Identification of a Chitin Synthase Gene from Arma chinensis (Hemiptera: Pentatomidae) Under Temperature Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Insects
2.2. Identification and Physicochemical Characterization of Chitin Synthase Genes in Arma chinensis
2.3. Sequence Alignment and Phylogenetic Analysis
2.4. Analysis of Protein Three-Dimensional Structures
2.5. Total RNA Extraction, Reverse Transcription, and qRT-PCR Validation
2.6. Statistical Analysis
3. Results
3.1. Identification and Physicochemical Characterization of CHS Genes in Arma chinensis
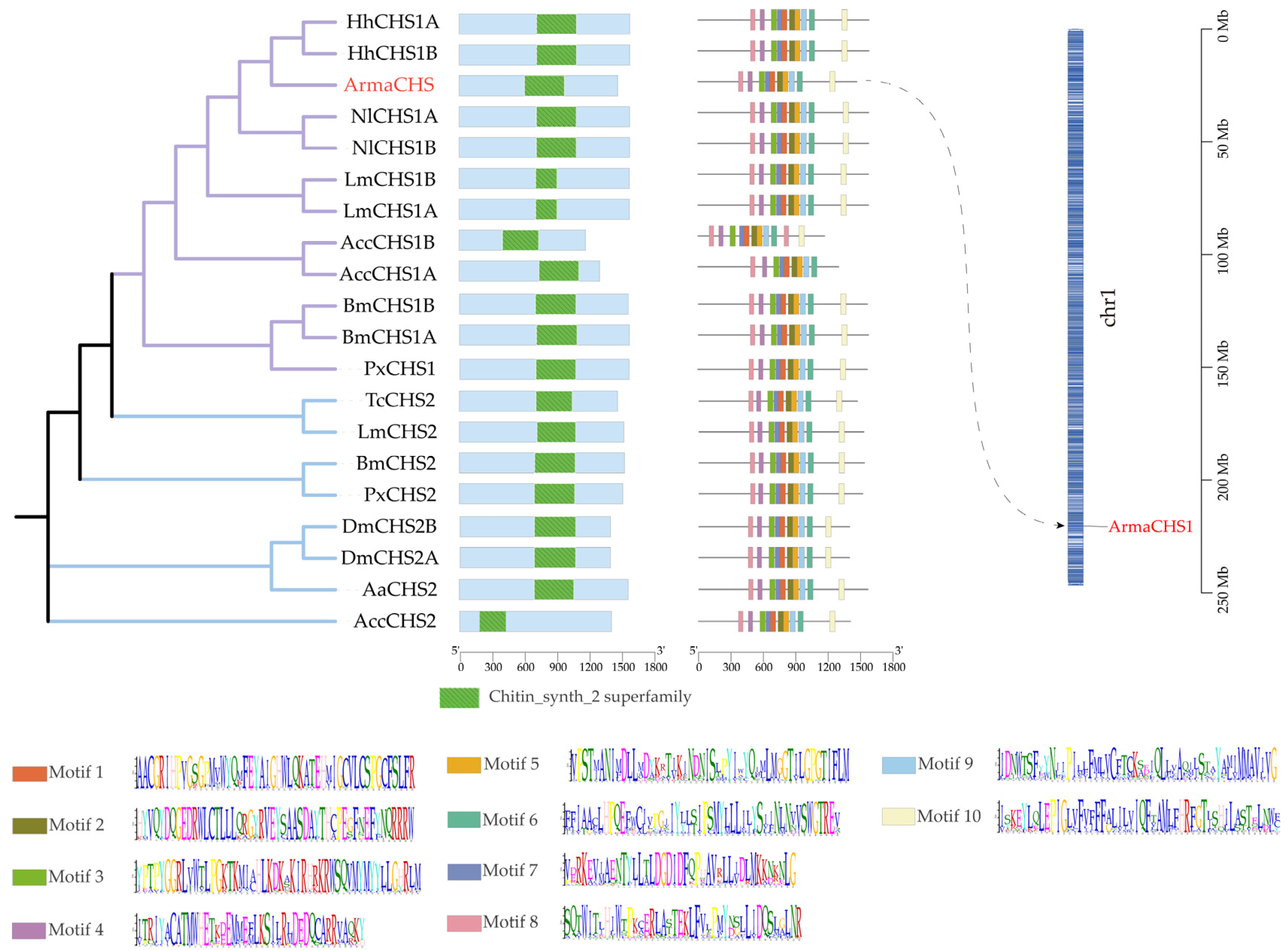
3.2. Sequence Conservation and Phylogenetic Analysis of A. chinensis CHS
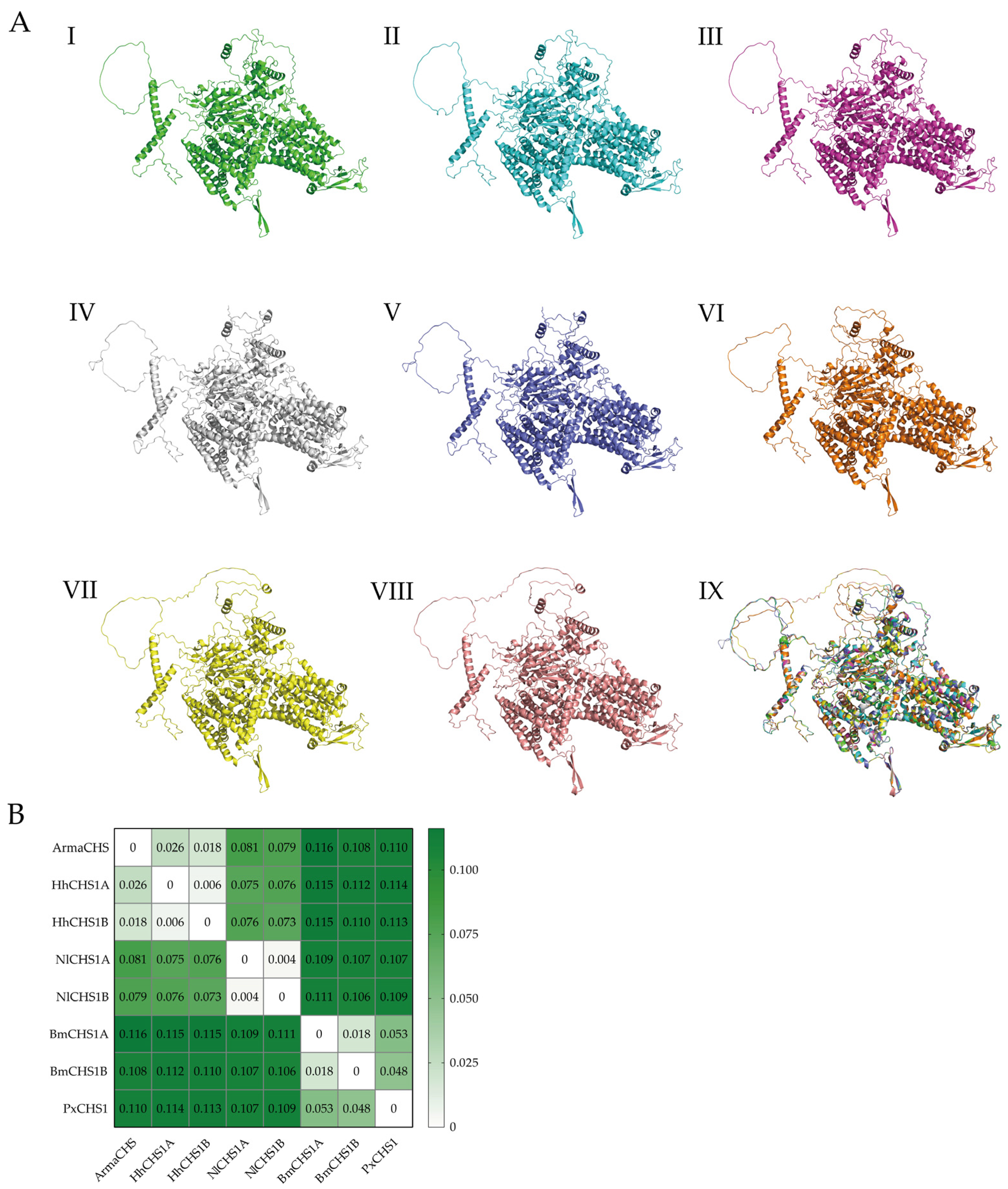
3.3. Three-Dimensional Structure Prediction and Conservation Analysis of ArmaCHS1
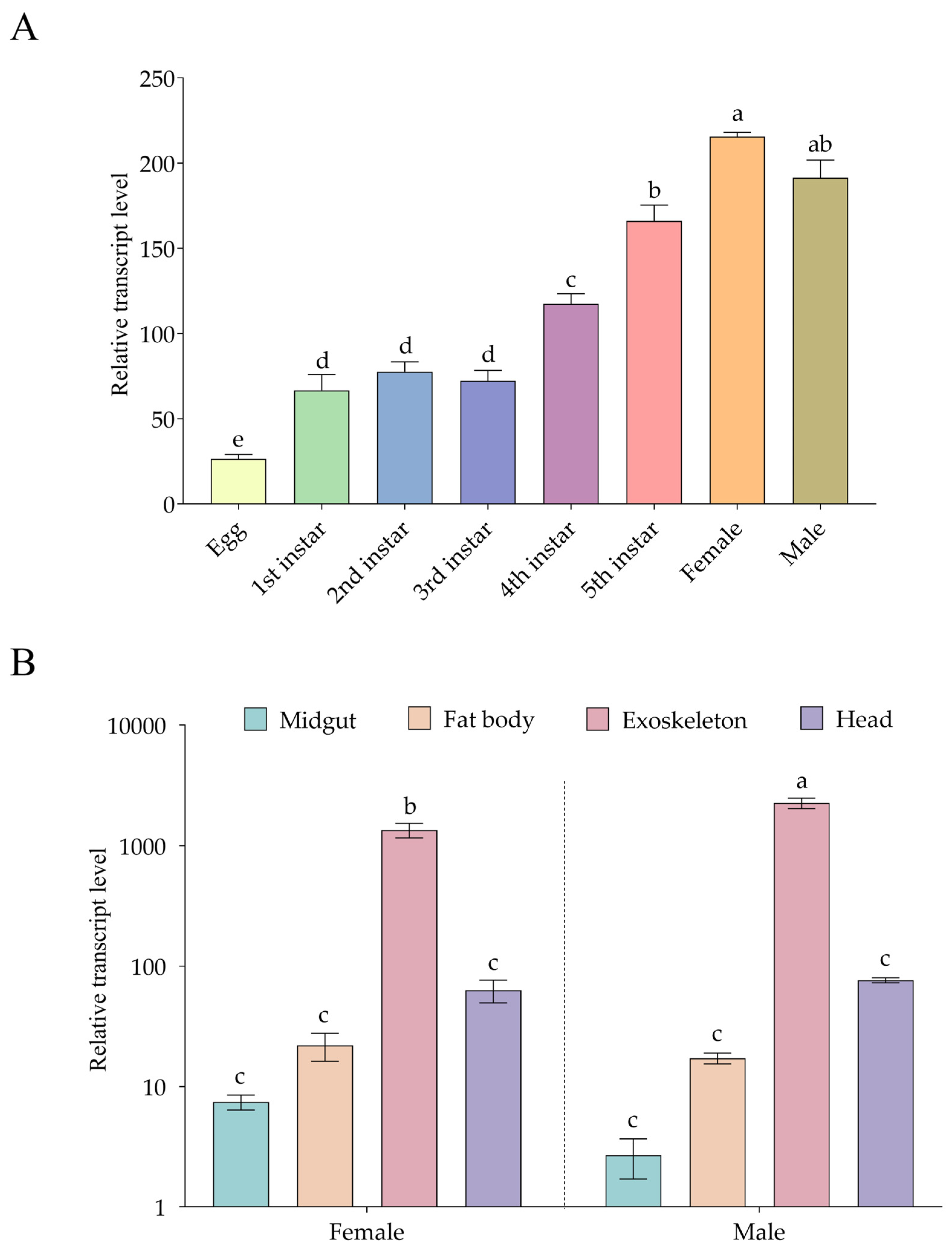
3.4. Tissue-, Stage-, and Sex-Specific Expression Profiles of ArmaCHS1
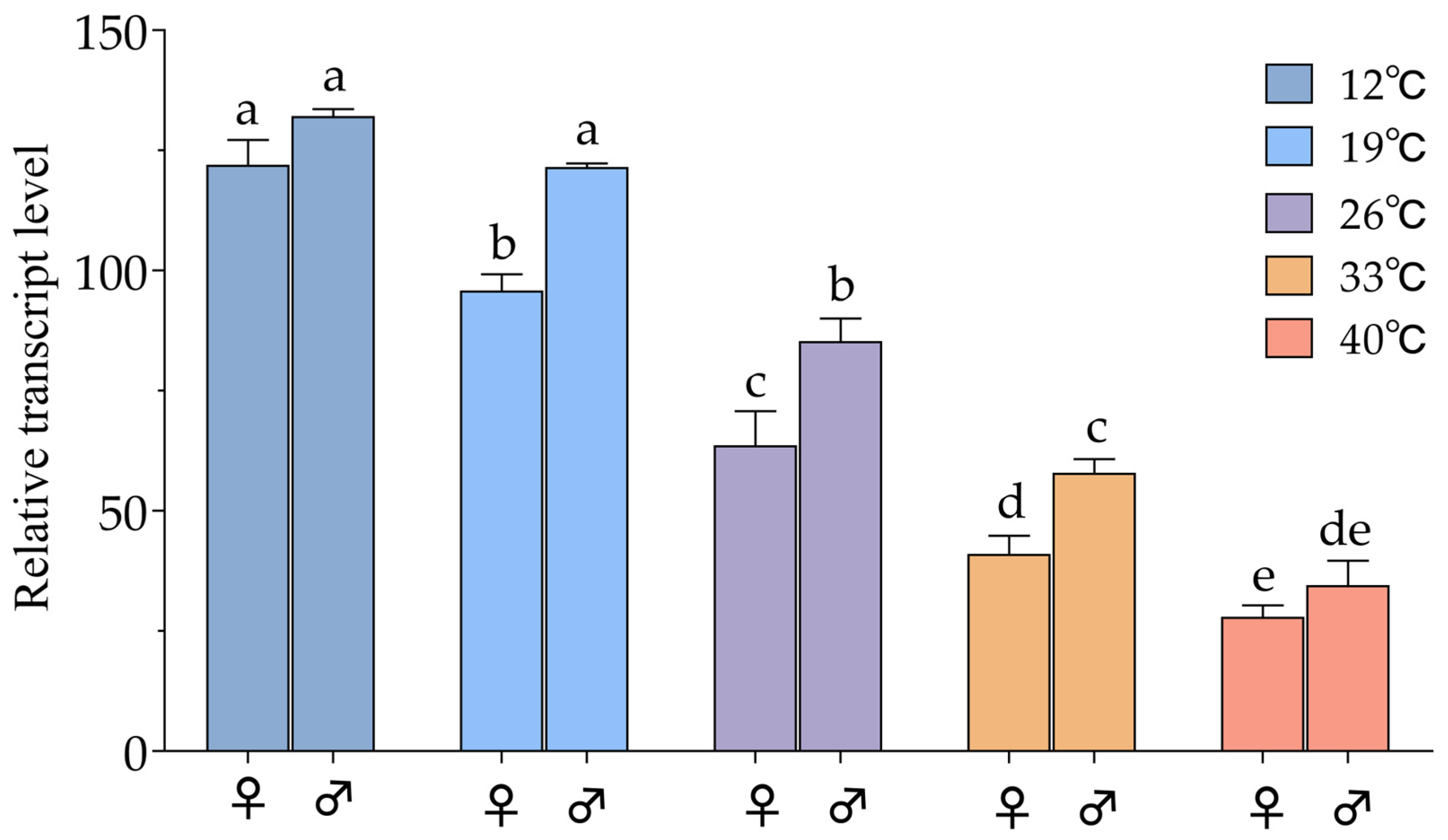
3.5. Temperature Stress Response of ArmaCHS1
4. Discussion
4.1. Gene Identification and Phylogenetic Analysis
4.2. Spatiotemporal Expression Patterns of ArmaCHS1
4.3. The Role of ArmaCHS1 Under Thermal Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PM | the peritrophic membrane |
| CHS | Chitin synthase |
| GlcNAc | β-1,4-linked N-acetyl-d-glucosamine |
| UDP-GlcNAc | Chitin 4-β-N-acetylglucosaminyltransferase |
| TRE | Trehalase |
| HK | Hexokinase |
| G6PI | Glucosamine-6-phosphate isomerase |
| GFAT | Glutamine-fructose-6-phosphate aminotransferase |
| GNA | Glucosamine-6-phosphate aminotransferase |
| PGM | Phosphoacetylglucosamine mutase |
| UAP | UDP-N-acetylglucosamine pyrophosphorylase |
| NCBI | National Center for Biotechnology Information |
| RMSD | Root mean square deviation |
| SEM | Standard error of the mean |
| qRT-PCR | Quantitative Reverse-Transcription PCR |
| ORF | Open reading frame |
| RNAi | RNA interference |
References
- Stork, N.E. How Many Species of Insects and Other Terrestrial Arthropods Are There on Earth? Annu. Rev. Entomol. 2018, 63, 31–45. [Google Scholar] [CrossRef]
- Yang, Q. Targeting Chitin-Containing Organisms. In Advances in Experimental Medicine and Biology; Springer Singapore: Singapore, 2019; Volume 1142, ISBN 978-981-13-7317-6. [Google Scholar]
- Sugumaran, M. (Ed.) Chitin in Insect Cuticle. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–110. ISBN 978-0-323-99976-2. [Google Scholar]
- Alvarenga, E.S.L.; Mansur, J.F.; Justi, S.A.; Figueira-Mansur, J.; Dos Santos, V.M.; Lopez, S.G.; Masuda, H.; Lara, F.A.; Melo, A.C.A.; Moreira, M.F. Chitin Is a Component of the Rhodnius prolixus Midgut. Insect Biochem. Mol. Biol. 2016, 69, 61–70. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Chitin and Chitinase: Role in Pathogenicity, Allergenicity and Health. Int. J. Biol. Macromol. 2017, 97, 331–338. [Google Scholar] [CrossRef]
- Merzendorfer, H. Insect Chitin Synthases: A Review. J. Comp. Physiol. B 2006, 176, 1–15. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.Q.; Zhang, J.Z.; Muthukrishnan, S. Biosynthesis, Turnover, and Functions of Chitin in Insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Beck, M.; Merzendorfer, H.; Yang, Q. Advances in Understanding Insect Chitin Biosynthesis. Insect Biochem. Mol. Biol. 2024, 164, 104058. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, P.M.; Deleury, E.; Davies, G.J.; Henrissat, B. An Evolving Hierarchical Family Classification for Glycosyltransferases. J. Mol. Biol. 2003, 328, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Lairson, L.L.; Henrissat, B.; Davies, G.J.; Withers, S.G. Glycosyltransferases: Structures, Functions, and Mechanisms. Annu. Rev. Biochem. 2008, 77, 521–555. [Google Scholar] [CrossRef]
- Ren, M.; Lu, J.; Li, D.; Yang, J.; Zhang, Y.; Dong, J.; Niu, Y.; Zhou, X.; Zhang, X. Identification and Functional Characterization of Two Chitin Synthases in the Black Cutworm, Agrotis ipsilon (Hufnagel) (Lepidoptera: Noctuidae). J. Econ. Entomol. 2023, 116, 574–583. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Y.; Yin, Q.; Zhang, H.; Yin, H.; Sun, Y.; Ma, L.; Zhou, D.; Shen, B. Physiological Characterization of Chitin Synthase a Responsible for the Biosynthesis of Cuticle Chitin in Culex pipiens pallens (Diptera: Culicidae). Parasites Vectors 2021, 14, 234. [Google Scholar] [CrossRef]
- Jiang, L.H.; Mu, L.L.; Jin, L.; Anjum, A.A.; Li, G.Q. RNAi for Chitin Synthase 1 Rather than 2 Causes Growth Delay and Molting Defect in Henosepilachna Vigintioctopunctata. Pestic. Biochem. Physiol. 2021, 178, 104934. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, M.; Wang, H.; Liang, X.; Yin, M.; Lin, T. Characterization of Chitin Synthase B Gene (HvChsb) and the Effects on Feeding Behavior in Heortia vitessoides Moore. Insects 2023, 14, 608. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yin, Q.; Xu, Y.; Li, X.; Sun, Y.; Ma, L.; Zhou, D.; Shen, B. Molecular and Physiological Characterization of the Chitin Synthase B Gene Isolated from Culex pipiens pallens (Diptera: Culicidae). Parasites Vectors 2019, 12, 614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, W.; Yu, Z.; Liu, X.; Wang, J.; Xin, T.; Zou, Z.; Xia, B. Characterization of Chitin Synthase a cDNA from Diaphorina citri (Hemiptera: Liviidae) and Its Response to Diflubenzuron. Insects 2022, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Mian, M.A.R.; Mittapalli, O.; Michel, A.P. Characterization of a Chitin Synthase Encoding Gene and Effect of Diflubenzuron in Soybean Aphid, Aphis glycines. Int. J. Biol. Sci. 2012, 8, 1323–1334. [Google Scholar] [CrossRef]
- Shang, F.; Xiong, Y.; Xia, W.K.; Wei, D.D.; Wei, D.; Wang, J.J. Identification, Characterization and Functional Analysis of a Chitin Synthase Gene in the Brown Citrus Aphid, Toxoptera citricida (Hemiptera, Aphididae). Insect Mol. Biol. 2016, 25, 422–430. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Wang, X.; Ding, Q.; Said, F.; Gao, X.; Desneux, N.; Song, D. RNAi-Mediated Knockdown of Chitin Synthase 1 (CHS1) Gene Causes Mortality and Decreased Longevity and Fecundity in Aphis gossypii. Insects 2019, 11, 22. [Google Scholar] [CrossRef]
- Mohammed, A.M.A.; Diab, M.R.; Abdelsattar, M.; Khalil, S.M.S. Characterization and RNAi-Mediated Knockdown of Chitin Synthase a in the Potato Tuber Moth, Phthorimaea operculella. Sci. Rep. 2017, 7, 9502. [Google Scholar] [CrossRef]
- Yu, H.-Z.; Li, N.-Y.; Xie, Y.-X.; Zhang, Q.; Wang, Y.; Lu, Z.-J. Identification and Functional Analysis of Two Chitin Synthase Genes in the Common Cutworm, Spodoptera litura. Insects 2020, 11, 253. [Google Scholar] [CrossRef]
- Gong, C.; Hasnain, A.; Wang, Q.; Liu, D.; Xu, Z.; Zhan, X.; Liu, X.; Pu, J.; Sun, M.; Wang, X. Eco-Friendly Deacetylated Chitosan Base siRNA Biological-Nanopesticide Loading Cyromazine for Efficiently Controlling Spodoptera frugiperda. Int. J. Biol. Macromol. 2023, 241, 124575. [Google Scholar] [CrossRef]
- Zhai, Y.; Fan, X.; Yin, Z.; Yue, X.; Men, X.; Zheng, L.; Zhang, W. Identification and Functional Analysis of Chitin Synthase a in Oriental armyworm, Mythimna separata. Proteomics 2017, 17, 1700165. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Jiang, Y.-D.; An, X.; Yang, L.; Shang, F.; Niu, J.; Wang, J.-J. Effects of RNAi-Based Silencing of Chitin Synthase Gene on Moulting and Fecundity in Pea Aphids (Acyrthosiphon pisum). Sci. Rep. 2019, 9, 3694. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Guo, Z.; Wu, M.; Tang, J.; Lv, H.; Li, J.; Ma, K. miR-9a and miR-10482-5p Regulate the Expression of Chitin Synthase and Chitinase Genes, Enhancing Lufenuron Tolerance in Spodoptera frugiperda. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2025, 289, 110115. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xu, Y.; Yang, X.; Sun, X.; Sun, Y.; Zhou, D.; Ma, L.; Shen, B.; Zhu, C. TRE1 and CHS1 Contribute to Deltamethrin Resistance in Culex pipiens pallens. Arch. Insect Biochem. Physiol. 2019, 100, e21538. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Yang, X.; Sun, X.; Sun, Y.; Zhou, D.; Ma, L.; Shen, B.; Zhu, C. Protective Roles of Chitin Synthase Gene 1 in Nilaparvata lugens against Cordyceps Javanica and Insecticides. Pestic. Biochem. Physiol. 2025, 209, 106324. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Kato, D.; Kamiya, K.; Minakuchi, C.; Miura, K. Chitin Synthase 1 Gene Is Crucial to Antifungal Host Defense of the Model Beetle, Tribolium castaneum. J. Invertebr. Pathol. 2017, 143, 26–34. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Zhang, D.; Zhang, Z.; Meng, S.; Li, Z.; Liu, X. miR-252 Targeting Temperature Receptor CcTRPM to Mediate the Transition from Summer-Form to Winter-Form of Cacopsylla chinensis. eLife 2023, 12, RP88744. [Google Scholar] [CrossRef]
- Grazia, J.; Schwertner, C.F.; Fernandes, J.A.M. Stink Bugs (Pentatomidae). In True Bugs (Heteroptera) of the Neotropics; Panizzi, A.R., Grazia, J., Eds.; Springer: Dordrecht, The Netherlands, 2015; Volume 2, pp. 681–756. [Google Scholar] [CrossRef]
- Fu, L.; Lin, C.; Xu, W.; Cheng, H.; Liu, D.; Ma, L.; Su, Z.; Yan, X.; Dong, X.; Liu, C. Chromosome-Level Genome Assembly of Predatory Arma chinensis. Sci. Data 2024, 11, 962. [Google Scholar] [CrossRef]
- Zou, D.; Wang, M.; Zhang, L.; Zhang, Y.; Zhang, X.; Chen, H. Taxonomic and Bionomic Notes on Arma chinensis (Fallou). Zootaxa 2012, 3382, 41–52. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Liao, J.; Li, C. Biological Performance of Arma Chinensis on Three Preys Antheraea pernyi, Plodia iInterpunctella and Leptinotarsa decemlineata. Int. J. Pest Manag. 2025, 71, 377–384. [Google Scholar] [CrossRef]
- Meng, J.-Y.; Yang, C.-L.; Wang, H.-C.; Cao, Y.; Zhang, C.-Y. Molecular Characterization of Six Heat Shock Protein 70 Genes from Arma chinensis and Their Expression Patterns in Response to Temperature Stress. Cell Stress Chaperones 2022, 27, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Zhang, H.; Zhang, L.; Chen, H. Effects of Starvation and Prey Availability on Predation and Dispersal of an Omnivorous Predator Arma chinensis Fallou. J. Insect Behav. 2019, 32, 134–144. [Google Scholar] [CrossRef]
- Liu, J.; Liao, J.; Li, C. Bottom-up Effects of Drought on the Growth and Development of Potato, Leptinotarsa decemlineata Say and Arma chinensis Fallou. Pest Manag. Sci. 2022, 78, 4353–4360. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, W.; Fu, L.; Cheng, H.; Lin, C.; Dong, X.; Liu, C. Detoxification and Neurotransmitter Clearance Drive the Recovery of Arma Chinensis from β-Cypermethrin-Triggered Knockdown. J. Hazard. Mater. 2024, 476, 135175. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lu, Z.-J.; Huang, Y.-L.; Yu, H.-Z.; Li, N.-Y.; Xie, Y.-X.; Zhang, Q.; Zeng, X.-D.; Hu, H.; Huang, A.-J.; Yi, L.; et al. Silencing of the Chitin Synthase Gene Is Lethal to the Asian Citrus Psyllid, Diaphorina citri. Int. J. Mol. Sci. 2019, 20, 3734. [Google Scholar] [CrossRef]
- Shao, Z.-M.; Li, Y.-J.; Ding, J.-H.; Liu, Z.-X.; Zhang, X.-R.; Wang, J.; Sheng, S.; Wu, F.-A. Identification, Characterization, and Functional Analysis of Chitin Synthase Genes in Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). Int. J. Mol. Sci. 2020, 21, 4656. [Google Scholar] [CrossRef]
- Chen, D.-D.; Wang, Z.-B.; Wang, L.-X.; Zhao, P.; Yun, C.-H.; Bai, L. Structure, Catalysis, Chitin Transport, and Selective Inhibition of Chitin Synthase. Nat. Commun. 2023, 14, 4776. [Google Scholar] [CrossRef]
- Chen, W.; Cao, P.; Liu, Y.; Yu, A.; Wang, D.; Chen, L.; Sundarraj, R.; Yuchi, Z.; Gong, Y.; Merzendorfer, H.; et al. Structural Basis for Directional Chitin Biosynthesis. Nature 2022, 610, 402–408. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Zhang, J.; Li, D.; Sun, Y.; Guo, Y.; Ma, E.; Zhu, K. Silencing of Two Alternative Splicing-Derived mRNA Variants of Chitin Synthase 1 Gene by RNAi Is Lethal to the Oriental Migratory Locust, Locusta migratoria manilensis (Meyen). Insect Biochem. Mol. Biol. 2010, 40, 824–833. [Google Scholar] [CrossRef]
- Pan, H.; Song, B.; Liao, J.; Zhang, Y.; Liu, Z. Buprofezin Delayed the Molting of Pardosa pseudoannulata, a Predatory Enemy for Insect Pests, by Suppressing Chitin Synthase 1 Expression. Pestic. Biochem. Physiol. 2024, 199, 105798. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Tada, A.; Musolin, D.L.; Hari, N.; Hosokawa, T.; Fujisaki, K.; Fukatsu, T. Collapse of Insect Gut Symbiosis under Simulated Climate Change. mBio 2016, 7, e01578-16. [Google Scholar] [CrossRef]
- Li, C.; Ul Haq, I.; Khurshid, A.; Tao, Y.; Quandahor, P.; Zhou, J.-J.; Liu, C.-Z. Effects of Abiotic Stresses on the Expression of Chitinase-like Genes in Acyrthosiphon pisum. Front. Physiol. 2022, 13, 1024136. [Google Scholar] [CrossRef]
- Tang, C.-F.; Li, F.-F.; Cao, Y.; Liao, H.-J. Universal Cooling Patterns of the Butterfly Wing Scales Hierarchy Deduced from the Heterogeneous Thermal and Structural Properties of Tirumala limniace (Lepidoptera: Nymphalidae, Danainae). Insect Sci. 2022, 29, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, H.; Zhou, C.; Yang, W.-J.; Jin, D.-C.; Long, G.-Y. Molecular Cloning, Expression, and Functional Analysis of the Chitin Synthase 1 Gene and Its Two Alternative Splicing Variants in the White-Backed Planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Sci. Rep. 2019, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.-L.; Xu, Z.-Y.; Li, M.-J.; Mbuji, A.L.; Gu, M.; Zhang, L.; Gao, X.-W. Detection of Chitin Synthase Mutations in Lufenuron-Resistant Spodoptera frugiperda in China. Insects 2022, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Masetti, A.; Rathé, A.; Robertson, N.; Anderson, D.; Walker, J.; Pasqualini, E.; Depalo, L. Effects of Three Chitin Synthesis Inhibitors on Egg Masses, Nymphs and Adults of Halyomorpha halys (Hemiptera: Pentatomidae). Pest Manag. Sci. 2023, 79, 2882–2890. [Google Scholar] [CrossRef]
- Gong, C.; Wang, X.; Huang, Q.; Zhang, J.; Zhang, Y.; Zhan, X.; Zhang, S.; Hasnain, A.; Ruan, Y.; Shen, L. The Fitness Advantages of Bistrifluron Resistance Related to Chitin Synthase a in Spodoptera litura (Fab.) (Noctuidae: Lepidoptera). Pest Manag. Sci. 2021, 77, 3458–3468. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Su, Z.; Lin, C.; Xu, W.; Yan, X.; Chen, Y.; Wang, Y.; Dong, X.; Liu, C. Identification of a Chitin Synthase Gene from Arma chinensis (Hemiptera: Pentatomidae) Under Temperature Stress. Agronomy 2025, 15, 2157. https://doi.org/10.3390/agronomy15092157
Liu D, Su Z, Lin C, Xu W, Yan X, Chen Y, Wang Y, Dong X, Liu C. Identification of a Chitin Synthase Gene from Arma chinensis (Hemiptera: Pentatomidae) Under Temperature Stress. Agronomy. 2025; 15(9):2157. https://doi.org/10.3390/agronomy15092157
Chicago/Turabian StyleLiu, Dianyu, Zhihan Su, Changjin Lin, Wenyan Xu, Xiaoyu Yan, Yu Chen, Yichen Wang, Xiaolin Dong, and Chenxi Liu. 2025. "Identification of a Chitin Synthase Gene from Arma chinensis (Hemiptera: Pentatomidae) Under Temperature Stress" Agronomy 15, no. 9: 2157. https://doi.org/10.3390/agronomy15092157
APA StyleLiu, D., Su, Z., Lin, C., Xu, W., Yan, X., Chen, Y., Wang, Y., Dong, X., & Liu, C. (2025). Identification of a Chitin Synthase Gene from Arma chinensis (Hemiptera: Pentatomidae) Under Temperature Stress. Agronomy, 15(9), 2157. https://doi.org/10.3390/agronomy15092157





