Interlinked Temperature and Light Effects on Lettuce Photosynthesis and Transpiration: Insights from a Dynamic Whole-Plant Gas Exchange System
Abstract
1. Introduction
2. Materials and Methods
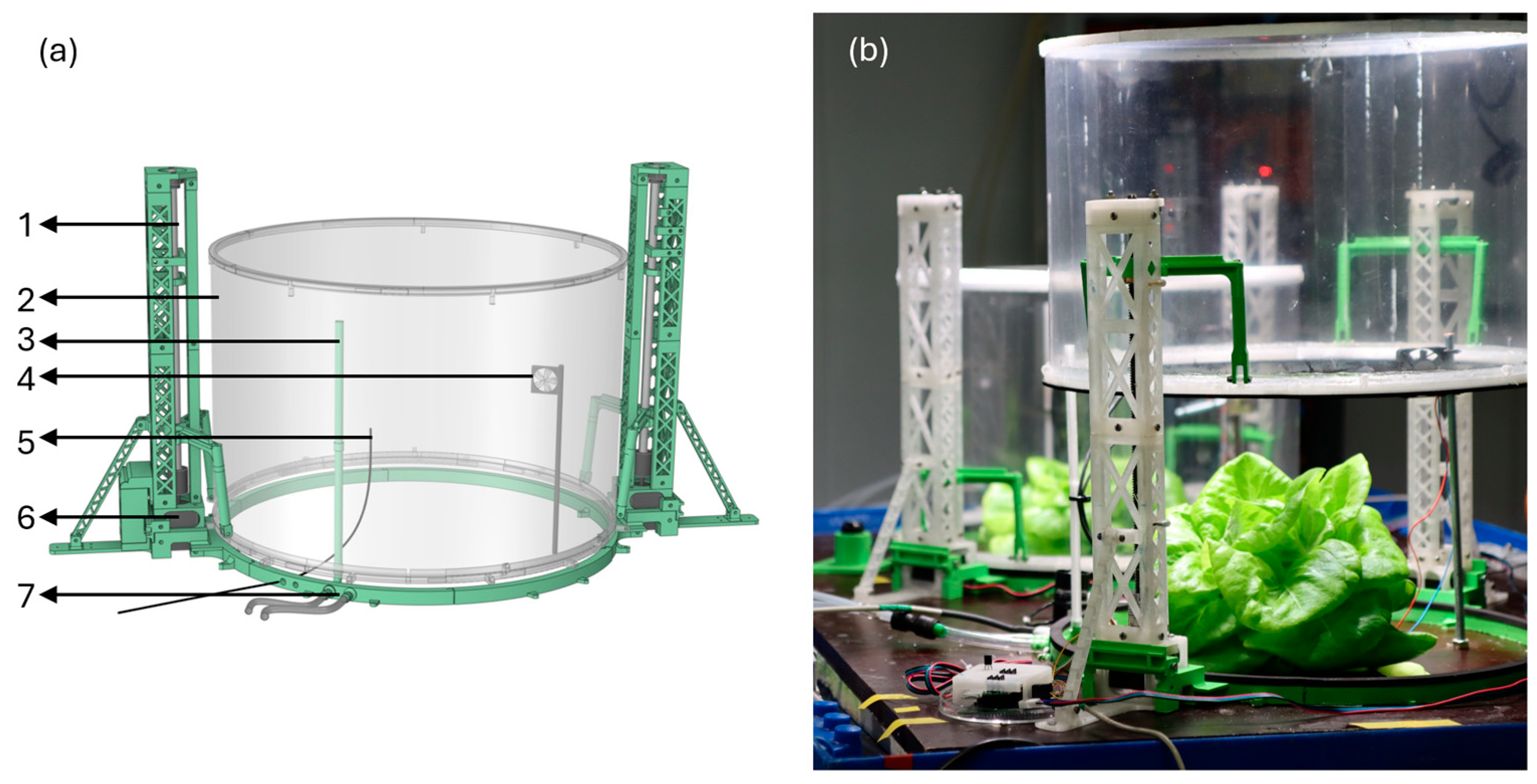
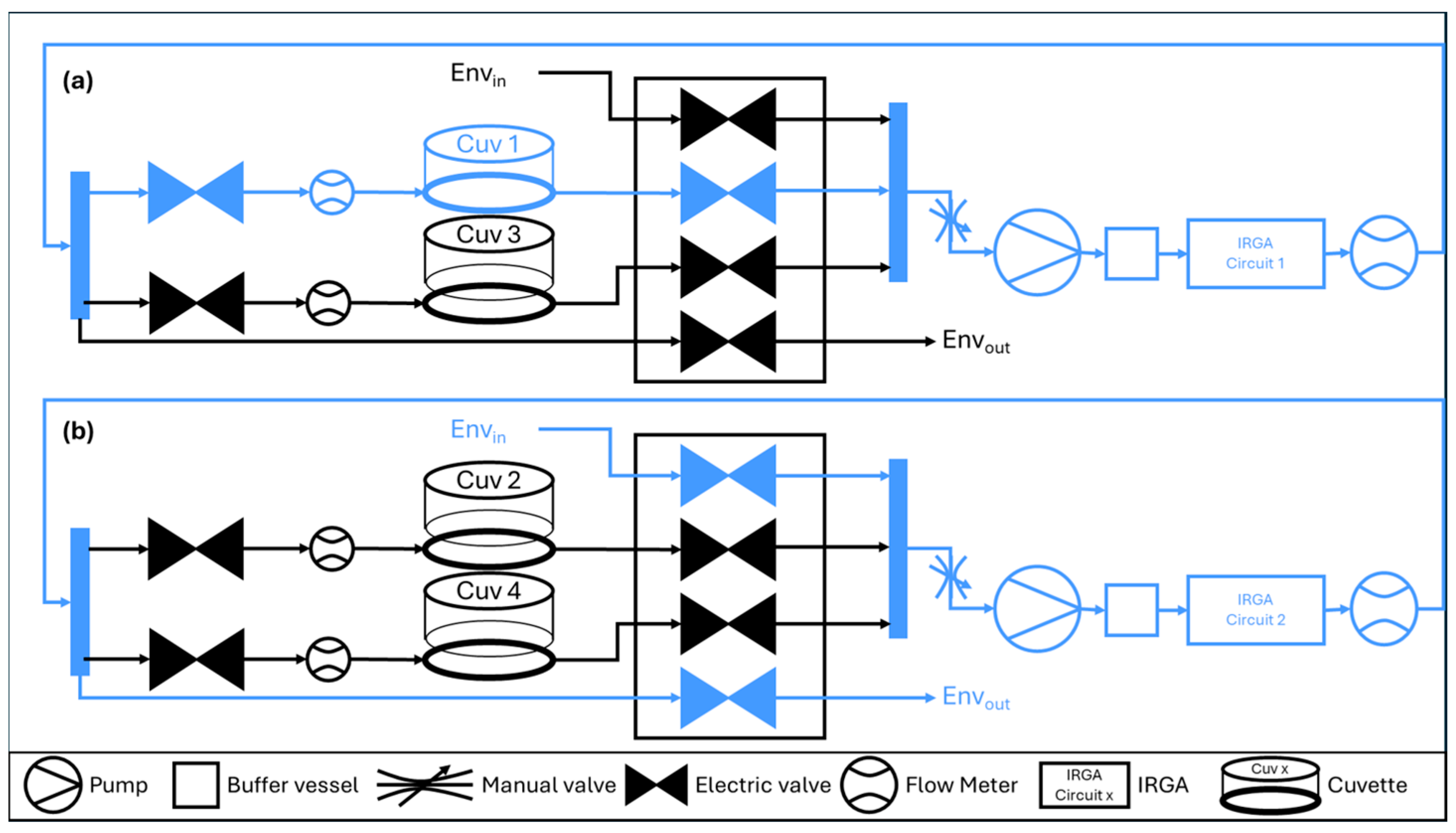
2.1. Dynamic Whole-Plant Gas Exchange System
2.2. Plant Material and Growing Conditions
2.3. Experimental Design
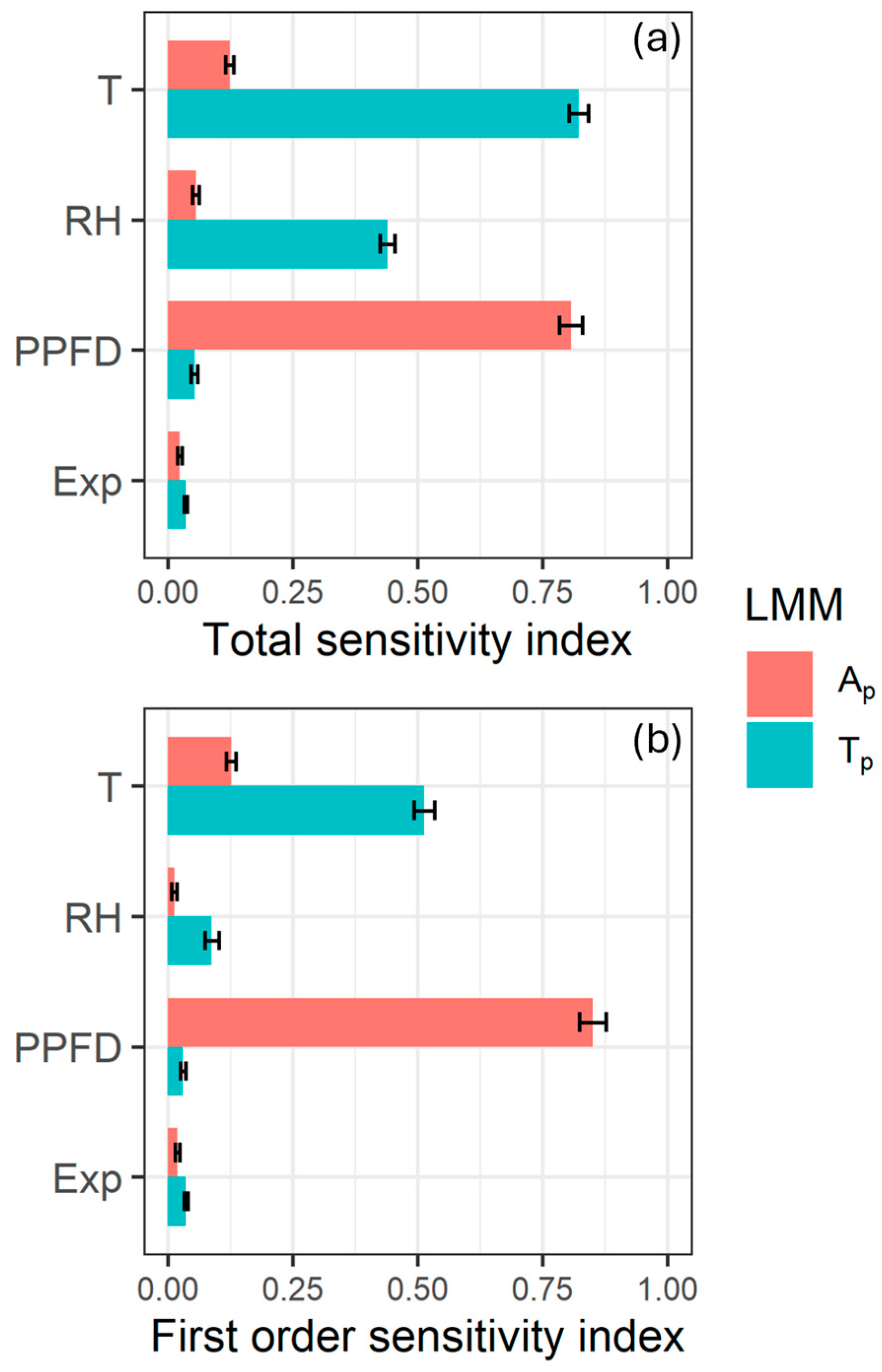
2.4. Data Processing and Statistical Analysis
3. Results
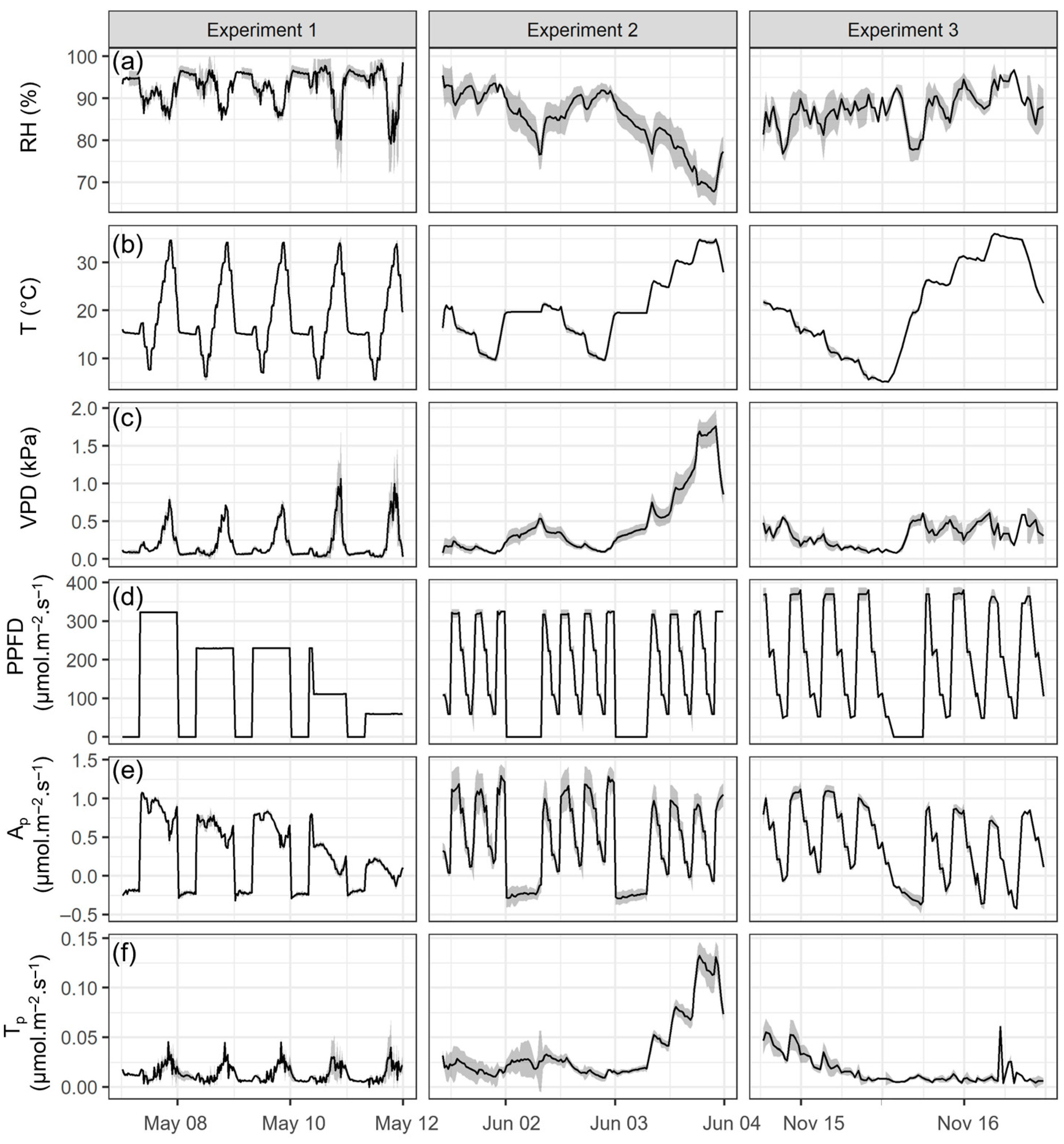
3.1. Environmental Data and Whole-Plant Photosynthesis and Transpiration Across Three Experiments
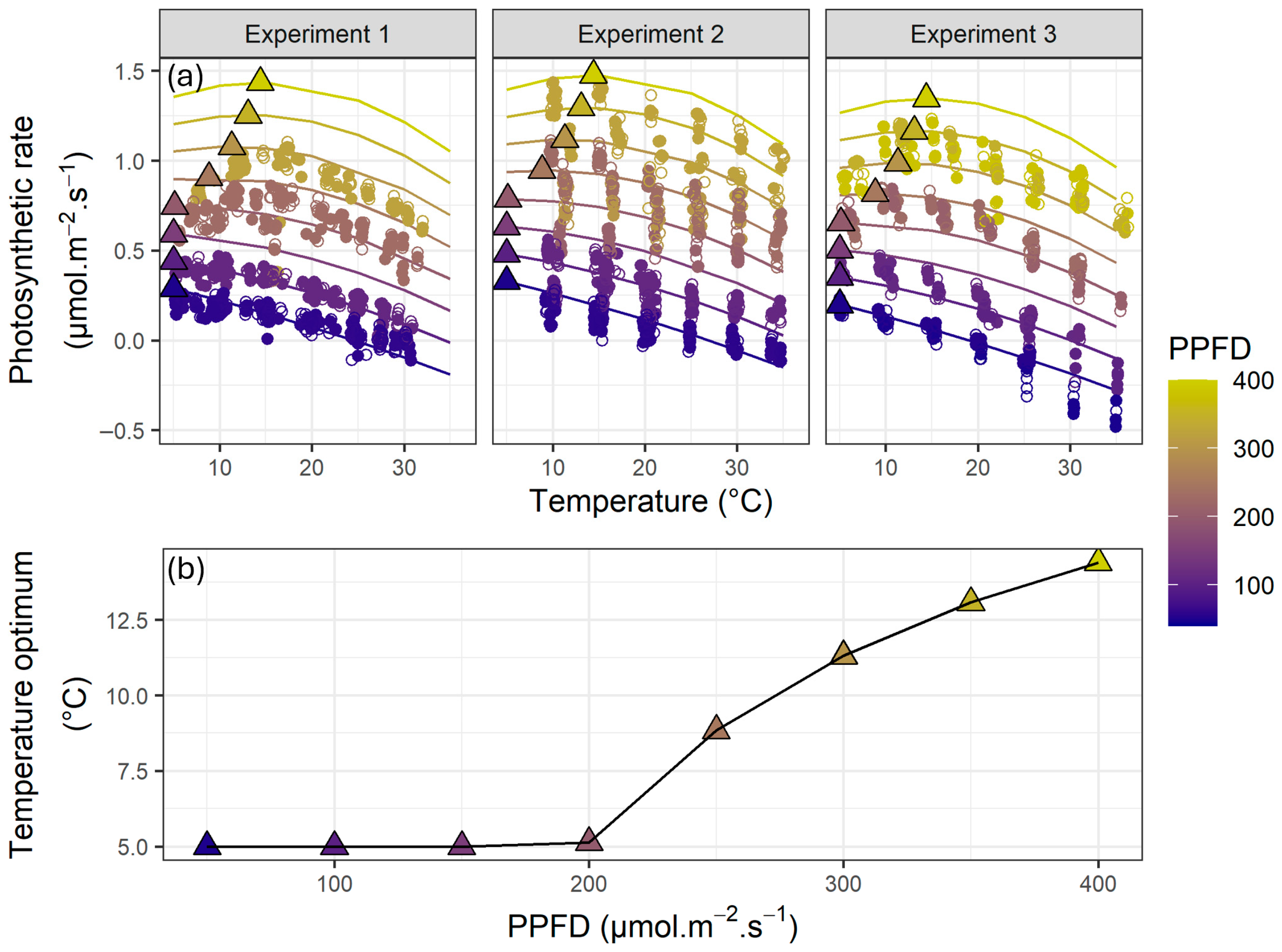
3.2. Whole-Plant Photosynthetic Rates Across Temperature, VPD and PPFD Levels
3.3. Whole-Plant Transpiration Rates Across VPD and PPFD Levels
4. Discussion
4.1. Optimizing Whole-Plant Photosynthesis in Lettuce by Balancing Temperature and Light Dynamics for More Energy-Efficient Production
4.2. The Decoupling of Whole-Plant Transpiration and Photosynthesis Under High Relative Humidity in Lettuce
4.3. Dynamic Closed Gas-Exchange Systems Enable Accurate Whole-Plant Response Analysis Under Prevailing Phylloclimate Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CEA | Closed environment agriculture |
| DynGES | Dynamic gas exchange system |
| IRGA | Infra-red gas analyzer |
| LMM | Linear mixed models |
| PPFD | Photosynthetic photon flux density |
| RH | Relative humidity |
| VPD | Vapour pressure deficit |
Appendix A
| Term | Coefficient | SE | p-Value | ||
|---|---|---|---|---|---|
| Fixed effects | Main effect | Intercept | 2.03 × 10−1 | 4.29 × 10−2 | * |
| T | −1.68 × 10−2 | 9.14 × 10−4 | *** | ||
| PPFD | 4.59 × 10−3 | 2.98 × 10−4 | *** | ||
| Interaction effect | PPFD: RH | −2.69 × 10−5 | 3.18 × 10−6 | *** | |
| PPFD: T | −1.07 × 10−4 | 1.19 × 10−5 | *** | ||
| PPFD: T2 | −2.26 × 10−6 | 2.68 × 10−7 | *** | ||
| Random effects | Experiment (Intercept) | 4.44 × 10−4 |
| Term | Coefficient | SE | p-Value | ||
|---|---|---|---|---|---|
| Fixed effects | Main effect | Intercept | −9.59 × 10−1 | 2.13 × 10−1 | *** |
| T | 4.73 × 10−2 | 6.84 × 10−3 | *** | ||
| PPFD | 4.74 × 10−4 | 5.41 × 10−5 | *** | ||
| RH | 1.93 × 10−2 | 4.73 × 10−3 | *** | ||
| RH2 | −9.60 × 10−5 | 2.62 × 10−5 | *** | ||
| Interaction effect | PPFD: RH | −5.18 × 10−6 | 6.20 × 10−7 | *** | |
| PPFD: T | −9.43 × 10−4 | 1.55 × 10−4 | *** | ||
| PPFD: T2 | 4.3 × 10−6 | 8.84 × 10−7 | *** | ||
| Random effects | Experiment (Intercept) | 4.96 × 10−5 |
References
- Despommier, D.D. The Vertical Farm: Feeding the World in the 21st Century, 1st ed.; Picador: New York, NY, USA, 2011; ISBN 978-0-312-61069-2. [Google Scholar]
- Benke, K.; Tomkins, B. Future Food-Production Systems: Vertical Farming and Controlled-Environment Agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Van Gerrewey, T.; Boon, N.; Geelen, D. Vertical Farming: The Only Way Is Up? Agronomy 2022, 12, 2. [Google Scholar] [CrossRef]
- Vatistas, C.; Avgoustaki, D.D.; Bartzanas, T. A Systematic Literature Review on Controlled-Environment Agriculture: How Vertical Farms and Greenhouses Can Influence the Sustainability and Footprint of Urban Microclimate with Local Food Production. Atmosphere 2022, 13, 1258. [Google Scholar] [CrossRef]
- Graamans, L.; Baeza, E.; van den Dobbelsteen, A.; Tsafaras, I.; Stanghellini, C. Plant Factories versus Greenhouses: Comparison of Resource Use Efficiency. Agric. Syst. 2018, 160, 31–43. [Google Scholar] [CrossRef]
- Tuomisto, H.L. Vertical Farming and Cultured Meat: Immature Technologies for Urgent Problems. One Earth 2019, 1, 275–277. [Google Scholar] [CrossRef]
- Blom, T.; Jenkins, A.; Pulselli, R.M.; van den Dobbelsteen, A.A.J.F. The Embodied Carbon Emissions of Lettuce Production in Vertical Farming, Greenhouse Horticulture, and Open-Field Farming in the Netherlands. J. Clean. Prod. 2022, 377, 134443. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature Extremes: Effect on Plant Growth and Development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Ahamed, M.S.; Sultan, M.; Monfet, D.; Rahman, M.S.; Zhang, Y.; Zahid, A.; Bilal, M.; Ahsan, T.M.A.; Achour, Y. A Critical Review on Efficient Thermal Environment Controls in Indoor Vertical Farming. J. Clean. Prod. 2023, 425, 138923. [Google Scholar] [CrossRef]
- Talbot, M.-H.; Monfet, D. Analysing the Influence of Growing Conditions on Both Energy Load and Crop Yield of a Controlled Environment Agriculture Space. Appl. Energy 2024, 368, 123406. [Google Scholar] [CrossRef]
- Choi, K.Y.; Paek, K.Y.; Lee, Y.B. Effect of Air Temperature on Tipburn Incidence of Butterhead and Leaf Lettuce in a Plant Factory. In Transplant Production in the 21st Century; Kubota, C., Chun, C., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 166–171. ISBN 978-90-481-5570-5. [Google Scholar]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of Environment Lighting on the Growth, Photosynthesis, and Quality of Hydroponic Lettuce in a Plant Factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Frantz, J.M.; Ritchie, G.; Cometti, N.N.; Robinson, J.; Bugbee, B. Exploring the Limits of Crop Productivity: Beyond the Limits of Tipburn in Lettuce. J. Am. Soc. Hortic. Sci. 2004, 129, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, H.; Mozafari, V.; Roosta, H.R.; Shirani, H.; Farhangi, M. Optimizing Growth Conditions in Vertical Farming: Enhancing Lettuce and Basil Cultivation through the Application of the Taguchi Method. Sci. Rep. 2023, 13, 6717. [Google Scholar] [CrossRef]
- Lange, O.L.; Geiger, I.L.; Schulze, E.-D. Ecophysiological Investigations on Lichens of the Negev Desert. V. A Model to Simulate Net Photosynthesis and Respiration of Ramalina Maciformis. Oecologia 1977, 28, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Von Caemmerer, S.; Berry, J.A. A Biochemical Model of Photosynthetic CO2 Assimilation in Leaves of C3 Species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Cannell, M.; Thornley, J.H.M. Temperature and CO2 Responses of Leaf and Canopy Photosynthesis: A Clarification Using the Non-Rectangular Hyperbola Model of Photosynthesis. Ann. Bot. 1998, 82, 883–892. [Google Scholar] [CrossRef]
- Körner, O.; Heuvelink, E.; Niu, Q. Quantification of Temperature, CO2, and Light Effects on Crop Photosynthesis as a Basis for Model-Based Greenhouse Climate Control. J. Hortic. Sci. Biotechnol. 2009, 84, 233–239. [Google Scholar] [CrossRef]
- Yamori, W.; Evans, J.R.; Von Caemmerer, S. Effects of Growth and Measurement Light Intensities on Temperature Dependence of CO2 Assimilation Rate in Tobacco Leaves. Plant Cell Environ. 2010, 33, 332–343. [Google Scholar] [CrossRef]
- Schulze, E.-D.; Beck, E.; Buchmann, N.; Clemens, S.; Müller-Hohenstein, K.; Scherer-Lorenzen, M. Plant Ecology; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 978-3-662-56231-4. [Google Scholar]
- Kim, S.H.; Lieth, J.H. A Coupled Model of Photosynthesis, Stomatal Conductance and Transpiration for a Rose Leaf (Rosa hybrida L.). Ann. Bot. 2003, 91, 771–781. [Google Scholar] [CrossRef]
- Decoteau, D.R. Vegetable Crops; Prentice Hall: Upper Saddle River, NJ, USA, 2000; ISBN 978-0-13-956996-8. [Google Scholar]
- Carotti, L.; Graamans, L.; Puksic, F.; Butturini, M.; Meinen, E.; Heuvelink, E.; Stanghellini, C. Plant Factories Are Heating Up: Hunting for the Best Combination of Light Intensity, Air Temperature and Root-Zone Temperature in Lettuce Production. Front. Plant Sci. 2021, 11, 592171. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal Size, Speed, and Responsiveness Impact on Photosynthesis and Water Use Efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Carvalho, D.R.A.; Torre, S.; Kraniotis, D.; Almeida, D.P.F.; Heuvelink, E.; Carvalho, S.M.P. Elevated Air Movement Enhances Stomatal Sensitivity to Abscisic Acid in Leaves Developed at High Relative Air Humidity. Front. Plant Sci. 2015, 6, 383. [Google Scholar] [CrossRef]
- Naranjani, B.; Najafianashrafi, Z.; Pascual, C.; Agulto, I.; Chuang, P.-Y.A. Computational Analysis of the Environment in an Indoor Vertical Farming System. Int. J. Heat Mass Transf. 2022, 186, 122460. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Yu-Xin, T.; Qi-Chang, Y. Optimal Control of Environmental Conditions Affecting Lettuce Plant Growth in a Controlled Environment with Artificial Lighting: A Review. S. Afr. J. Bot. 2020, 130, 75–89. [Google Scholar] [CrossRef]
- Shamshiri, R.R.; Jones, J.W.; Thorp, K.R.; Ahmad, D.; Man, H.C.; Taheri, S. Review of Optimum Temperature, Humidity, and Vapour Pressure Deficit for Microclimate Evaluation and Control in Greenhouse Cultivation of Tomato: A Review. Int. Agrophysics 2018, 32, 287–302. [Google Scholar] [CrossRef]
- Graamans, L.; van den Dobbelsteen, A.; Meinen, E.; Stanghellini, C. Plant Factories; Crop Transpiration and Energy Balance. Agric. Syst. 2017, 153, 138–147. [Google Scholar] [CrossRef]
- Bugbee, B. Steady-State Canopy Gas Exchange: System Design and Operation. HortSci 1992, 27, 770–776. [Google Scholar] [CrossRef]
- Takahashi, N.; Ling, P.P.; Frantz, J.M. Considerations for Accurate Whole Plant Photosynthesis Measurement. Environ. Control Biol. 2008, 46, 91–101. [Google Scholar] [CrossRef]
- Kölling, K.; George, G.M.; Künzli, R.; Flütsch, P.; Zeeman, S.C. A Whole-Plant Chamber System for Parallel Gas Exchange Measurements of Arabidopsis and Other Herbaceous Species. Plant Methods 2015, 11, 48. [Google Scholar] [CrossRef]
- Ferraz, T.M.; Rodrigues, W.P.; Netto, A.T.; De Oliveira Reis, F.; Peçanha, A.L.; De Assis Figueiredo, F.A.M.M.; De Sousa, E.F.; Glenn, D.M.; Campostrini, E. Comparison between Single-Leaf and Whole-Canopy Gas Exchange Measurements in Papaya (Carica papaya L.) Plants. Sci. Hortic. 2016, 209, 73–78. [Google Scholar] [CrossRef]
- Impron, I.; Hemming, S.; Bot, G.P.A. Simple Greenhouse Climate Model as a Design Tool for Greenhouses in Tropical Lowland. Biosyst. Eng. 2007, 98, 79–89. [Google Scholar] [CrossRef]
- Vanthoor, B.H.E.; Stanghellini, C.; van Henten, E.J.; Visser, P.H.B. de A Methodology for Model-Based Greenhouse Design: Part 1, a Greenhouse Climate Model for a Broad Range of Designs and Climates. Biosyst. Eng. 2011, 110, 363–377. [Google Scholar] [CrossRef]
- Defraeye, T.; Derome, D.; Verboven, P.; Carmeliet, J.; Nicolai, B. Cross-Scale Modelling of Transpiration from Stomata via the Leaf Boundary Layer. Ann. Bot. 2014, 114, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Plas, W.; Demeester, T.; Ahmed, Z.Y.; Coussement, J.R.; Steppe, K.; De Paepe, M. A Detailed Plant Model in CFD That Resolves the Microclimate around Individual Leaves. Biosyst. Eng. 2025, 254, 104144. [Google Scholar] [CrossRef]
- Gu, X.; Goto, E. Evaluation of Plant Canopy Microclimates with Realistic Plants in Plant Factories with Artificial Light Using a Computational Fluid Dynamics Model. Build. Environ. 2024, 264, 111876. [Google Scholar] [CrossRef]
- Van Iersel, M.W.; Bugbee, B. A Multiple Chamber, Semicontinuous, Crop Carbon Dioxide Exchange System: Design, Calibration, and Data Interpretation. J. Am. Soc. Hortic. Sci. 2000, 125, 86–92. [Google Scholar] [CrossRef]
- Frantz, J.M.; Bugbee, B. Acclimation of Plant Populations to Shade: Photosynthesis, Respiration, and Carbon Use Efficiency. J. Am. Soc. Hortic. Sci. 2005, 130, 918–927. [Google Scholar] [CrossRef]
- Zhen, S.; Bugbee, B. Far-red Photons Have Equivalent Efficiency to Traditional Photosynthetic Photons: Implications for Redefining Photosynthetically Active Radiation. Plant Cell Environ. 2020, 43, 1259–1272. [Google Scholar] [CrossRef]
- Liu, J.; Van Iersel, M.W. Far-Red Photons Increase Light Capture but Have Lower Photosynthetic Capacity Than Red Photons. J. Amer. Soc. Hort. Sci. 2023, 148, 253–265. [Google Scholar] [CrossRef]
- Song, Q.; Xiao, H.; Xiao, X.; Zhu, X.-G. A New Canopy Photosynthesis and Transpiration Measurement System (CAPTS) for Canopy Gas Exchange Research. Agric. For. Meteorol. 2016, 217, 101–107. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- R Core Team. R, version 2023.12.0.369; A Language and Environment for Statistical Computing; Posit Software, PBC: Boston, MA, USA, 2023.
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Soft. 2015, 67. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Soft. 2017, 82. [Google Scholar] [CrossRef]
- Caporn, S.J.M.; Hand, D.W.; Mansfield, T.A.; Wellburn, A.R. Canopy Photosynthesis of CO2-Enriched Lettuce (Lactuca sativa L.). Response to Short-Term Changes in CO2, Temperature and Oxides of Nitrogen. New Phytol. 1994, 126, 45–52. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J. Effects of Light Intensity and Temperature on the Photosynthesis Characteristics and Yield of Lettuce. Horticulturae 2022, 8, 178. [Google Scholar] [CrossRef]
- Iooss, B.; Da Veiga, S.; Janon, A.; Pujol, G. Sensitivity, version 1.30.0; Software for Global Sensitivity Analysis of Model Outputs and Importance Measures, R 2024; Microsoft: Washington, WA, USA, 2024.
- Sobol’, I.M.; Tarantola, S.; Gatelli, D.; Kucherenko, S.S.; Mauntz, W. Estimating the Approximation Error When Fixing Unessential Factors in Global Sensitivity Analysis. Reliab. Eng. Syst. Saf. 2007, 92, 957–960. [Google Scholar] [CrossRef]
- Locke, A.M.; Ort, D.R. Leaf Hydraulic Conductance Declines in Coordination with Photosynthesis, Transpiration and Leaf Water Status as Soybean Leaves Age Regardless of Soil Moisture. J. Exp. Bot. 2014, 65, 6617–6627. [Google Scholar] [CrossRef]
- Mayorga-Gomez, A.M.; Van Iersel, M.W.; Ferrarezi, R.S. Varying Light Intensities Affect Lettuce Growth and Physiology in Controlled Indoor Environments. Horticulturae 2024, 10, 931. [Google Scholar] [CrossRef]
- Gavhane, K.P.; Hasan, M.; Singh, D.K.; Kumar, S.N.; Sahoo, R.N.; Alam, W. Determination of Optimal Daily Light Integral (DLI) for Indoor Cultivation of Iceberg Lettuce in an Indigenous Vertical Hydroponic System. Sci. Rep. 2023, 13, 10923. [Google Scholar] [CrossRef]
- Albornoz, F.; Heinrich Lieth, J.; Gonzalez-Fuentes, J.A. Effect of Different Day and Night Nutrient Solution Concentrations on Growth, Photosynthesis, and Leaf NO3- Content of Aeroponically Grown Lettuce. Chil. J. Agric. Res. 2014, 74, 240–245. [Google Scholar] [CrossRef]
- Bunce, J.A. Does Transpiration Control Stomatal Responses to Water Vapour Pressure Deficit? Plant Cell Environ. 1997, 20, 131–135. [Google Scholar] [CrossRef]
- Kaiser, E.; Morales, A.; Harbinson, J.; Kromdijk, J.; Heuvelink, E.; Marcelis, L.F.M. Dynamic Photosynthesis in Different Environmental Conditions. J. Exp. Bot. 2015, 66, 2415–2426. [Google Scholar] [CrossRef]
- Merilo, E.; Jõesaar, I.; Brosché, M.; Kollist, H. To Open or to Close: Species-specific Stomatal Responses to Simultaneously Applied Opposing Environmental Factors. New Phytol. 2014, 202, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Ben-Asher, J.; Garcia Y Garcia, A.; Flitcroft, I.; Hoogenboom, G. Effect of Atmospheric Water Vapor on Photosynthesis, Transpiration and Canopy Conductance: A Case Study in Corn. Plant Soil Environ. 2013, 59, 549–555. [Google Scholar] [CrossRef]
- Brechner, M.; Both, A.J.; CEA Staff. Hydroponic Lettuce Handbook. Cornell Control. Environ. Agric. 1996, 834, 504–509. [Google Scholar]



| Species | Ca/PPFD | TOpt | Reference |
|---|---|---|---|
| Lichen | Ambient/20–400 | 4–20 | [15] |
| Tobacco-Leaves | 380/100–450 | 18–28 | [19] |
| Tomato-Canopy | 400/300–600 | 25–26 | [18] |
| Tomato-Leaves | 350/Light saturation | 22 | [17] |
| Lettuce-Leaves | 400/100–350 | 19–25 | Figure 1 |
| RH | TDay | VPDDay | TNight | VPDNight | |
|---|---|---|---|---|---|
| Experiment 1 | 82.5 ± 8.2 | 19.8 ± 0.5 | 0.43 ± 0.20 | 17.1 ± 0.7 | 0.30 ± 0. 14 |
| Experiment 2 | 83.4 ± 7.9 | 19.8 ± 0.5 | 0.41 ± 0.20 | 17.1 ± 0.7 | 0.28 ± 0.13 |
| Experiment 3 | 60.2 ± 5.3 | 18.9 ± 1.9 | 0.92 ± 0.19 | 15.9 ± 1.4 | 0.61 ± 0.11 |
| R2 | RMSE | NRMSE | ||||
|---|---|---|---|---|---|---|
| Ap | Tp | Ap | Tp | Ap | Tp | |
| Experiment 1 | 0.94 | 0.11 | 0.08 | 0.01 | 0.06 | 0.11 |
| Experiment 2 | 0.92 | 0.87 | 0.13 | 0.01 | 0.08 | 0.09 |
| Experiment 3 | 0.94 | 0.05 | 0.10 | 0.01 | 0.06 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauwers, S.; Coussement, J.R.; Steppe, K. Interlinked Temperature and Light Effects on Lettuce Photosynthesis and Transpiration: Insights from a Dynamic Whole-Plant Gas Exchange System. Agronomy 2025, 15, 2180. https://doi.org/10.3390/agronomy15092180
Lauwers S, Coussement JR, Steppe K. Interlinked Temperature and Light Effects on Lettuce Photosynthesis and Transpiration: Insights from a Dynamic Whole-Plant Gas Exchange System. Agronomy. 2025; 15(9):2180. https://doi.org/10.3390/agronomy15092180
Chicago/Turabian StyleLauwers, Simon, Jonas R. Coussement, and Kathy Steppe. 2025. "Interlinked Temperature and Light Effects on Lettuce Photosynthesis and Transpiration: Insights from a Dynamic Whole-Plant Gas Exchange System" Agronomy 15, no. 9: 2180. https://doi.org/10.3390/agronomy15092180
APA StyleLauwers, S., Coussement, J. R., & Steppe, K. (2025). Interlinked Temperature and Light Effects on Lettuce Photosynthesis and Transpiration: Insights from a Dynamic Whole-Plant Gas Exchange System. Agronomy, 15(9), 2180. https://doi.org/10.3390/agronomy15092180






