Preharvest Far-Red Light Affects Respiration Rate and Carbohydrate Status in Lettuce Grown in a Vertical Farm and Stored Under Modified Atmosphere Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Setup
2.2. Assessment of Plant Status at Harvest
2.2.1. Measurement of Biomass Accumulation and Processing of Fresh Lettuce
2.2.2. Photosynthetic Pigment Analysis
2.2.3. Vitamin C Analysis
2.3. Packaging and Storage Conditions
2.4. Evaluation of Postharvest Quality
2.4.1. Respiration Measurements
2.4.2. Sampling of the Packaged Lettuce
2.4.3. Sensory Quality Assessment
2.4.4. Soluble Sugars
2.4.5. Microbial Contamination
2.5. Statistical Analysis
3. Results
3.1. Plant Quality at Harvest
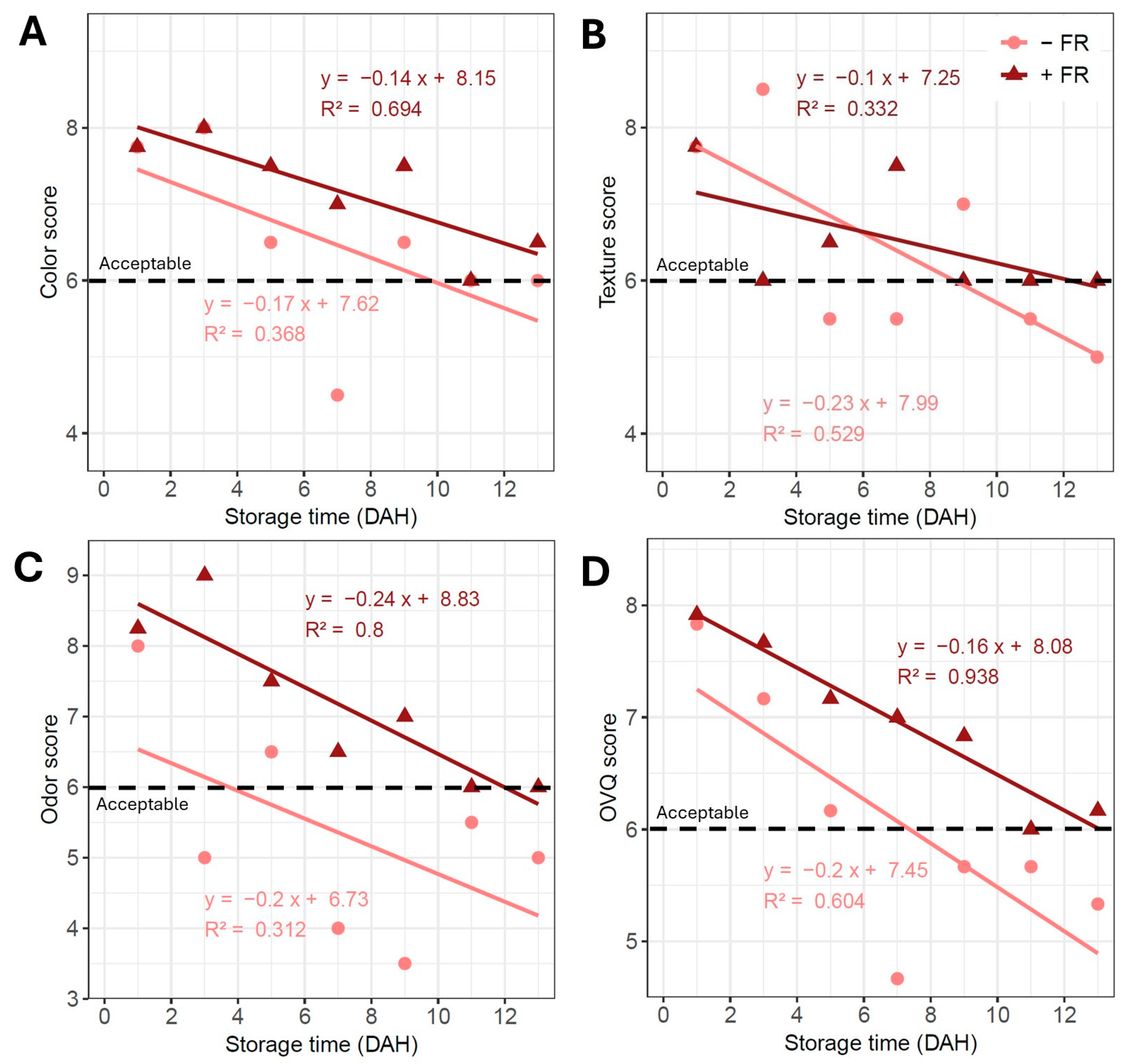
3.2. Overall Visual Quality
3.3. Respiration Rate
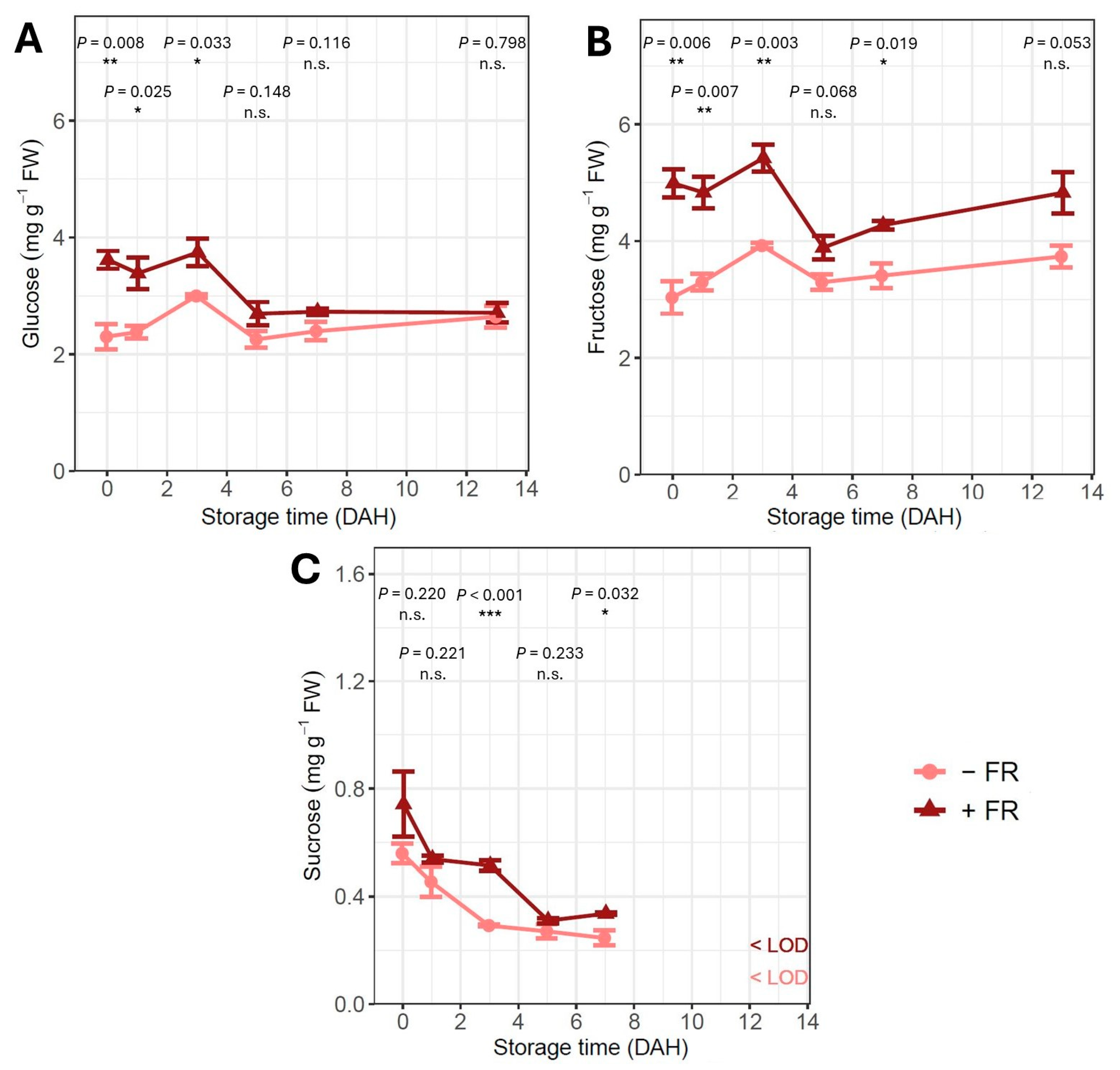
3.4. Soluble Sugar Content
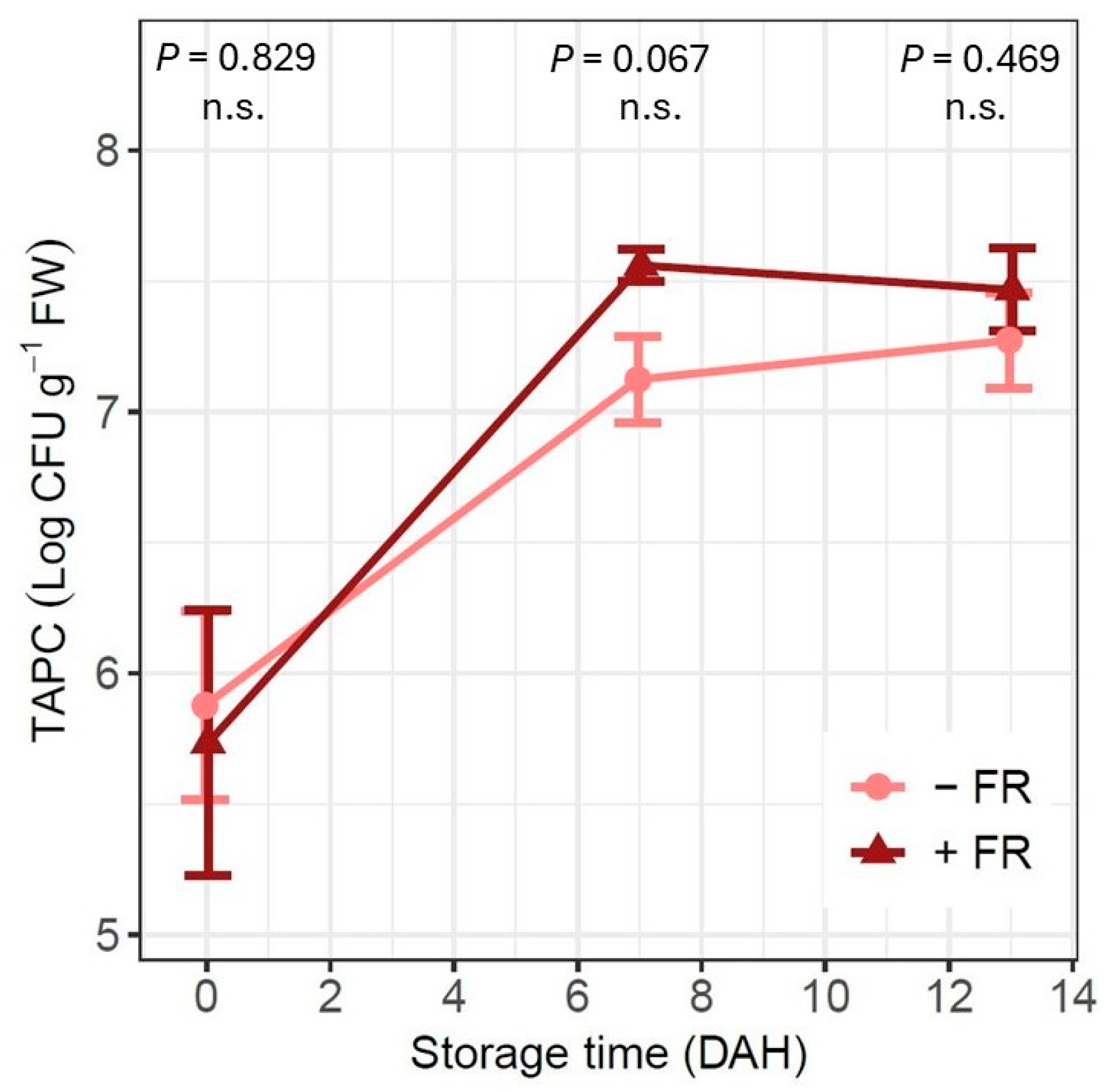
3.5. Microbial Contamination
4. Discussion
4.1. Preharvest Far-Red Light Minimally Affects Plant Quality at Harvest
4.2. Limited Impact of Preharvest Far-Red Light on Visual Quality
4.3. Far-Red Light Addition Affects Carbohydrate Metabolism but Not Microbial Growth
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LED | Light-emitting diode |
| (E)MAP | (Equilibrium) modified atmosphere packaging |
| (e)PPFD | (Extended) photosynthetic photon flux density |
| EC | Electrical conductivity |
| PSS | Phytochrome photostationary state |
| FW | Fresh weight |
| DW | Dry weight |
| EDTA | Ethylenediaminetetraacetic acid |
| OPD | Orthophenylenediamine |
| DAH | Days after harvest |
| OVQ | Overall visual quality |
| UPLC | Ultra performance liquid chromatography |
| TAPC | Total aerobic psychrotrophic count |
| CFU | Colony forming units |
| LOD | Limit of detection |
References
- WayBeyond and Agritecture Consulting. 2021 Global CEA Census Report; WayBeyond: Auckland, New Zealand, 2021. [Google Scholar]
- Zhen, S.; van Iersel, M.W. Far-Red Light Is Needed for Efficient Photochemistry and Photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef]
- Zhen, S.; Bugbee, B. Far-red Photons Have Equivalent Efficiency to Traditional Photosynthetic Photons: Implications for Redefining Photosynthetically Active Radiation. Plant Cell Environ. 2020, 43, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Sheerin, D.J.; Hiltbrunner, A. Molecular Mechanisms and Ecological Function of Far-Red Light Signalling. Plant Cell Environ. 2017, 40, 2509–2529. [Google Scholar] [CrossRef]
- Sharrock, R.A. The Phytochrome Red/Far-Red Photoreceptor Superfamily. Genome Biol. 2008, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A. Shade Avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kubota, C. Effects of Supplemental Light Quality on Growth and Phytochemicals of Baby Leaf Lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Zou, J.; Fanourakis, D.; Tsaniklidis, G.; Cheng, R.; Yang, Q.; Li, T. Lettuce Growth, Morphology and Critical Leaf Trait Responses to Far-Red Light during Cultivation Are Low Fluence and Obey the Reciprocity Law. Sci. Hortic. 2021, 289, 110455. [Google Scholar] [CrossRef]
- van Brenk, J.B.; Courbier, S.; Kleijweg, C.L.; Verdonk, J.C.; Marcelis, L.F.M. Paradise by the Far-Red Light: Far-Red and Red:Blue Ratios Independently Affect Yield, Pigments, and Carbohydrate Production in Lettuce, Lactuca Sativa. Front. Plant Sci. 2024, 15, 1383100. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Far-Red Radiation Interacts with Relative and Absolute Blue and Red Photon Flux Densities to Regulate Growth, Morphology, and Pigmentation of Lettuce and Basil Seedlings. Sci. Hortic. 2019, 255, 269–280. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S.; Vose, J.M.; Volin, J.C.; Gresham, C.; Bowman, W.D. Relationships of Leaf Dark Respiration to Leaf Nitrogen, Specific Leaf Area and Leaf Life-Span: A Test across Biomes and Functional Groups. Oecologia 1998, 114, 471–482. [Google Scholar] [CrossRef]
- Van de Velde, E.; Steppe, K.; Van Labeke, M.-C. Leaf Morphology, Optical Characteristics and Phytochemical Traits of Butterhead Lettuce Affected by Increasing the Far-Red Photon Flux. Front. Plant Sci. 2023, 14, 1129335. [Google Scholar] [CrossRef]
- Liu, J.; van Iersel, M.W. Far-Red Light Effects on Lettuce Growth and Morphology in Indoor Production Are Cultivar Specific. Plants 2022, 11, 2714. [Google Scholar] [CrossRef]
- Li, Y.; Wu, L.; Jiang, H.; He, R.; Song, S.; Su, W.; Liu, H. Supplementary Far-Red and Blue Lights Influence the Biomass and Phytochemical Profiles of Two Lettuce Cultivars in Plant Factory. Molecules 2021, 26, 7405. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and Physiological Properties of Indoor Cultivated Lettuce in Response to Additional Far-Red Light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Ares, G.; Martínez, I.; Lareo, C.; Lema, P. Failure Criteria Based on Consumers’ Rejection to Determine the Sensory Shelf Life of Minimally Processed Lettuce. Postharvest Biol. Technol. 2008, 49, 255–259. [Google Scholar] [CrossRef]
- Ragaert, P.; Verbeke, W.; Devlieghere, F.; Debevere, J. Consumer Perception and Choice of Minimally Processed Vegetables and Packaged Fruits. Food Qual. Prefer. 2004, 15, 259–270. [Google Scholar] [CrossRef]
- Peng, H.; Simko, I. Extending Lettuce Shelf Life through Integrated Technologies. Curr. Opin. Biotechnol. 2023, 81, 102951. [Google Scholar] [CrossRef]
- Min, Q.; Marcelis, L.F.M.; Nicole, C.C.S.; Woltering, E.J. High Light Intensity Applied Shortly before Harvest Improves Lettuce Nutritional Quality and Extends the Shelf Life. Front. Plant Sci. 2021, 12, 615355. [Google Scholar] [CrossRef]
- Okawa, K. Market and Trade Impacts of Food Loss and Waste Reduction. In OECD Food, Agriculture and Fisheries Papers; OECD Publishing: Paris, France, 2015; Volume 75. [Google Scholar] [CrossRef]
- Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Basile, F.; Cannata, C.; Abdelkhalik, A.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.R. Postharvest Changes in the Nutritional Properties of Commercial and Traditional Lettuce Varieties in Relation with Overall Visual Quality. Agronomy 2022, 12, 403. [Google Scholar] [CrossRef]
- Granger, M.; Eck, P. Dietary Vitamin C in Human Health. In Advances in Food and Nutrition Research; Academic Press Inc: Cambridge, MA, USA, 2018; Volume 83, pp. 281–310. [Google Scholar]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional Value, Bioactive Compounds and Health Benefits of Lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The Effects of Red, Blue, and White Light-Emitting Diodes on the Growth, Development, and Edible Quality of Hydroponically Grown Lettuce (Lactuca sativa L. Var. Capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- O’Leary, B.M.; Plaxton, W.C. Plant Respiration. In Encyclopedia of Life Sciences; Wiley: Hoboken, NJ, USA, 2016; pp. 1–11. [Google Scholar]
- Agüero, M.V.; Barg, M.V.; Yommi, A.; Camelo, A.; Roura, S.I. Postharvest Changes in Water Status and Chlorophyll Content of Lettuce (Lactuca sativa L.) and Their Relationship with Overall Visual Quality. J. Food Sci. 2008, 73, S47–S55. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Fanourakis, D.; Tsaniklidis, G.; Woltering, E.J.; Cheng, R.; Li, T. Far-Red Radiation during Indoor Cultivation Reduces Lettuce Nutraceutical Quality and Shortens the Shelf-Life When Stored at Supra Optimal Temperatures. Postharvest Biol. Technol. 2023, 198, 112269. [Google Scholar] [CrossRef]
- Lonchamp, J.; Barry-Ryan, C.; Devereux, M. Identification of Volatile Quality Markers of Ready-to-Use Lettuce and Cabbage. Food Res. Int. 2009, 42, 1077–1086. [Google Scholar] [CrossRef]
- Beaudry, R.M. Responses of Horticultural Commodities to Low Oxygen: Limits to the Expanded Use of Modified Atmosphere Packaging. HortTechnology 2000, 10, 491–500. [Google Scholar] [CrossRef]
- Ragaert, P.; Devlieghere, F.; Devuyst, E.; Dewulf, J.; Van Langenhove, H.; Debevere, J. Volatile Metabolite Production of Spoilage Micro-Organisms on a Mixed-Lettuce Agar during Storage at 7 °C in Air and Low Oxygen Atmosphere. Int. J. Food Microbiol. 2006, 112, 162–170. [Google Scholar] [CrossRef]
- Paillart, M.J.M.; van der Vossen, J.M.B.M.; Levin, E.; Lommen, E.; Otma, E.C.; Snels, J.C.M.A.; Woltering, E.J. Bacterial Population Dynamics and Sensorial Quality Loss in Modified Atmosphere Packed Fresh-Cut Iceberg Lettuce. Postharvest Biol. Technol. 2017, 124, 91–99. [Google Scholar] [CrossRef]
- Ioannidis, A.G.; Kerckhof, F.M.; Riahi Drif, Y.; Vanderroost, M.; Boon, N.; Ragaert, P.; De Meulenaer, B.; Devlieghere, F. Characterization of Spoilage Markers in Modified Atmosphere Packaged Iceberg Lettuce. Int. J. Food Microbiol. 2018, 279, 1–13. [Google Scholar] [CrossRef]
- Lareo, C.; Ares, G.; Ferrando, L.; Lema, P.; GÁmbaro, A.; Soubes, M. Influence of Temperature on Shelf Life of Butterhead Lettuce Leaves under Passive Modified Atmosphere Packaging. J. Food Qual. 2009, 32, 240–261. [Google Scholar] [CrossRef]
- Woltering, E.J.; Paillart, M.J.M. Effect of Low Oxygen Modified- and Controlled-Atmospheres on Quality Attributes and Microbial Population Dynamics in Fresh-Cut Iceberg Lettuce. Acta Hortic. 2024, 1386, 169–173. [Google Scholar] [CrossRef]
- Ragaert, P.; Devlieghere, F.; Debevere, J. Role of Microbiological and Physiological Spoilage Mechanisms during Storage of Minimally Processed Vegetables. Postharvest Biol. Technol. 2007, 44, 185–194. [Google Scholar] [CrossRef]
- Woltering, E.J.; Witkowska, I.M. Effects of Pre-and Postharvest Lighting on Quality and Shelf Life of Fresh-Cut Lettuce. Acta Hortic. 2016, 1134, 357–365. [Google Scholar] [CrossRef]
- Kusuma, P.; Bugbee, B. Far-Red Fraction: An Improved Metric for Characterizing Phytochrome Effects on Morphology. J. Am. Soc. Hortic. Sci. 2021, 146, 3–13. [Google Scholar] [CrossRef]
- Holmes, M.G.; Smith, H. The Function of Phytochrome in the Natural Environment—Characterization of Daylight for Studies in Photomorphogenesis and Photoperiodism. Photochem. Photobiol. 1977, 25, 533–538. [Google Scholar] [CrossRef]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic Efficiency and Phytochrome Photoequilibria Determination Using Spectral Data. Am. Soc. Agric. Eng. 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Zapata, S.; Dufour, J.-P. Ascorbic, Dehydroascorbic and Isoascorbic Acid Simultaneous Determinations by Reverse Phase Ion Interaction HPLC. J. Food Sci. 1992, 57, 506–511. [Google Scholar] [CrossRef]
- Dodson, K.Y.; Young, E.R.; Soliman, A.-G.M. Determination of Total Vitamin C in Various Food Matrixes by Liquid Chromatography and Fluorescence Detection. J. AOAC Int. 1992, 75, 887–890. [Google Scholar] [CrossRef]
- Jacxsens, L.; Devlieghere, F.; De Rudder, T.; Debevere, J. Designing Equilibrium Modified Atmosphere Packages for Fresh-Cut Vegetables Subjected to Changes in Temperature. LWT 2000, 33, 178–187. [Google Scholar] [CrossRef]
- Zagory, D.; Kader, A.A. Modified Atmosphere Packaging of Fresh Produce. Food Technol. 1988, 42, 70–77. [Google Scholar]
- Belgian Official Gazette. Koninklijk Besluit van 13 Juli 2014 Betreffende Levensmiddelenhygiëne; Belgian Official Gazette: Brussels, Belgium, 2014. [Google Scholar]
- Kader, A.A.; Lipton, W.J.; Morris, L.L. Systems for Scoring Quality of Harvested Lettuce. HortScience 1973, 8, 408–409. [Google Scholar] [CrossRef]
- Christiaens, A.; De Keyser, E.; Lootens, P.; Pauwels, E.; Roldán-Ruiz, I.; De Riek, J.; Gobin, B.; Van Labeke, M.C. Cold Storage to Overcome Dormancy Affects the Carbohydrate Status and Photosynthetic Capacity of Rhododendron Simsii. Plant Biol. 2015, 17, 97–105. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Mickens, M.A.; Skoog, E.J.; Reese, L.E.; Barnwell, P.L.; Spencer, L.E.; Massa, G.D.; Wheeler, R.M. A Strategic Approach for Investigating Light Recipes for ‘Outredgeous’ Red Romaine Lettuce Using White and Monochromatic LEDs. Life Sci. Space Res. 2018, 19, 53–62. [Google Scholar] [CrossRef]
- Wong, C.E.; Teo, Z.W.N.; Shen, L.; Yu, H. Seeing the Lights for Leafy Greens in Indoor Vertical Farming. Trends Food Sci. Technol. 2020, 106, 48–63. [Google Scholar] [CrossRef]
- Evans, J.R.; Poorter, H. Photosynthetic Acclimation of Plants to Growth Irradiance: The Relative Importance of Specific Leaf Area and Nitrogen Partitioning in Maximizing Carbon Gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Chen, Y.; Li, T.; Yang, Q.; Zhang, Y.; Zou, J.; Bian, Z.; Wen, X. UVA Radiation Is Beneficial for Yield and Quality of Indoor Cultivated Lettuce. Front. Plant Sci. 2019, 10, 1563. [Google Scholar] [CrossRef] [PubMed]
- Rijk Zwaan KnoxTM. Available online: https://www.rijkzwaan.com/en/page/knox (accessed on 25 March 2025).
- Clarkson, G.J.J.; O’Byrne, E.E.; Rothwell, S.D.; Taylor, G. Identifying Traits to Improve Postharvest Processability in Baby Leaf Salad. Postharvest Biol. Technol. 2003, 30, 287–298. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Li, T.; Yang, Q.; Guo, W. Sugar Accumulation and Growth of Lettuce Exposed to Different Lighting Modes of Red and Blue LED Light. Sci. Rep. 2019, 9, 6926. [Google Scholar] [CrossRef]
- Kim, J. Sugar Metabolism as Input Signals and Fuel for Leaf Senescence. Genes Genom. 2019, 41, 737–746. [Google Scholar] [CrossRef]
- Jacxsens, L.; Devlieghere, F.; Debevere, J. Quality of Equilibrium Modified Atmosphere Packaged (EMAP) Fresh-Cut Vegetables. In Production Practices and Quality Assessment of Food Crops; Dris, R., Mohan Jain, S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; Volume 3, pp. 473–523. [Google Scholar]
- Hüve, K.; Bichele, I.; Ivanova, H.; Keerberg, O.; Pärnik, T.; Rasulov, B.; Tobias, M.; Niinemets, Ü. Temperature Responses of Dark Respiration in Relation to Leaf Sugar Concentration. Physiol. Plant 2012, 144, 320–334. [Google Scholar] [CrossRef]
- Chaix, E.; Broyart, B.; Couvert, O.; Guillaume, C.; Gontard, N.; Guillard, V. Mechanistic Model Coupling Gas Exchange Dynamics and Listeria Monocytogenes Growth in Modified Atmosphere Packaging of Non Respiring Food. Food Microbiol. 2015, 51, 192–205. [Google Scholar] [CrossRef]


| Treatment | Blue (µmol m−2 s−1) | Green (µmol m−2 s−1) | Red (µmol m−2 s−1) | Far-Red (µmol m−2 s−1) | PPFD (µmol m−2 s−1) | ePPFD (µmol m−2 s−1) | R/FR | PSS | FR Fraction |
|---|---|---|---|---|---|---|---|---|---|
| −FR | 21.6 ± 0.2 | 0.8 ± 0.0 | 169.7 ± 1.3 | 0.2 ± 0.0 | 192.1 ± 1.5 | 192.3 ± 1.5 | ∞ | 0.885 | 0.0 |
| +FR | 22.1 ± 0.2 | 1.4 ± 0.1 | 168.4 ± 1.6 | 51.5 ± 0.5 | 192.2 ± 1.9 | 238.8 ± 2.3 | 4.9 | 0.817 | 19.3 |
| Score | Description |
|---|---|
| 9—Excellent | Bright and typical natural color of leaf blade and petiole, no browning, firm and crispy with fresh grass-like smell. |
| 8—Very good | One slightly discolored or browning or pinking features are shown at the leaf cut edge or blade. Leaves are firm and crisp and with a fresh grass-like smell. |
| 7—Good | Few slightly discolored leaves and brown edges are allowed. Leaves still crisp, reduced fresh smell. |
| 6—Acceptable | The defined consumer acceptance threshold. Slightly discolored leaves and moderate brown edges are allowed. No unpleasant odor or texture decay. |
| 5—Mediocre | Some yellowing and browning of leaf blade, slightly brown petiole, darker brown cut edge, texture decay but still acceptable, slightly unpleasant odor emerged. |
| 4—Borderline | Obvious discoloration on leaf blades, browning of leaf blade and petiole, clearly mild soft in texture, unpleasant odor. |
| 3—Poor | Strong discoloration, browning of leaves, wilted texture, obvious unpleasant odor. |
| 2—Bad | Complete yellow or brown leaf, texture decay with liquid leakage, strong off-odor. |
| 1—Very bad | Complete discolored leaf, liquid leaking from leaf material, fermented smell. |
| Treatment | FW (g) | DW (g) | Chla (µg g−1 FW) | Chlb (µg g−1 FW) | Cx+c (µg g−1 FW) | Vitamin C (µg g−1 FW) |
|---|---|---|---|---|---|---|
| −FR | 107.08 ± 8.43 | 4.36 ± 0.33 | 260 ± 41 | 94 ± 13 | 70 ± 10 | 118 ± 10 |
| +FR | 113.53 ± 2.68 | 5.12 ± 0.16 | 193 ± 11 | 76 ± 3 | 59 ± 3 | 141 ±11 |
| p-value | 0.241 | 0.033 | 0.907 | 0.076 | 0.817 | 0.168 |
| Significance | n.s. | * | n.s. | n.s. | n.s. | n.s. |
| N | 6 | 6 | 3 | 3 | 3 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van de Velde, E.; Van Wilder, L.; Van Labeke, M.-C.; De Meulenaer, B.; Steppe, K.; Devlieghere, F.; Dhooghe, E. Preharvest Far-Red Light Affects Respiration Rate and Carbohydrate Status in Lettuce Grown in a Vertical Farm and Stored Under Modified Atmosphere Conditions. Agronomy 2025, 15, 1957. https://doi.org/10.3390/agronomy15081957
Van de Velde E, Van Wilder L, Van Labeke M-C, De Meulenaer B, Steppe K, Devlieghere F, Dhooghe E. Preharvest Far-Red Light Affects Respiration Rate and Carbohydrate Status in Lettuce Grown in a Vertical Farm and Stored Under Modified Atmosphere Conditions. Agronomy. 2025; 15(8):1957. https://doi.org/10.3390/agronomy15081957
Chicago/Turabian StyleVan de Velde, Ellen, Lauriane Van Wilder, Marie-Christine Van Labeke, Bruno De Meulenaer, Kathy Steppe, Frank Devlieghere, and Emmy Dhooghe. 2025. "Preharvest Far-Red Light Affects Respiration Rate and Carbohydrate Status in Lettuce Grown in a Vertical Farm and Stored Under Modified Atmosphere Conditions" Agronomy 15, no. 8: 1957. https://doi.org/10.3390/agronomy15081957
APA StyleVan de Velde, E., Van Wilder, L., Van Labeke, M.-C., De Meulenaer, B., Steppe, K., Devlieghere, F., & Dhooghe, E. (2025). Preharvest Far-Red Light Affects Respiration Rate and Carbohydrate Status in Lettuce Grown in a Vertical Farm and Stored Under Modified Atmosphere Conditions. Agronomy, 15(8), 1957. https://doi.org/10.3390/agronomy15081957






