Interaction Regulation Mechanism of Soil Organic Carbon Fraction and Greenhouse Gases by Organic and Inorganic Fertilization
Abstract
1. Introduction
2. Materials and Methods
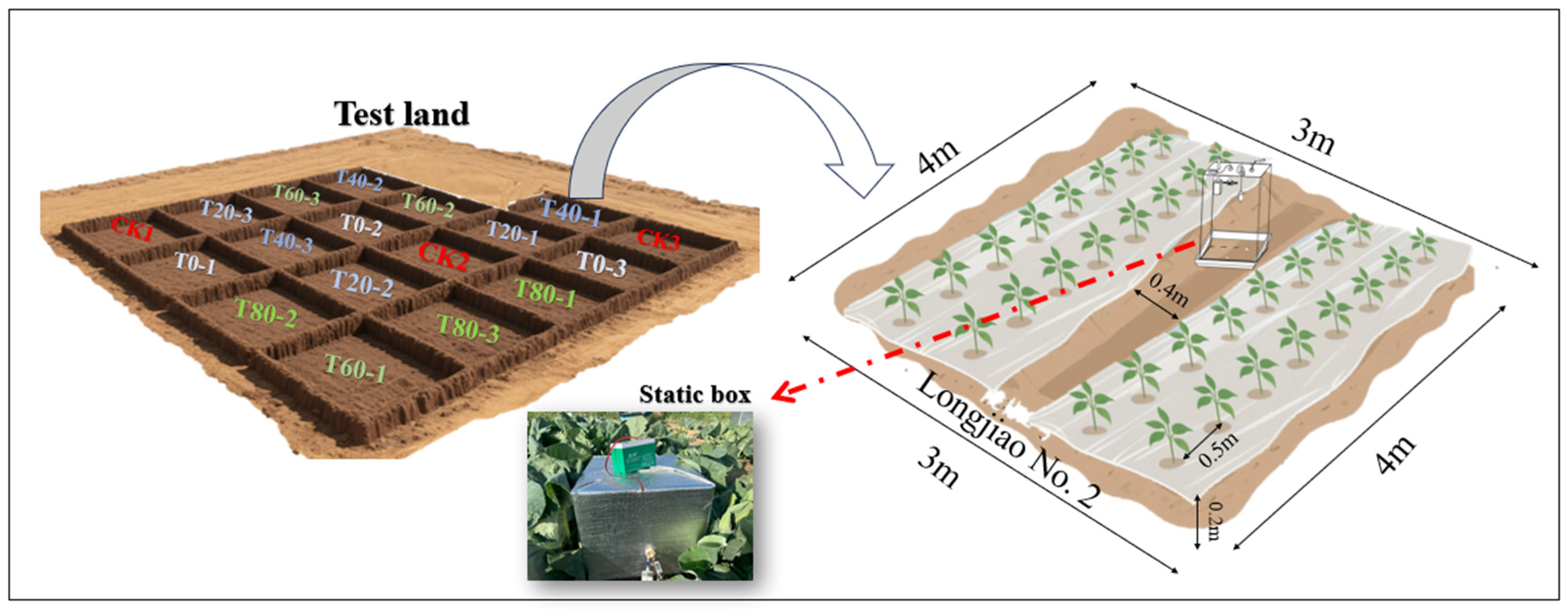
2.1. Study Sites
2.2. Experimental Design
2.3. Sample Collection and Measurement
2.3.1. Soil Sample Collection and Determination
2.3.2. Measurement of GHGS
2.4. Calculation Formulae
2.4.1. Calculation of Gas Samples
2.4.2. Calculation of the Carbon Pool Management Index
2.5. Data Analysis
3. Results
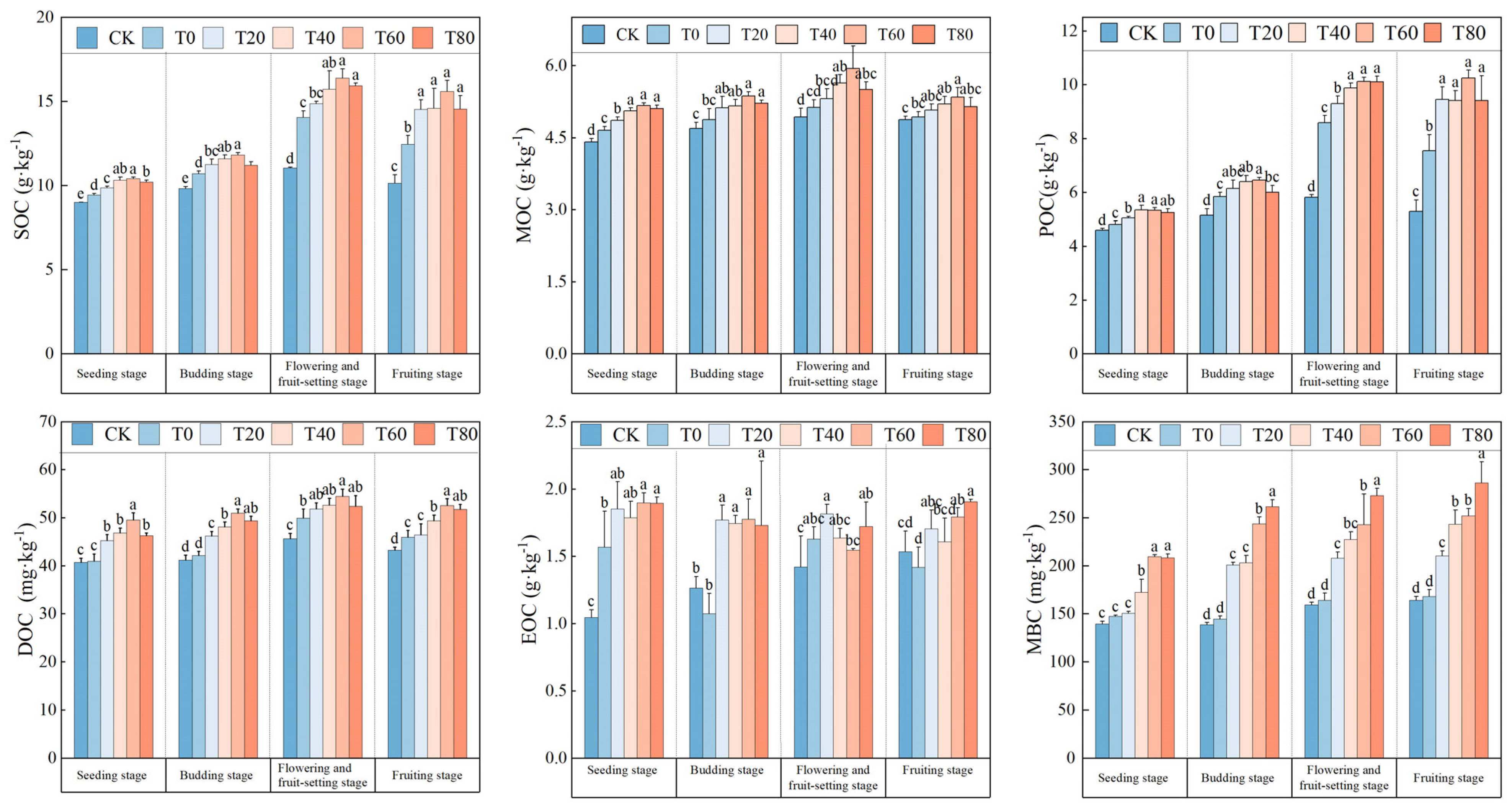
3.1. Effect of Fertilization Practices on Soil Total Organic Carbon and Its Fractions
3.2. Effect of Fertilization Practices on the Proportion of Soil Organic Carbon Fractions
3.3. Effect of Fertilization Practices on Soil Carbon Pool Management Indices
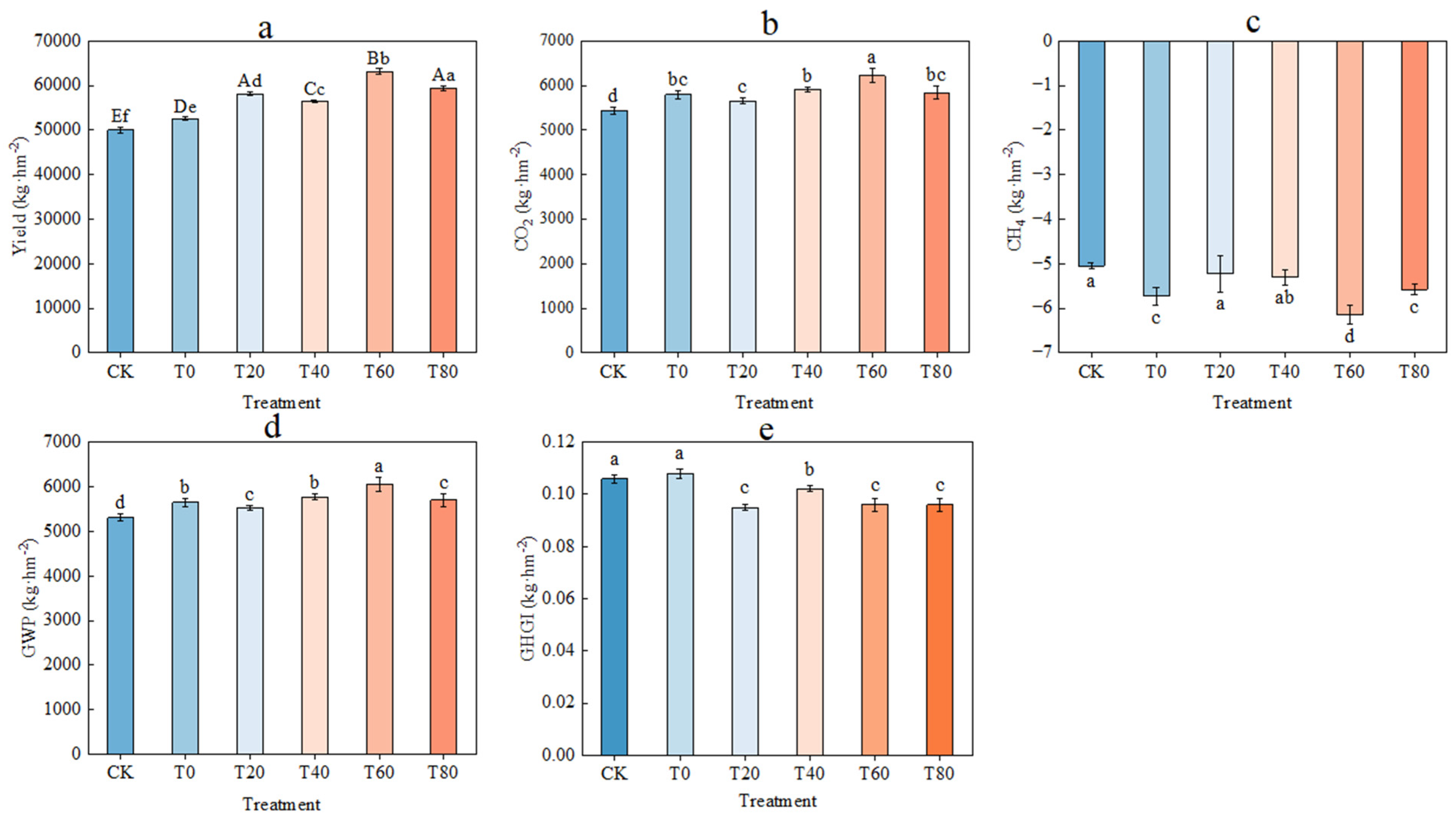
3.4. Effect of Fertilization Practices on Yield and Cumulative CH4 and CO2 Emissions
3.5. Correlation Analysis of Soil Organic Carbon Fractions and Nutrients with Cumulative CH4 and CO2 Emissions
4. Discussion
4.1. Effect of Fertilization Practices on the Dynamics of Soil Carbon Pools and Their Indices During the Reproductive Period
4.2. Effect of Fertilization Practices on Cumulative CO2 and CH4 Emissions and Warming Potential During the Reproductive Period
4.3. Mechanisms of Soil Nutrient–Carbon Component–Greenhouse Gas Interactions During the Reproductive Period
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, S.; Jiang, S.; Wang, L.; Rui, L.; Qi, Z.; Yu, J.; Zhou, Y. Effects of Supplemental Far-Red Light on Growth and Abiotic Stress Tolerance of Pepper Seedlings. J. Integr. Agric. 2022, 55, 1189–1198. [Google Scholar] [CrossRef]
- Wang, N.; Ai, Z.; Zhang, Q.; Leng, P.; Qiao, Y.; Li, Z.; Tian, C.; Cheng, H.; Li, F. Influence of long-term inorganic fertilization and straw incorporation on soil organic carbon: Roles of enzyme activity, labile organic carbon fractions, soil aggregates, and microbial traits. Agric. Ecosyst. Environ. 2025, 392, 109758. [Google Scholar] [CrossRef]
- Bai, Y.; Cotrufo, M. Grassland soil carbon sequestration: Current understanding, challenges, and solutions. Science 2022, 377, 603–608. [Google Scholar] [CrossRef]
- Georgiou, K.; Jackson, R.; Vaidišová, O.; Rose, Z.; Anders, A.; Wentling, F.; Jennifer, W.; Adam, F.; Polley, H.W.; Jennifer, L.; et al. Global stocks and capacity of mineral-associated soil organic carbon. Nat. Commun. 2022, 13, 3797. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Keenan, T.F. Tropical extreme droughts drive long-term increase in atmospheric CO2 growth rate variability. Nat. Commun. 2022, 13, 1193. [Google Scholar] [CrossRef]
- Berger, S.K.; Morales, R.C.; McCown, K.A.; Wilson, K.C.; Jobson, B.T.; Johnston, N. Analysis of Volatile Organic Compounds from Compost. Atmosphere 2025, 16, 591. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, K.; Tian, Y.; Li, Y.; Zhao, H.; Zhang, N. Greenhouse Gas Response to Simulated Precipitation Extremes in Alpine River Source Wetlands During the Growing Season. Atmosphere 2025, 16, 526. [Google Scholar] [CrossRef]
- Wang, H.; Hu, R.; Lin, S.; Gao, H.; Xu, M.; Zhang, W.; Wu, L. Effects of Long-term Application of Organic and Chemical Fertilizers on N2O Emissions from Black Soils. Huan Jing Ke Xue = Huanjing Kexue 2025, 46, 2621–2628. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mondal, B.; Linda, A.; Baruah, K.; Baruah, N. Enhancing Sustainability in Indian Rice–Wheat Agroecosystem: A Review and Analysis of Fertilizer Management Practices and Greenhouse Gas (N2O) Emission Mitigation. Water Air Soil Pollut. 2025, 236, 358. [Google Scholar] [CrossRef]
- Rahim, A.; Peng, Q.; Chen, H.; Liu, Y. The impact of carbon emissions from lag fertilization on wheat production. PLoS ONE 2024, 19, e0299299. [Google Scholar] [CrossRef]
- Zhang, C.; Song, X.; Zhang, Y.; Wang, D.; Rees, R.; Ju, X. Using nitrification inhibitors and deep placement to tackle the trade-offs between NH3 and N2O emissions in global croplands. Glob. Change Biol. 2022, 28, 4409–4422. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Tan, X.; Tan, X.; Zhu, J. County-level Agricultural Non-CO2 Greenhouse Gas Emissions and Scenario Simulation in Hunan Province, China. Chin. Geogr. Sci. 2025, 35, 914–928. [Google Scholar] [CrossRef]
- Eden, M.; Gerke, H.; Houot, S. Organic waste recycling in agriculture and related effects on soil water retention and plant available water: A review. Agron. Sustain. Dev. 2017, 37, 11. [Google Scholar] [CrossRef]
- Rampura, S.; Velayudhan Nair, K.; Radhakrishnan, D. Influence of fruit and vegetable waste substrates on the nutritional profile of black soldier fly (Hermetic ilicins) larvae and prepupa. Int. J. Trop. Insect Sci. 2025, 45, 433–445. [Google Scholar] [CrossRef]
- Huang, Y.; Mei, J.; Duan, E.; Zhu, Y.; Wu, Y. Effect and its mechanism of potassium persulfate on aerobic composting process of vegetable wastes. Environ. Sci. Pollut. Res. Int. 2024, 31, 7111–7121. [Google Scholar] [CrossRef]
- Muktamar, Z.; Sinaga, D.; Widiyono, H.; Gusmara, H.; Mucitro, B. Performance of Sweet Corn and Increasing Soil Total Nitrogen after the Application of Vegetable Waste-Based Liquid Organic Fertilizer in Coastal Entisols. Int. J. Plant Soil Sci. 2023, 35, 221–231. [Google Scholar] [CrossRef]
- Mirzaei, M.; Saunders, M.; Murphy, R.; Richards, K.; Mousavi, S.; Aghamir, F.; Horák, J.; Mancinelli, R.; Li, Y.; Radicetti, E. Integrating organic fertilizers in maize-mung bean intercropping: Implications for soil carbon dynamics and greenhouse gas reduction. Nutr. Cycl. Agroecosyst. 2025, 130, 197–211. [Google Scholar] [CrossRef]
- Liu, F.; Shen, X.; Gao, X.; Zhang, F.; Luo, X.; Liu, Y.; Yang, Y.; Yang, W.; Liang, T. An innovative integrated management strategy drives sustainable vegetable production in southwest China: Higher yield with reduced net GHG emissions. Eur. J. Agron. 2025, 169, 127703. [Google Scholar] [CrossRef]
- Prasad, A.; Sachan, H.; Krishna, D. Nutrient composition of Capsicum annuum L. fruits under integrated organic and inorganic fertilizer regimes. Vegetos 2025, 38, 1–7. [Google Scholar] [CrossRef]
- Eduah, J.; Arthur, A.; Attah, I.; Manso, E.; Quaye, A.; Dogbatse, J.; Padi, F. Differential impacts of organic and chemical fertilization on soil organic carbon pools and stability, and soil quality in cacao agroforestry. Soil Environ. Health 2025, 3, 100147. [Google Scholar] [CrossRef]
- Song, J.; Xu, W.; Song, J.; Bai, J.; Gao, G.; Zhang, Z.; Yu, Q.; Hao, J.; Ren, G.; Feng, Y.; et al. Straw return with fertilizer improves soil CO2 emissions by mitigating microbial nitrogen limitation during the winter wheat season. CATENA 2024, 241, 108050. [Google Scholar] [CrossRef]
- Bao, S. Soil Agrochemical Analysis; Agricultural Press: Beijing, China, 2000; pp. 30–34. [Google Scholar]
- Wu, J. Methods for the Determination of Soil Microbial Biomass and Its Application; Meteorological Press: Beijing, China, 2011. [Google Scholar]
- Zhang, J.; Song, C.; Wang, S. Dynamics of soil organic carbon and its fractions after abandonment of cultivated wetlands in northeast China. Soil Tillage Res. 2007, 96, 350–360. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Xu, M.; Huang, Q.; Luo, K. Response of red soil organic carbon and its particulate fractions to different fertilization patterns under long-term fertilization. J. Plant Nutr. Fertil. 2012, 18, 868–875. [Google Scholar]
- Wang, K.; Li, F.; Fang, Z.; Dong, Y.; Liu, J.; Huang, Z.; Luo, W. Soil CH4 emission and its relationship with organic carbon fraction under different irrigation methods and nitrogen rates. J. Agric. Environ. Sci. 2017, 36, 1012–1020. [Google Scholar]
- Dong, Y.; Huang, J.; Li, F.; Wang, K.; Fang, Z.; Liu, J.; Huang, Z.; Luo, W. Emissions of CH4 and N2Ounder different irrigation methods and nitrogen treatment. J. Plant Nutr. Fertil. 2017, 23, 578–588. [Google Scholar]
- Blair, G.; Lefroy, R.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Chen, Q.; Xie, J.; Li, L.; Khan, K.S.; Wang, L.; Chang, L.; Du, C. Partial substitution of chemical fertilizer with organic fertilizer: A promising circular economy approach for improvement soil physical and chemical properties and sustainable crop yields. Front. Plant Sci. 2025, 16, 1565081. [Google Scholar] [CrossRef]
- Wei, J.; Yang, S.; Wang, X.; Duan, J.; Mei, T.; Li, M.; Yang, S.; Wang, F. Effects of organic fertilizer replacing chemical fertilizer on organic carbon mineralization and active carbon fractions in yellow paddy soil of Guizhou Province. PLoS ONE 2025, 20, e0323801. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, P.; Angmo, P.; Satpute, S. Total and labile pools of organic carbon in relation to soil biological properties under contrasting land-use systems in a dry mountainous region. Carbon Manag. 2022, 13, 352–371. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, M. Effect of bio-organic fertilizers partially substituting chemical fertilizers on labile organic carbon and bacterial community of citrus orchard soils. Plant Soil 2023, 483, 255–272. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Q.; Ran, H.; Lu, W.; Xu, W.; Ali, W.; Yang, Q.; Liu, W.; Fang, M.; Yang, H. Daily Variation of Soil Greenhouse Gas Fluxes in Rubber Plantations Under Different Levels of Organic Fertilizer Substitution. Forests 2025, 16, 706. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Li, R.; Wang, H.; Wu, G.; Wen, X.; Huang, S.; Wang, X.; Liu, C. Long-Term Organic Substitution Regimes Affect Open-Field Vegetable Yields and Soil Organic Carbon Stability by Regulating Soil Labile Organic Carbon Fractions’ Changes. Agronomy 2025, 15, 396. [Google Scholar] [CrossRef]
- Mon, W.; Toma, Y.; Ueno, H. Residual Effects of Rice Husk Biochar and Organic Manure Application after 1 Year on Soil Chemical Properties, Rice Yield, and Greenhouse Gas Emissions from Paddy Soils. Soil Syst. 2024, 8, 91. [Google Scholar] [CrossRef]
- Han, Z.; Hou, H.; Yao, X.; Qian, X.; Zhou, M. Substituting Partial Chemical Fertilizers with Bio-Organic Fertilizers to Reduce Greenhouse Gas Emissions in Water-Saving Irrigated Rice Fields. Agronomy 2024, 14, 544. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, Y.; Liu, Y.; Huang, X.; Yang, Y.; Xiong, H.; Zhu, H.; Li, Y. Characteristics of Greenhouse Gas Emissions from Yellow Paddy Soils under Long-Term Organic Fertilizer Application. Sustainability 2022, 14, 12574. [Google Scholar] [CrossRef]
- Lagomarsino, A.; Verga, E.; Valagussa, M.; Rispoli, S.; Rocchi, F.; Becagli, C.; Tosca, A. Optimizing Livestock By-Products Storage to Reduce Ammonia and Greenhouse Gas Emissions Using Biochar and Wood Vinegar. Atmosphere 2025, 16, 509. [Google Scholar] [CrossRef]
- Yan, C.; An, D.; Ma, H.; Zhao, B.; Liu, Y.; Hu, X.; Ma, Z. Soil carbon pool dynamics, microbial community diversity and pineapple (Ananas comosus) productivity response to partial organic substitution strategies: A four-year study. Sci. Hortic. 2025, 343, 114096. [Google Scholar] [CrossRef]
- Zhang, A.; Zheng, Z.; Zhao, Z.; Feng, T.; Zheng, W.; Zhai, B. Effects of Long-term Combined Application of Organic and Inorganic Fertilizers on Soil Carbon Pool and Greenhouse Gas Emissions in Orchards. Huan Jing Ke Xue 2023, 44, 5823–5831. [Google Scholar] [CrossRef]
- He, H.; Peng, M.; Lu, W.; Ru, S.; Hou, Z.; Li, J. Organic fertilizer substitution promotes soil organic carbon sequestration by regulating permanganate oxidizable carbon fractions transformation in oasis wheat fields. Catena 2023, 221 Pt A, 106784. [Google Scholar] [CrossRef]
- Wang, H.; Dong, W.; Shao, D.; Liu, L.; Liao, B.; Gu, W.; Tang, C.; Liu, J.; Hu, W.; Feng, J.; et al. Biochar Enhances Paddy Productivity, Carbon Sequestration, and Reduces Greenhouse Gas Emissions in the Middle Yangtze River Region. Agronomy 2024, 14, 3067. [Google Scholar] [CrossRef]
- Lee, Y.; An, H.; Yoon, S.; Tak, S.; Jeong, S.Y.; Lee, C.H.; Das, S.; Kim, S. Simultaneous reduction of greenhouse gas and NH3 emissions by combined application of organic and inorganic fertilizers in maize-cabbage cropping systems. J. Environ. Manag. 2025, 373, 123629. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, D.; Wu, Y.; Liu, S.; Chen, W.; Wu, S.; Meng, Q.; Feng, H.; Siddique, K. Mitigating greenhouse gas emissions by replacing inorganic fertilizer with organic fertilizer in wheat–maize rotation systems in China. J. Environ. Manag. 2023, 344, 118494. [Google Scholar] [CrossRef] [PubMed]
- Pramono, A.; Adriany, T.; Susilawati, H.; Jumari; Yunianti, I. Alternate wetting and drying combined farmyard manure for reducing greenhouse gas while improving rice yield. IOP Conf. Ser. Earth Environ. Sci. 2022, 950, 012012. [Google Scholar] [CrossRef]
- Cui, Z.; Wei, J.; Pan, Y.; Zhang, W.; Lv, J.; Yang, Y. Straw return enhances the global warming potential in paddy soil under the regulation of functional genes. Environ. Technol. Innov. 2025, 39, 104293. [Google Scholar] [CrossRef]
- Aumtong, S.; Somyo, C.; Kanchai, K.; Chuephudee, T.; Chotamonsak, C. Relationships Between Carbon Fractions and Soil Nutrients in Organic Cassava Cultivation in the Sandy Soil of Northeastern Thailand. Agronomy 2025, 15, 1069. [Google Scholar] [CrossRef]
- Ansabayeva, A.; Makhambetov, M.; Rebouh, N.; Abdelkader, M.; Saudy, H.; Hassan, K.; Nasser, M.; Ali, M.; Ebrahim, M. Plant Growth-Promoting Microbes for Resilient Farming Systems: Mitigating Environmental Stressors and Boosting Crops Productivity-A Review. Horticulturae 2025, 11, 260. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, Y.; Li, Y.; Yan, C.; Shi, M.; Fan, L.; Wei, C. Dominant Role of Irrigation Regime over Biochar in Controlling GHG Emissions from Paddy Fields. Agronomy 2025, 15, 1127. [Google Scholar] [CrossRef]


| Indicator | pH | SOC (g·kg−1) | TN (g·kg−1) | TP (g·kg−1) | TK (g·kg−1) | AP (mg·kg−1) | AN (mg·kg−1) | AK (mg·kg−1) |
|---|---|---|---|---|---|---|---|---|
| Value | 8.12 | 8.32 | 0.56 | 0.82 | 28.00 | 117.40 | 76.42 | 237.70 |
| Treatment | Chemical Fertilizer | Organic Fertilizer (kg·hm−2) | ||
|---|---|---|---|---|
| N (kg·hm−2) | P2O5 (kg·hm−2) | K2O (kg·hm−2) | ||
| CK | 0 | 0 | 0 | 0 |
| T0 | 277.33 | 487.17 | 142.42 | 0 |
| T20 | 221.87 | 389.73 | 113.93 | 1916.67 |
| T40 | 166.40 | 292.30 | 85.45 | 3691.67 |
| T60 | 110.93 | 194.87 | 56.97 | 5541.67 |
| T80 | 55.47 | 97.43 | 28.48 | 7391.67 |
| Period | Treatment | L | LI | CPI | CPMI |
|---|---|---|---|---|---|
| Seedling stage | CK | 0.143 ± 0.003 b | 1.000 ± 0.032 a | 1.000 ± 0.000 c | 100.033 ± 1.998 d |
| T0 | 0.151 ± 0.002 b | 0.922 ± 0.023 c | 1.090 ± 0.026 b | 114.230 ± 3.885 c | |
| T20 | 0.161 ± 0.002 a | 0.979 ± 0.014 ab | 1.083 ± 0.012 b | 121.753 ± 2.124 b | |
| T40 | 0.162 ± 0.001 a | 0.95 ± 0.031 bc | 1.150 ± 0.010 a | 129.777 ± 1.420 a | |
| T60 | 0.165 ± 0.01 a | 0.988 ± 0.015 ab | 1.160 ± 0.026 a | 133.173 ± 4.997 a | |
| T80 | 0.163 ± 0.008 a | 0.949 ± 0.02 bc | 1.137 ± 0.015 a | 128.577 ± 5.770 a | |
| Budding stage | CK | 0.194 ± 0.006 a | 1.000 ± 0.032 a | 1.000 ± 0.010 d | 99.980 ± 2.370 d |
| T0 | 0.179 ± 0.005 c | 0.922 ± 0.023 c | 1.093 ± 0.006 c | 100.903 ± 2.153 c | |
| T20 | 0.190 ± 0.007 a | 0.979 ± 0.014 ab | 1.147 ± 0.021 b | 112.127 ± 1.642 c | |
| T40 | 0.184 ± 0.006 bc | 0.950 ± 0.031 bc | 1.180 ± 0.020 a | 112.005 ± 4.495 b | |
| T60 | 0.189 ± 0.004 a | 0.988 ± 0.015 ab | 1.203 ± 0.015 a | 118.690 ± 3.118 a | |
| T80 | 0.197 ± 0.002 a | 0.949 ± 0.020 bc | 1.153 ± 0.012 b | 109.747 ± 2.222 b | |
| Flowering and fruit-setting stage | CK | 0.183 ± 0.005 c | 1.000 ± 0.028 c | 0.997 ± 0.006 c | 100.007 ± 2.962 c |
| T0 | 0.195 ± 0.005 ab | 1.061 ± 0.027 ab | 1.317 ± 0.032 b | 139.837 ± 3.547 b | |
| T20 | 0.194 ± 0.003 ab | 1.058 ± 0.016 ab | 1.347 ± 0.015 ab | 142.400 ± 2.981 b | |
| T40 | 0.187 ± 0.006 bc | 1.019 ± 0.031 bc | 1.460 ± 0.062 a | 148.84 ± 9.991 b | |
| T60 | 0.199 ± 0.002 a | 1.084 ± 0.010 a | 1.487 ± 0.050 a | 160.880 ± 4.118 a | |
| T80 | 0.188 ± 0.008 bc | 1.028 ± 0.042 bc | 1.443 ± 0.015 a | 148.470 ± 7.412 b | |
| Fruiting stage | CK | 0.161 ± 0.009 a | 1.000 ± 0.057 a | 0.997 ± 0.049 c | 99.927 ± 5.851 c |
| T0 | 0.128 ± 0.005 bc | 0.797 ± 0.031 bc | 1.227 ± 0.051 b | 97.927 ± 5.580 c | |
| T20 | 0.133 ± 0.007 bc | 0.825 ± 0.042 bc | 1.430 ± 0.056 a | 118.057 ± 10.320 abc | |
| T40 | 0.124 ± 0.002 c | 0.768 ± 0.015 c | 1.437 ± 0.119 a | 110.607 ± 10.868 bc | |
| T60 | 0.131 ± 0.005 bc | 0.877 ± 0.092 b | 1.533 ± 0.061 a | 135.047 ± 19.970 a | |
| T80 | 0.139 ± 0.005 b | 0.866 ± 0.030 b | 1.433 ± 0.081 a | 123.85 ± 2.996 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Han, G.; Li, C.; He, M.; Chen, J. Interaction Regulation Mechanism of Soil Organic Carbon Fraction and Greenhouse Gases by Organic and Inorganic Fertilization. Agronomy 2025, 15, 2166. https://doi.org/10.3390/agronomy15092166
Wang J, Han G, Li C, He M, Chen J. Interaction Regulation Mechanism of Soil Organic Carbon Fraction and Greenhouse Gases by Organic and Inorganic Fertilization. Agronomy. 2025; 15(9):2166. https://doi.org/10.3390/agronomy15092166
Chicago/Turabian StyleWang, Jing, Guojun Han, Chunbin Li, Mingzhu He, and Jianjun Chen. 2025. "Interaction Regulation Mechanism of Soil Organic Carbon Fraction and Greenhouse Gases by Organic and Inorganic Fertilization" Agronomy 15, no. 9: 2166. https://doi.org/10.3390/agronomy15092166
APA StyleWang, J., Han, G., Li, C., He, M., & Chen, J. (2025). Interaction Regulation Mechanism of Soil Organic Carbon Fraction and Greenhouse Gases by Organic and Inorganic Fertilization. Agronomy, 15(9), 2166. https://doi.org/10.3390/agronomy15092166





