada, J.A.; Balcells, J. Effect of ammonia treatment and carbohydrate supplementation of barley straw on rumen liquid characteristics and substrate degradation by sheep. Anim. Feed Sci. Technol. 1994, 50, 137–155. [Google Scholar] [CrossRef]
- Song, G.; Hayes, M.H.B. Isolation, fractionation, and characterization of humic substances from a maize-amended soil. In Humic Substances and Soil and Water Environment, Proceedings of XII International Meeting of IHSS, São Pedro, Brazil, 25–30 July 2004; Martin-Neto, L., Milori, D.M.B., da Silva, W.T.L., Eds.; International Humic Substances Society: São Paulo, Brazil, 2004; pp. 327–331. [Google Scholar]
- Hayes, M.H.B.; Graham, C.L. Procedures for the isolation and fractionation of humic substances. In Humic Substances. Versatile Components of Plants, Soils and Waters; Davies, G., Ghabbour, E.A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2000; pp. 91–109. [Google Scholar]
- Snyder, L.R. Solvent selection for separation processes. In Separation and Purification. Techniques of Chemistry, 3rd ed.; Perry, E.S., Weissberger, A., Eds.; Wiley: New York, NY, USA, 1978; Volume XII, pp. 25–75. [Google Scholar]
- Parker, A.J. The effects of solvation on the properties of anions in dipolar aprotic solvents. Q. Rev. Chem. Soc. 1962, 16, 163–187. [Google Scholar] [CrossRef]
- Pimentel, G.C.; McClellan, A.L. The Hydrogen Bond; W.H. Freeman and Co.: San Francisco, CA, USA, 1960. [Google Scholar]
- Taft, R.W.; Gurka, D.; Joris, L.; Schleyer, P.vR.; Rakshys, J.W. Studies of hydrogen-bonded complex formation with p-fluorophenol. V. Linear free energy relationships with OH reference acids. J. Am. Chem. Soc. 1969, 91, 4801–4808. [Google Scholar] [CrossRef]
- Karger, B.L.; Snyder, L.R.; Horvath, C. An Introduction to Separation Science; Wiley-Interscience: New York, NY, USA, 1973. [Google Scholar]
- Rice, J.; MacCarthy, P. Isolation of humin by liquid-liquid partitioning. Sci. Total Environ. 1989, 81–82, 61–69. [Google Scholar] [CrossRef]
- Song, G.; Hayes, M.H.B. Isolation and fractionation of organic matter from soils and waters. Adv. Agron. 2023, 177, 169–214. [Google Scholar]
- Song, G.; Simpson, A.J.; Hayes, M.H.B. Compositional changes in the humin fraction resulting from the long-term cultivation of an Irish grassland soil: Evidence from FTIR and multi-NMR spectroscopies. Adv. Agron. 2023, 880, 163280. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Novotny, E.H.; Hayes, M.H.B. Vindication for uses of urea-amended aqueous alkali and of concentrated H2SO4-amended DMSO in an exhaustive extraction sequence for the isolation of humic and of humin components of SOM. J. Soils Sediments 2023, 23, 1146–1155. [Google Scholar] [CrossRef]
- Song, G.; Hayes, M.H.B.; Novotny, E.H. A two-year incubation study of transformations of crop residues into soil organic matter (SOM) and a procedure for the sequential isolation and the fractionation of components of SOM. Sci. Total Environ. 2021, 763, 143034. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Mylotte, R.; Swift, R.S. Humin: Its composition and importance in soil organic matter. Adv. Agron. 2017, 143, 47–138. [Google Scholar]
- Song, G.; Novotny, E.H.; Richards, K.G.; Hayes, M.H.B. Characteristics of hydrophobic and hydrophilic acid fractions in drainage waters of undisturbed soil lysimeters. J. Soils Sediments 2018, 18, 3197–3214. [Google Scholar] [CrossRef]
- Olk, D.C.; Bloom, P.R.; Perdue, E.M.; McKnight, D.M.; Chen, Y.; Farenhorst, A.; Senesi, N.; Chin, Y.-P.; Schmitt-Koplin, P.; Hertkorn, N.; et al. Environmental and agricultural relevance of humic fractions extracted by alkali from soils and natural waters. J. Env. Qual. 2019, 48, 217–232. [Google Scholar] [CrossRef]
- Olk, D.C.; Bloom, P.R.; de Nobili, M.; Chen, Y.; McKnight, D.M.; Wells, M.J.M.; Weber, J. Using humic fractions to understand natural organic matter processes in soil and water: Selected studies and applications. J. Env. Qual. 2019, 48, 1633–1643. [Google Scholar] [CrossRef]
- Bagherifam, S.; Brown, T.C.; Baglieri, A.; Sarkar, B.; Rinklebe, J. Fractionation of water-soluble organic matter (WSOM) with polyvinylpyrrolidone: A study on antimony associated with WSOM in contaminated soils. J. Environ. Sci. 2025, 157, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Hirose, T.; Tamori, R.; Fujitake, N.; Nakashima, S.; Yamamura, H.; Satoh, H. Solid-phase fluorescence excitation-emission matrix spectroscopy of soil, fulvic acid fractions, and clay mineral complexes: Evidence from red shift of fluorescence maxima associated with aggregation. Environ. Res. 2025, 279, 121900. [Google Scholar] [CrossRef] [PubMed]
- De Nobili, M.; Bravoa, C.; Chen, Y. The spontaneous secondary synthesis of soil organic matter components: A critical examination of the soil continuum model theory. Appl. Soil Ecol. 2020, 154, 103655. [Google Scholar] [CrossRef]
- Dick, D.P.; Burba, P. Extraction kinetics and molecular size fractionation of humic substances from two Brazilian soils. J. Braz. Chem. Soc. 1999, 10, 146–152. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Clapp, C.E. Humic substances: Considerations of compositions, aspects of structure, and environmental influences. Soil Sci. 2001, 166, 723–737. [Google Scholar] [CrossRef]
- Rho, H.; Chon, K.; Park, J.; Cho, J. Rapid and Effective Isolation of Dissolved Organic Matter Using Solid-Phase Extraction Cartridges Packed with Amberlite XAD 8/4 Resins. Water 2019, 11, 67. [Google Scholar] [CrossRef]
- Swift, R.S. Fractionation of soil humic substances. In Humic Substances in Soil, Sediment, and Water; Aiken, G.R., McKnight, D.M., Wershaw, R.L., MacCarthy, P., Eds.; John Wiley and Sons: New York, NY, USA, 1985; pp. 387–408. [Google Scholar]
- Piccolo, A. The supramolecular structure of humic substances: A novel understanding of humus chemistry and implications in soil science. Adv. Agron. 2002, 75, 57–134. [Google Scholar]
- Cameron, R.S.; Thornton, B.K.; Swift, R.S.; Posner, A.M. Molecular weight and shape of of humic acid from sedimentation and diffusion measurements on fractionated extracts. J. Soil Sci. 1972, 23, 394–408. [Google Scholar] [CrossRef]
- Swift, R.S.; Leonard, R.L.; Newman, R.H.; Theng, B.K.G. Changes in humic acid composition with molecular weight as detected by 13C-nuclear magnetic resonance spectroscopy. Sci. Total Environ. 1992, 117, 53–61. [Google Scholar] [CrossRef]
- Chefetz, B.; Tarchitzky, J.; Deshmukh, A.P.; Hatcher, P.G.; Chen, Y. Structural characterization of soil organic matter and humic acids in particle-size fractions of an agricultural soil. Soil Sci. Soc. Am. J. 2002, 66, 129–141. [Google Scholar] [CrossRef]
- Lowe, L.E. Fractionation of acid-soluble components of soil organic matter using PVP. Can. J. Soil Sci. 1975, 55, 109–126. [Google Scholar] [CrossRef]
- Peuravuori, J.; Monteiro, A.; Eglite, L.; Pihlaja, K. Comparative study for separation of aquatic humic-type organic constituents by DAX-8, PVP and DEAE sorbing solids and tangential ultrafiltration: Elemental composition, size-exclusion chromatography, UV-vis and FT-IR. Talanta 2005, 65, 408–422. [Google Scholar] [CrossRef]
- Watanabe, A.; Kuwatsuka, S. Fractionation of soil fulvic acids using polyvinyl-pyrrolidone and their ionization difference spectra. Soil Sci. Plant Nutr. 1991, 37, 611–617. [Google Scholar] [CrossRef]
- Her, N.; Amy, G.; Foss, D.; Cho, J.; Yoon, Y.; Kosenka, P. Optimization of method for detecting and characterizing NOM by HPLC−Size Exclusion Chromatography with UV and on-line DOC detection. Environ. Sci. Technol. 2002, 36, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.P.; Ludwichowski, K.-U.; Kattner, G.; Dittmar, T.; Witt, M. Advanced characterization of marine dissolved organic matter by combining reversed-phase liquid chromatography and FT-ICR-MS. Mar. Chem. 2008, 111, 233–241. [Google Scholar] [CrossRef]
- Shimotori, K.; Satou, T.; Imai, A.; Kawasaki, N.; Komatsu, K.; Kohzu, A.; Tomioka, N.; Shinohara, R.; Miura, S. Quantification and characterization of coastal dissolved organic matter by high-performance size exclusion chromatography with ultraviolet absorption, fluorescence, and total organic carbon analyses. Limnol. Oceanogr-Meth. 2016, 14, 637–648. [Google Scholar] [CrossRef]
- Woods, G.C.; Simpson, M.J.; Kelleher, B.P.; McCaul, M.; Kingery, W.L.; Simpson, A.J. Online High-Performance Size Exclusion Chromatography−Nuclear Magnetic Resonance for the characterization of dissolved organic matter. Environ. Sci. Technol. 2010, 44, 624–630. [Google Scholar] [CrossRef]
- Balabanova-Radonova, E.M.; Stefanova, M.D.; Nikolov, R.N. Use of HPLC in fractionation of lignite humic substances. Fuel 1980, 59, 271–272. [Google Scholar] [CrossRef]
- Fievre, A.; Solouki, T.; Marshall, A.G.; Cooper, W.T. High-Resolution Fourier Transform Ion Cyclotron Resonance Mass Spectrometry of humic and fulvic acids by Laser Desorption/Ionization and Electrospray Ionization. Energy Fuels 1997, 11, 554–560. [Google Scholar] [CrossRef]
- Hutta, M.; Gora, R. Novel stepwise gradient reversed-phase liquid chromatography separations of humic substances, air particulate humic-like substances and lignins. J. Chromatogr. A 2003, 1012, 67–79. [Google Scholar] [CrossRef]
- Jaroszewski, J.W. Hyphenated NMR methods in natural products research, part 1: Direct hyphenation. Planta Med. 2005, 71, 691–700. [Google Scholar] [CrossRef]
- Jaroszewski, J.W. Hyphenated NMR methods in natural products research, Part 2: HPLC-SPE-NMR and other new trends in NMR hyphenation. Planta Med. 2005, 71, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.J.; Lefebvre, B.; Moser, A.; Williams, A.; Larin, N.; Kvasha, M.; Kingery, W.L.; Kelleher, B. Identifying residues in natural organic matter through spectral prediction and pattern matching of 2-D NMR datasets. Magn. Reson. Chem. 2004, 22, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, M. Separation and spectroscopic characterization of soil fulvic acid constituents by hydrophilic interaction chromatography and reversed-phase high-performance liquid chromatography with π–π interactions. Soil Sci. Plant Nutr. 2025, 71, 18–26. [Google Scholar] [CrossRef]
- Brown, T.A.; Jackson, B.A.; Bythell, B.J.; Stenson, A.C. Benefits of multidimensional fractionation for the study and characterization of natural organic matter. J. Chromatogr. A 2016, 1470, 84–96. [Google Scholar] [CrossRef]
- Haleem, J.I. A decade of capillary electrophoresis. Electrophoresis 2000, 21, 1921–1939. [Google Scholar] [CrossRef]
- De Nobili, M.; Leita, L.; Sequi, P. 2D electrophoresis of humic substances: Application of a high resolution technique to polyanionic polydisperse systems. Sci. Total Environ. 1987, 62, 85–88. [Google Scholar] [CrossRef]
- Dunkel, R.; Ruttinger, H.H.; Peisker, K. Comparative study for the separation of aquatic humic substances. J. Chromatogr. A 1997, 777, 355–362. [Google Scholar] [CrossRef]
- Kovacs, P.; Posta, J. Separation of humic acids using capillary isoelectric focusing. Microchem. J. 2005, 79, 49–54. [Google Scholar] [CrossRef]
- Castagnol, M.; Heras, R.G.; Marini-Bettòlo, G.B. Effect of urea on eleectrophoretic pattern of soil humic acids. J. Chromatog. A 1978, 147, 438–442. [Google Scholar] [CrossRef]
- Schmitt, P.; Garrison, A.W.; Freitag, D.; Kettrup, A. Capillary isoelectric focusing (CIEF) for the characterization of humic substances. Water Res. 1997, 31, 2037–2049. [Google Scholar] [CrossRef]
- Sandron, S.; Rojas, A.; Wilson, R.; Davies, N.W.; Haddad, P.R.; Shellie, R.A.; Nesterenko, P.N.; Kelleher, B.P.; Paull, B. Chromatographic methods for the isolation, separation and characterisation of dissolved organic matter. Environ. Sci. Process. Impacts 2015, 17, 1531–1567. [Google Scholar] [CrossRef]
- Trubetskaya, O.E.; Selivanova, O.M.; Rogachevskii, V.V.; Trubetskoj, O.A. Electron Microscopy of Stable Electrophoretic Fractions of Natural Humic Acids as a Key to the Understanding of Their Structural Organization. Russ. J. Bioorg. Chem. 2024, 50, 766–777. [Google Scholar] [CrossRef]
- Hejzlar, J.; Szpakowska, B.; Wershaw, R.L. Comparison of humic substances isolated from peatbog water by sorption on DEAE-cellulose and Amberlite XAD-2. Water Res. 1994, 28, 1961–1970. [Google Scholar] [CrossRef]
- Tuschall, J.R.; Miles, C.J.; Brezonik, P.L. Efficiency of isolating humus from natural waters using DEAE cellulose. Org. Geochem. 1985, 8, 137–139. [Google Scholar] [CrossRef]
- Boult, S.; Jugdaohsingh, R.; White, K.; Smith, B.; Powell, J. Evidence that polysaccharide and humic and fulvic acids are co-extracted during analysis but have different roles in the chemistry of natural waters. Appl. Geochem. 2001, 16, 1261–1267. [Google Scholar] [CrossRef]
- Finch, P.; Hayes, M.H.B.; Stacey, M. Studies of soil polysaccharides and on their interactions with clay preparations. Int. Soc. Soil Sci. Trans. Meet. Comm. IV VI 1967, 1966, 19–32. [Google Scholar]
- Barker, S.A.; Hayes, M.H.B.; Simmonds, R.G.; Stacey, M. Studies on soil polysaccharides I. Carbohyd. Res. 1967, 5, 13–24. [Google Scholar] [CrossRef]
- Watt, B.E.; Malcolm, R.L.; Hayes, M.H.B.; Clark, N.W.E.; Chipman, J.K. Chemistry and potential mutagenicity of humic substances in waters from different watersheds in Britain and Ireland. Water Res. 1996, 30, 1502–1516. [Google Scholar] [CrossRef]
- Maurice, P.A.; Pullin, M.J.; Cabaniss, S.E.; Zhou, Q.; Namjesnik-Dejanovic, K.; Aiken, G.R. A comparison of surface water natural organic matter in raw filtered water samples, XAD, and reverse osmosis isolates. Water Res. 2002, 36, 2357–2371. [Google Scholar] [CrossRef]
- Croue, J. Isolation of humic and non-humic NOM fractions: Structural characterization. Environ. Monit. Assess. 2004, 92, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Swift, R.S.; Posner, A.M. Gel chromatography of humic acid. J. Soil Sci. 1971, 22, 237–249. [Google Scholar] [CrossRef]
- De Nobili, M.; Chen, Y. Size exclusion chromatography of humic substances: Limits, perspectives and prospectives. Soil Sci. 1999, 164, 825–833. [Google Scholar] [CrossRef]
- Swift, R.S. Molecular weight, size, shape, and charge characteristics of humic substances: Some basic considerations. In Humic Substances II: In Search of Structure; Hayes, M.H.B., MacCarthy, P., Malcolm, R.L., Swift, R.S., Eds.; John Wiley and Sons: Chichester, UK, 1989; pp. 449–466. [Google Scholar]
- Swift, R.S. Molecular weight, shape, and size of humic substances by ultracentrifugation. In Humic Substances II: In Search of Structure; Hayes, M.H.B., MacCarthy, P., Malcolm, R.L., Swift, R.S., Eds.; John Wiley and Sons: Chichester, UK, 1989; pp. 467–495. [Google Scholar]
- Swift, R.S. Macromolecular properties of soil humic substances: Fact, fiction and opinion. Soil Sci. 1999, 164, 790–802. [Google Scholar] [CrossRef]
- Wershaw, R.L. Molecular aggregation of humic substances. Soil Sci. 1999, 164, 803–813. [Google Scholar] [CrossRef]
- Engebretson, R.R.; von Wandruszka, R. Kinetic aspects of cation-enhanced aggregation in aqueous humic acids. Environ. Sci. Technol. 1998, 32, 488–493. [Google Scholar] [CrossRef]
- Kenworthy, I.P.; Hayes, M.H.B. Investigations of some structural properties of humic substances by fluorescence quenching. In Humic Substances, Peats, and Sludges. Health and Environmental Aspects; Hayes, M.H.B., Wilson, W.S., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1997; pp. 39–45. [Google Scholar]
- Piccolo, A. The supramolecular structure of humic substances. Soil Sci. 2001, 166, 810–832. [Google Scholar] [CrossRef]
- Piccolo, A.; Conte, P. Molecular size of humic substances. Supramolecular associations versus macromolecular polymers. Adv. Environ. Res. 2000, 3, 508–521. [Google Scholar]
- Piccolo, A.; Conte, P.; Trivellone, E.; van Lagen, B.; Buurman, P. Reduced heterogeneity of a lignite humic acid by preparative HPSEC following interaction with an organic acid. Characterization of size-separates by Pyr-GC-MS and 1H-NMR spectroscopy. Environ. Sci. Technol. 2002, 36, 76–84. [Google Scholar] [CrossRef]
- Wells, M.J.M.; Stretz, H.A. Supramolecular architectures of natural organic matter. Sci. Total Environ. 2019, 67, 1125–1133. [Google Scholar] [CrossRef]
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond. Outline of a Comprehensive Bond Theory; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Stevenson, F.J. Humus Chemistry: Genesis, Compositions, Reactions, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1994; pp. 325–348. [Google Scholar]
- Averett, R.C.; Leenheer, J.A.; McKnight, D.M.; Thorn, K.A. Humic Substances in the Suwannee River, Georgia: Interactions, Properties, and Proposed Structures; US Geological Survey Water Supply: Reston, VA, USA, 1994; p. 2373. [Google Scholar]
- Derrien, M.; Lee, Y.K.; Park, J.-E.; Li, P.; Chen, M.; Lee, S.H.; Lee, J.-B.; Hur, J. Spectroscopic and molecular characterization of humic substances (HS) from soils and sediment in a watershed: Comparative study of HS chemical fractions and the origins. Environ. Sci. Pollut. Res. 2017, 24, 16933–16945. [Google Scholar] [CrossRef]
- Waggoner, D.C.; Hatcher, P.G. Hydroxyl radical alteration of HPLC fractionated lignin: Formation of new compounds from terrestrial organic matter. Org. Geochem. 2017, 113, 315–325. [Google Scholar] [CrossRef]
- Fragouli, P.G.; Roulia, M.; Vassiliadis, A.A. Macromolecular Size and Architecture of Humic Substances Used in the Dyes’ Adsorptive Removal from Water and Soil. Agronomy 2023, 13, 2926. [Google Scholar] [CrossRef]
- Poncet-Legrand, C.; Cabane, B.; Bautista-Ortín, A.B.; Carrillo, S.; Fulcrand, H.; Pérez, J.; Vernhet, A. Tannin oxidation: Intra- versus intermolecular reactions. Biomacromolecules 2010, 11, 2376–2386. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, A.; Piccolo, A. Polymerization of dissolved humic substances catalyzed by peroxidase. Effects of pH and humic composition. Org. Geochem. 2002, 33, 281–294. [Google Scholar] [CrossRef]
- Brown, G.; Newman, A.C.D.; Rayner, J.H.; Weir, A.H. The structures and chemistry of soil clay minerals. In The Chemistry of Soil Constituents; Greenland, D.J., Hayes, M.H.B., Eds.; New India Publishing Agency: New Delhi, India, 2016; pp. 29–178. [Google Scholar]
- Greenland, D.J.; Hayes, M.H.B. Soils and soil chemistry. In The Chemistry of Soil Constituents; Greenland, D.J., Hayes, M.H.B., Eds.; New India Publishing Agency: New Delhi, India, 2016; pp. 1–27. [Google Scholar]
- Jastrow, J.D. Soil aggregate formation and the accrual of particulate and mineral-associated organic matter. Soil Biol. Biochem. 1996, 28, 665–676. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Miller, R.M. Soil aggregate stabilization and carbon sequestration: Feedbacks through organomineral associations. In Soil Processes and the Carbon Cycle; Lal, R., Kimble, J.M., Follett, R.F., Stewart, B.A., Eds.; Taylor and Fancies group: Boston, MA, USA, 1997; pp. 207–224. [Google Scholar]
- Edwards, A.P.; Bremner, J.M. Use of sonic vibration for separation of soil particles. Canad. J. Soil Sci. 1964, 44, 366. [Google Scholar] [CrossRef]
- Edwards, A.P.; Bremner, J.M. Microaggregates in soils. Eur. J. Soil Sci. 1967, 18, 64–73. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. Eur.J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Martin, H.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Nief, G.K.; Lehndorff, E.; Mikutta, R.; Peth, S.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2017, 181, 104–136. [Google Scholar] [CrossRef]
- Quirk, J.P. Some physico-chemical aspects of soil structural stability-a review. In Modification of Soil Structure; Emerson, W.W., Bond, R.D., Dexter, A.R., Eds.; John Wiley & Sons: Chichester, UK; New York, NY, USA, 1978; pp. 3–16. [Google Scholar]
- Jenkinson, D.S.; Ladd, J.N. Microbial biomass in soil: Measurement and turnover. In Soil Biochemistry; Paul, E.A., Ladd, J.N., Eds.; Marcel Dekker: Roca Baton, FL, USA, 1981; Volume 5, pp. 415–471. [Google Scholar]
- Crouzet, O.; Consentino, L.; Pétraud, J.-P.; Marrauld, C.; Aguer, J.-P.; Bureau, S.; Le Bourvellec, C.; Touloumet, L.; Bérard, A. Soil photosynthetic microbial communities mediate aggregate stability: Influence of cropping systems and herbicide use in an agricultural soil. Front. Microbiol. 2019, 10, 1319. [Google Scholar] [CrossRef]
- Stotzky, G. Surface interactions between clay minerals and microbes, viruses and soluble organics, and the probable importance of these interactions to the ecology of microbes in soil surfaces. In Microbial Adhesion to Surfaces; Berkeley, R.C.W., Lynch, J.M., Melling, J., Rutter, P.R., Vincent, B., Eds.; Ellis Horwood: Chichester, UK, 1980; pp. 231–247. [Google Scholar]
- Burns, R.G. Microbial adhesion to soil surfaces: Consequences for growth and enzyme activities. In Microbial Adhesion to Surfaces; Berkeley, R.C.W., Lynch, J.M., Melling, J., Rutter, P.R., Vincent, B., Eds.; Ellis Horwood: Chichester, UK, 1980; pp. 249–262. [Google Scholar]
- Chenu, C.; Stotzky, G. Interactions between microorganisms and soil particles: A review. In Interactions Between Soil Particles and Microorganisms—Impact on the Terrestrial Ecosystems; Huang, P.M., Bollag, J.-M., Senesi, N., Eds.; John Wiley& Sons: Chichester, UK, 2002; pp. 3–40. [Google Scholar]
- Kögel-Knabner, I.; Guggenberger, G.; Kleber, M.; Kandeler, E.; Kalbitz, K.; Scheu, S.; Eusterhues, K.; Leinweber, P. Organo-mineral associations in temperate soils: Integrating biology, mineralogy, and organic matter chemistry. J. Plant Nutr. Soil Sci. 2008, 171, 61–82. [Google Scholar] [CrossRef]
- Jones, D.D.; Morre, D.J. Golgi apparatus mediated polysaccharide secretion by outer root cap cells of Zea mays. II. Isolation and characterization of the secretory products. Z. Pflanzenphysiol. 1967, 56, 166–169. [Google Scholar]
- Liu, X.; Eusterhues, K.; Juergen, K.; Valerian, C.; Carmen, H.; Carsten, M.; Kirsten, K.; Kögel-Knabner, I.; Petra, R.; Popp, P.; et al. STXM and NanoSIMS Investigations on EPS Fractions before and after adsorption to Goethite. Env. Sci. Tech. 2013, 47, 3158–3166. [Google Scholar] [CrossRef] [PubMed]
- Morel, J.L.; Habib, L.; Plantureux, S.; Guckert, A. Influence of maize root mucilage on soil aggregate stability. Plant Soil 1991, 191, 111–119. [Google Scholar] [CrossRef]
- Agnihotri, R.; Sharma, M.P.; Prakash, A.; Ramesh, A.; Bhattacharjya, S.; Patra, A.K.; Mann, M.C.; Kurganova, I.; Yakov, Y. Glycoproteins of arbuscular mycorrhiza for soil carbon sequestration: Review of mechanisms and controls. Sci. Tot. Environ. 2022, 806, 150571. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Omoike, A.; Chorover, J. Adsorption to goethite of extracellular polymeric substances from Bacillus subtilis. Geochim. Cosmochim. Acta 2006, 70, 827–838. [Google Scholar] [CrossRef]
- McGill, W.B.; Paul, E.A. Fractionation of soil and 15N nitrogen to separate the organic and clay interactions of immobilized N. Can. J. Soil Sci. 1976, 56, 203–212. [Google Scholar] [CrossRef]
- Skjemstad, J.O.; Spouncer, L.R.; Cowie, B.; Swift, R.S. Calibration of the Rothamsted organic carbon turnover model (RothC ver. 26.3), using measurable soil organic carbon pools. Aust. J. Soil Res. 2004, 42, 79–88. [Google Scholar] [CrossRef]
- Knicker, H. Solid state CPMAS 13C and 15N NMR spectroscopy in organic geochemistry and how spin dynamics can either aggravate or improve spectra interpretation. Org. Geochem. 2011, 42, 867890. [Google Scholar] [CrossRef]
- Knicker, H.; González-Vila, F.J.; González-Vasquerez, R. Biodegradability of organic matter in fire-affected mineral soils of Southern Spain. Soil Biol. Biochem. 2011, 56, 31–39. [Google Scholar] [CrossRef]
- Novotny, E.H.; Hayes, M.H.B.; deAevedo, E.R.; Bonagamba, T.J. Characterisation of black carbon-rich samples by 13C solid-state nuclear magnetic resonance. Naturwissenschaften 2006, 93, 447–450. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Wiedemeier, D.B.; Abiven, S.; Hockaday, W.C.; Keiluweitc, M.; Kleber, M.; Masiello, C.A.; McBeath, A.V.; Nico, P.S.; Pyle, L.A.; Schneider, M.P.W.; et al. Aromaticity and degree of aromatic condensation of char. Org. Geochem. 2015, 78, 135–143. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. Glob. Change Biol. Biochem. 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Brodowski, S.; Amelung, W.; Haumaier, L.; Abetz, C.; Zech, W. Morphological and chemical properties of black carbon in physical soil fractions as revealed by scanning electron microscopy and energy-dispersive X-ray spectroscopy. Geoderma 2005, 128, 116–129. [Google Scholar] [CrossRef]
- Brodowski, S.; John, B.; Flessa, H.; Amelung, W. Aggregate-occluded black carbon in soil. Eur. J. Soil Sci. 2006, 57, 539–546. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Dos Santos, J.V.; Koenig, A.; Hatcher, P.G. Interaction between peptide and solid lignin suggest mechanisms of abiotic covalent bond formation. Org. Geochem. 2024, 197, 104861. [Google Scholar] [CrossRef]
- Sparling, G.P.; Cheshire, M.V. Effect of periodate oxidation on the polysaccharide content and microaggregate stability of rhizosphere and non-rhizosphere soils. Plant Soil 1985, 88, 113–122. [Google Scholar] [CrossRef]
- Martin, J.P. Microorganisms and soil aggregation. I. Origin and nature of some of the aggregating substances. Soil Sci. 1945, 59, 163–174. [Google Scholar] [CrossRef]
- Martin, J.P. Microorganisms and soil aggregation. II. Influence of bacterial polysaccharides on soil structure. Soil Sci. 1946, 61, 157–166. [Google Scholar] [CrossRef]
- Waksman, S.A.; Martin, J.P. The role of microorganisms in the conservation of the soil. Science 1939, 90, 304–305. [Google Scholar] [CrossRef]
- Haworth, W.N.; Pinkard, F.W.; Stacey, M. Function of bacterial polysaccharides in soil. Nature 1946, 158, 836–837. [Google Scholar] [CrossRef]
- Geoghegan, M.J.; Brian, R.C. Influence of bacterial polysaccharides on aggregate formation in soils. Nature 1946, 158, 837. [Google Scholar] [CrossRef]
- Geoghegan, M.J.; Brian, R.C. Aggregate formation in soil. 2. Influence of various carbohydrates and proteins on aggregation of soil particles. Biochem. J. 1948, 43, 5–13. [Google Scholar] [CrossRef]
- McLaren, A.D. The adsorption and reactions of enzymes and proteins on kaolinite. J. Phys. Chem. 1954, 58, 129–137. [Google Scholar] [CrossRef]
- McLaren, A.D.; Peterson, G.H.; Barshad, I. The reactions of enzymes and proteins on clay minerals. IV. Kaolinite and montmorillonite. Soil Sci. Soc. Am. Proc. 1958, 22, 239–244. [Google Scholar] [CrossRef]
- McLaren, A.D.; Puktie, A.H.; Barshad, I. Isolation of humus with enzymatic activity from soil. Soil Sci. 1975, 119, 178–180. [Google Scholar] [CrossRef]
- Christensen, B.T. Physical fractionation of soil and structural and functional complexity in organic matter turnover. Eur. J. Soil Sci. 2001, 52, 345–353. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Bucka, F.B.; Felde, V.J.; Peth, S.; Kögel-Knabner, I. Disentangling the effects of OM quality and soil texture on microbially mediated structure formation in artificial model soils. Geoderma 2021, 403, 115213. [Google Scholar] [CrossRef]
- Page, E.R. Cellulose xanthate as a soil conditioner: Laboratory experiments. J. Sci. Food Agric. 1980, 31, 1–6. [Google Scholar] [CrossRef]
- Wan, J.; Tang, Z.; Liu, Y.; Xiao, H.; Wang, H. Study on the improvement of clay properties by xanthan gum and its application on ecological slope protection engineering. Environ. Technol. 2023, 45, 2762–2775. [Google Scholar] [CrossRef]
- Harrison, R. A Study of Some Montmorillonite-Organic Complexes. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 1982. [Google Scholar]
- Clapp, C.E.; Harrison, R.; Hayes, M.H.B. Interactions between organic macro-molecules and soil inorganic colloids and soils. In Interactions at the Soil Colloid-Soil Solution Interface; Bolt, G.H., De Boodt, M.F., Hayes, M.H.B., McBride, M.B., Eds.; Springer+Business Media: Dordrecht, The Netherlands, 1991; pp. 409–468. [Google Scholar]
- Clapp, C.E.; Olness, A.E.; Hoffmann, D.J. Adsorption studies of a dextran by montmorillonite. Trans. 9th Int. Congr. Soil Sci. 1968, 1, 627–637. [Google Scholar]
- Olness, A.E.; Clapp, C.E. Occurrence of collapsed and expanded crystals in montmorillonite dextran complexes. Clays Clay Miner. 1973, 21, 289–293. [Google Scholar] [CrossRef]
- Olness, A.E.; Clapp, C.E. Influence of polysaccharide structure on dextran adsorption by montmorillonite. Soil Biol. Biochem. 1975, 7, 113–118. [Google Scholar] [CrossRef]
- Walshire, L.A.; Zhang, H.; Nick, Z.H.; Breland, B.R.; Runge, K.A.; Han, F.X. Modification of Surface Properties of Clay Minerals with Exopolysaccharides from Rhizobium Tropici. ACS Earth Space Chem. 2024, 8, 137–147. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Parfitt, R.L.; Greenland, D.J. Adsorption of polysaccharides by montmorillonite. Soil Sci. Soc. Am. Proc. 1970, 34, 862–866. [Google Scholar] [CrossRef]
- Burchill, S.; Hayes, M.H.B.; Greenland, D.G. Adsorption. In The Chemistry of Soil Processes; Greenland, D.J., Hayes, M.H.B., Eds.; Wiley: Chichester, UK; New York, NY, USA, 1981; New India Publishing Agency: New Delhi, India, 2016; pp. 221–400. [Google Scholar]
- Parfitt, R.L. Adsorption of charged sugars by montmorillonite. Soil Sci. 1972, 113, 417–421. [Google Scholar] [CrossRef]
- Theng, B.K.G. Formation and Properties of Clay-Polymer Complexes; Developments in Soil Science 9; Elsevier: Amsterdam, The Netherlands, 1979; pp. 255–257. [Google Scholar]
- Moavad, H.; Guzev, V.S.; Babyeva, I.P.; Zuyagintsev, D.G. Adsorption of the extracellular polysaccharide of the yeast Lipomyces lipofer on kaolinite. Pochvovedenie 1974, 11, 9–84. [Google Scholar]
- Liu, J.; Wang, Z.; Hu, G.; Xue, J.; Bu, F.; Jing, M.; Song, Z.; Che, W. Cracking and erosion behaviors of sand–clay mixtures stabilized with microbial biopolymer and palm fiber. Sci. Total Environ. 2023, 905, 166991. [Google Scholar] [CrossRef]
- Henry, E.A.; Montarges-Pelletier, E.; Bihannic, I.; Caillet, C.; Ghanbaja, J.; Gley, R.; Waldvogel, Y.; Duval, J.F.L. Controlled assembly of heterogeneous aggregates of clay, iron hydr(oxydes) and polysaccharide: Effects of preparation conditions. Appl. Clay Sci. 2022, 216, 106340. [Google Scholar] [CrossRef]
- Swincer, G.D.; Oades, J.M.; Greenland, D.J. Studies on soil polysaccharides. I. The isolation of polysaccharides from soil. Aust. J. Soil Res. 1968, 6, 211–224. [Google Scholar] [CrossRef]
- Greenland, D.J.; Oades, J.M. Saccharides. In Soil Components; Gieseking, J.E., Ed.; Organic Components; Springer: Berlin, Germany, 1975; Volume 1, pp. 213–257. [Google Scholar]
- Cheshire, M.V. Nature and Origin of Carbohydrates in Soils; Academic Press: London, UK, 1979; pp. 81–120. [Google Scholar]
- Cheshire, M.V.; Hayes, M.H.B. Composition, origins structures, and reactivities of soil polysaccharides. In Soil Colloids and Their Associations in Aggregates; De Boodt, M.F., Hayes, M.H.B., Herbillon, A., Eds.; Plenum: New York, NY, USA, 1990; pp. 307–336. [Google Scholar]
- Häusler, M.J.; Hayes, M.H.B. Uses of the XAD-8 resin and acidified dimethylsulfoxide in studies of humic acids. In Humic Substances and Organic Matter in Soil and Water Environments: Characterization, Transformations and Interactions; Clapp, C.E., Hayes, M.H.B., Senesi, N., Griffith, S.M., Eds.; IHSS, University of Minnesota: St. Paul, MN, USA, 1996; pp. 25–32. [Google Scholar]
- Cheshire, M.V.; Dumat, C.; Fraser, A.R.; Hillier, S.; Staunton, S. The interaction between soil organic matter and soil clay minerals by selective removal and controlled addition of organic matter. Eur. J. Soil Sci. 2000, 51, 497–509. [Google Scholar] [CrossRef]
- Chaney, K.; Swift, R.S. The influence of organic matter on aggregate stability in some British soils. J. Soil Sci. 1984, 35, 223–230. [Google Scholar] [CrossRef]
- Chaney, K.; Swift, R.S. Studies on aggregate stability. II. The effect of humic substances on the stability of re-formed soil aggregates. J. Soil Sci. 1986, 37, 337–343. [Google Scholar]
- Chaney, K.; Swift, R.S. Studies on aggregate stability. I. Re-formation of soil aggregates. J. Soil Sci. 1986, 37, 329–335. [Google Scholar] [CrossRef]
- Clapp, C.E.; Hayes, M.H.B. Characterization of humic substances isolated from clay- and silt-sized fractions of a corn residue-amended agricultural soil. Soil Sci. 1999, 164, 899–913. [Google Scholar] [CrossRef]
- Mao, J.-D.; Schmidt-Rohr, K. Recoupled long-range C–H dipolar dephasing in solid-state NMR, and its use for spectral selection of fused aromatic rings. J. Magn. Reson. 2003, 162, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Dal Ferro, N.; Berti, A.; Francioso, O.; Ferrari, E.; Matthews, G.P.; Morari, F. Investigating the effects of wettability and pore size distribution on aggregate stability: The role of soil organic matter and the humic fraction. Eur. J. Soil Sci. 2012, 63, 152–164. [Google Scholar] [CrossRef]
- Wershaw, R.L.; Hayes, T.M. Solubilization of Anthropogenic Compounds by Humic Substances. In Humic Substances and Chemical Contaminants, Clapp, C.E., Hayes, M.H.B., Senesi, N., Bloom, P.R., Jardine, P.M. Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 2001; Chapter 8; pp. 165–176. [Google Scholar]
- Roulia, M.; Vassiliadis, A.A. Water Purification by Potassium Humate–C.I. Basic Blue 3 Adsorption-Based Interactions. Agronomy 2021, 11, 1625. [Google Scholar] [CrossRef]
- Song, G. Acquisition of Essential Data for Assessments of Carbon Sequestration by Soils. Ph.D. Thesis, University of Limerick, Limerick, Ireland, 2007. [Google Scholar]
- Lal, R. Soil carbon sequestration impact on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Lal, R. Societal value of soil carbon. J. Soil Water Conserv. 2014, 69, 86A–192A. [Google Scholar] [CrossRef]
- Thompson, M.; Gamage, D.; Hirotsu, N.; Martin, A.; Seneweera, S. Effects of Elevated Carbon Dioxide on Photosynthesis and Carbon Partitioning: A Perspective on Root Sugar Sensing and Hormonal Crosstalk. Front Physiol. 2017, 8, 578. [Google Scholar] [CrossRef]
- Maffia, A.; Oliva, M.; Marra, F.; Mallamaci, C.; Nardi, S.; Muscolo, A. Humic Substances: Bridging Ecology and Agriculture for a Greener Future. Agronomy 2025, 15, 410. [Google Scholar] [CrossRef]
- De Boodt, M.F. Applications of Polymeric Substances as Physical Soil Conditioners. In Soil Colloids and Their Associations in Aggregates; DeBoodt, M.F., Hayes, M.H.B., Herbillon, A., Eds.; Springer: New York, NY, USA, 1990; Chapter 19; pp. 517–556. [Google Scholar]
- Ash, H. Translation of De Re Rustica Published by LJM Columella; Harvard University Press: Cambridge, MA, USA, 1941. [Google Scholar]
- Piccolo, A.; Spaccini, R.; Cozzolino, V.; Nuzzo, A.; Drosos, M.; Zavattaro, L.; Grignani, C.; Puglisi, E.; Trevisan, M. Effective carbon sequestration in Italian agricultural soils by in situ polymerization of soil organic matter under biomimetic photocatalysis. Land Degrad. Dev. 2018, 29, 485–494. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayes, M.H.B.; Roulia, M. Relevance of Organic Matter Compositions, Structures and Associations to Soil Aggregates and to Sustainable Productivity. Agronomy 2025, 15, 2182. https://doi.org/10.3390/agronomy15092182
Hayes MHB, Roulia M. Relevance of Organic Matter Compositions, Structures and Associations to Soil Aggregates and to Sustainable Productivity. Agronomy. 2025; 15(9):2182. https://doi.org/10.3390/agronomy15092182
Chicago/Turabian StyleHayes, Michael H. B., and Maria Roulia. 2025. "Relevance of Organic Matter Compositions, Structures and Associations to Soil Aggregates and to Sustainable Productivity" Agronomy 15, no. 9: 2182. https://doi.org/10.3390/agronomy15092182
APA StyleHayes, M. H. B., & Roulia, M. (2025). Relevance of Organic Matter Compositions, Structures and Associations to Soil Aggregates and to Sustainable Productivity. Agronomy, 15(9), 2182. https://doi.org/10.3390/agronomy15092182




