Preharvest Application of 1-Methylcyclopropene (1-MCP) to Schedule the Harvest and Maintain the Storage Quality of ‘Maxi Gala’ Apples
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
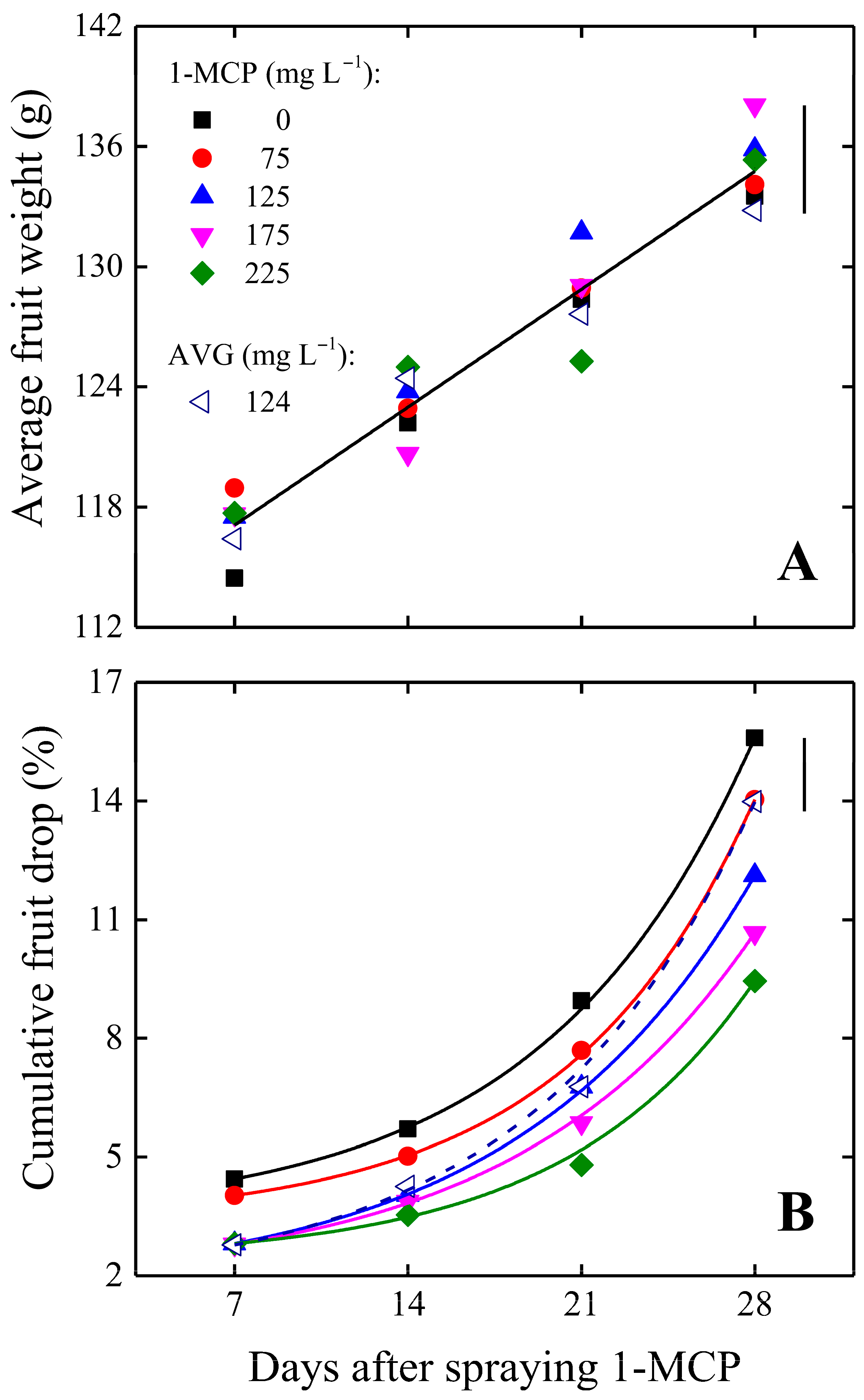
3.1. Preharvest Fruit Drop and Delay in Maturation
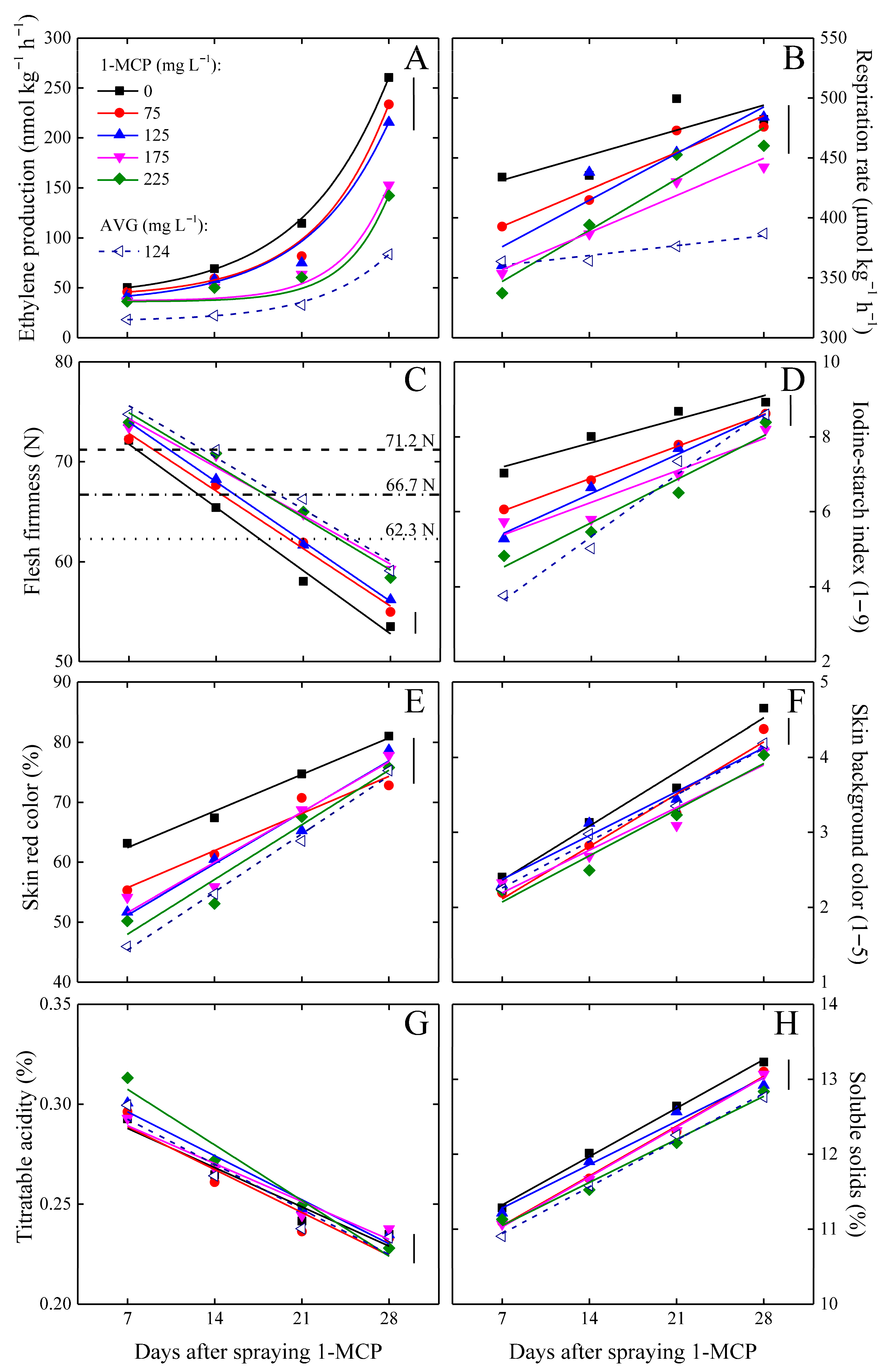
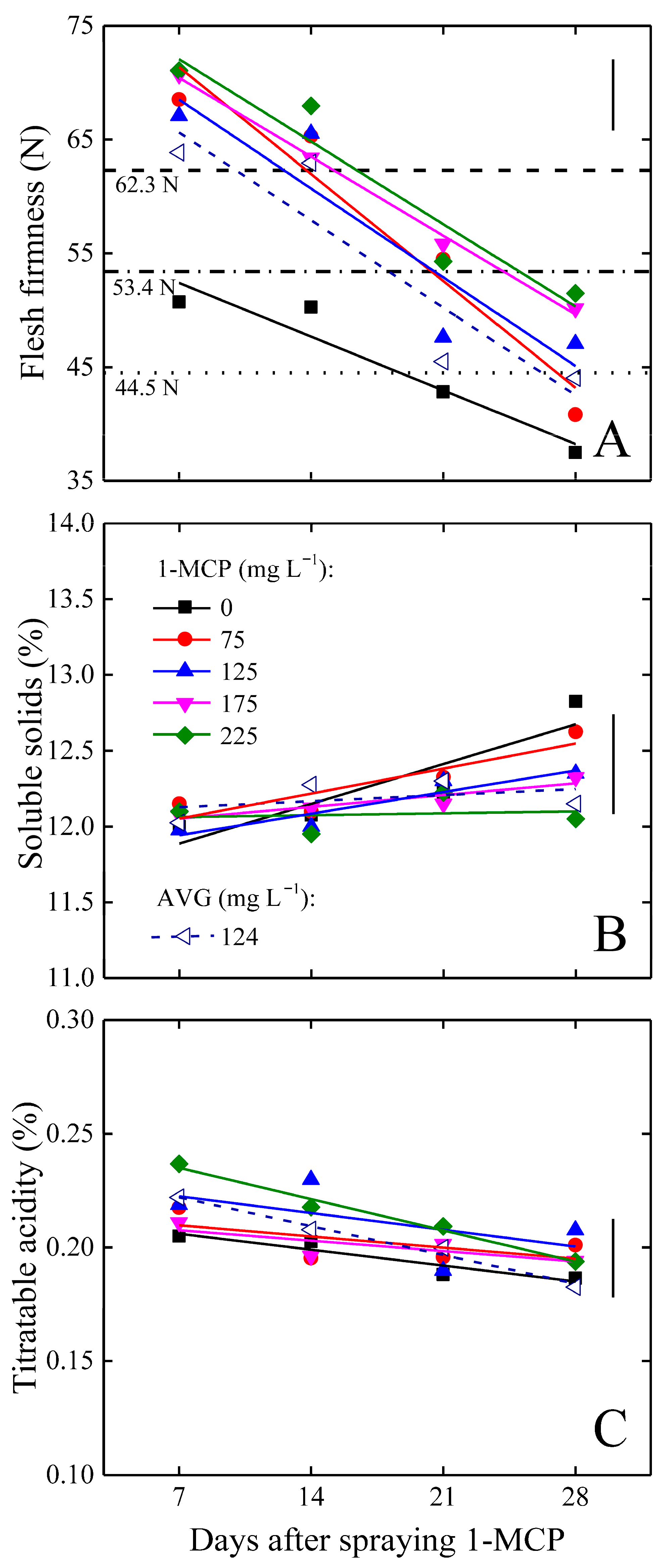
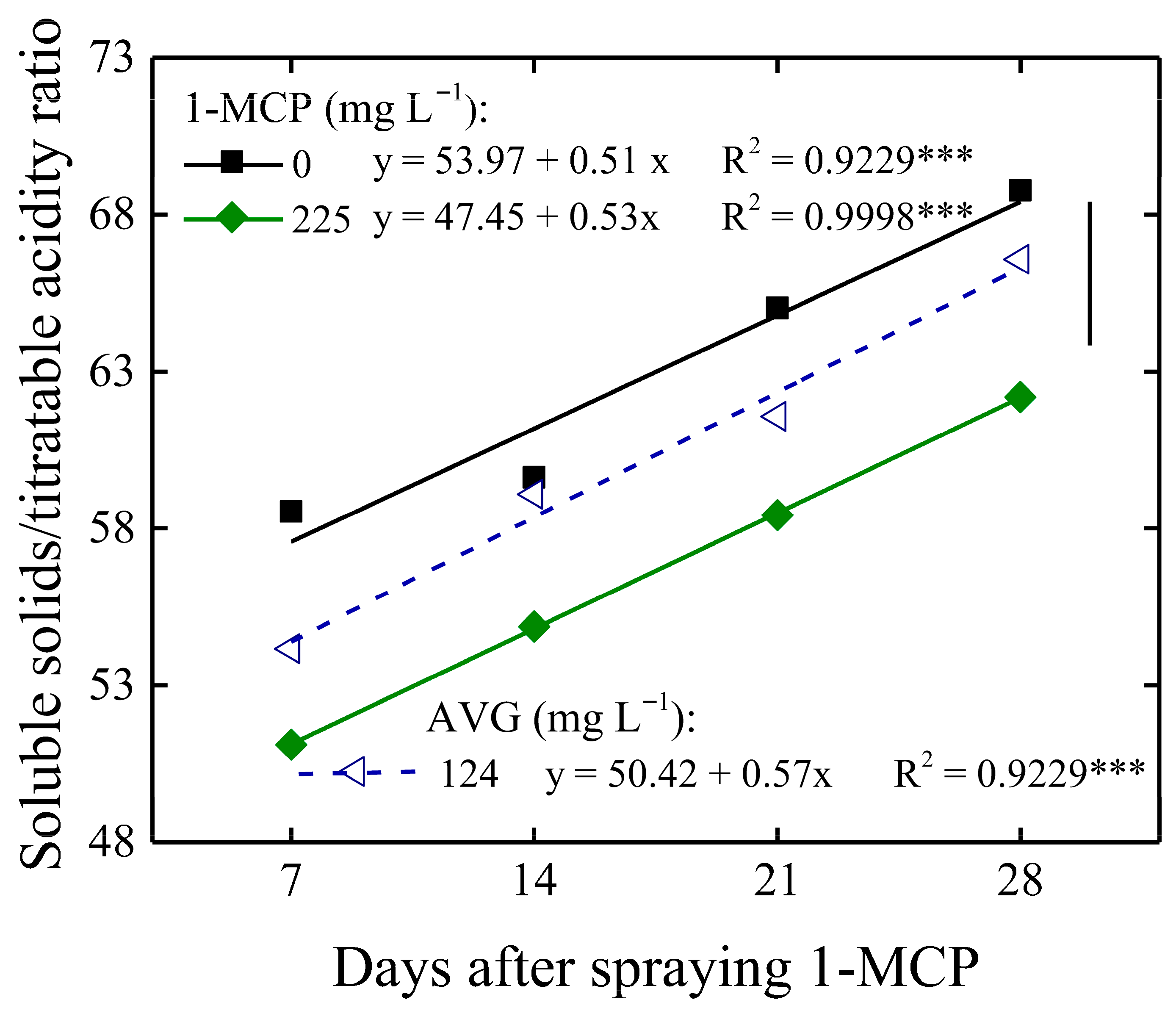
3.2. Preservation of Fruit Postharvest Quality
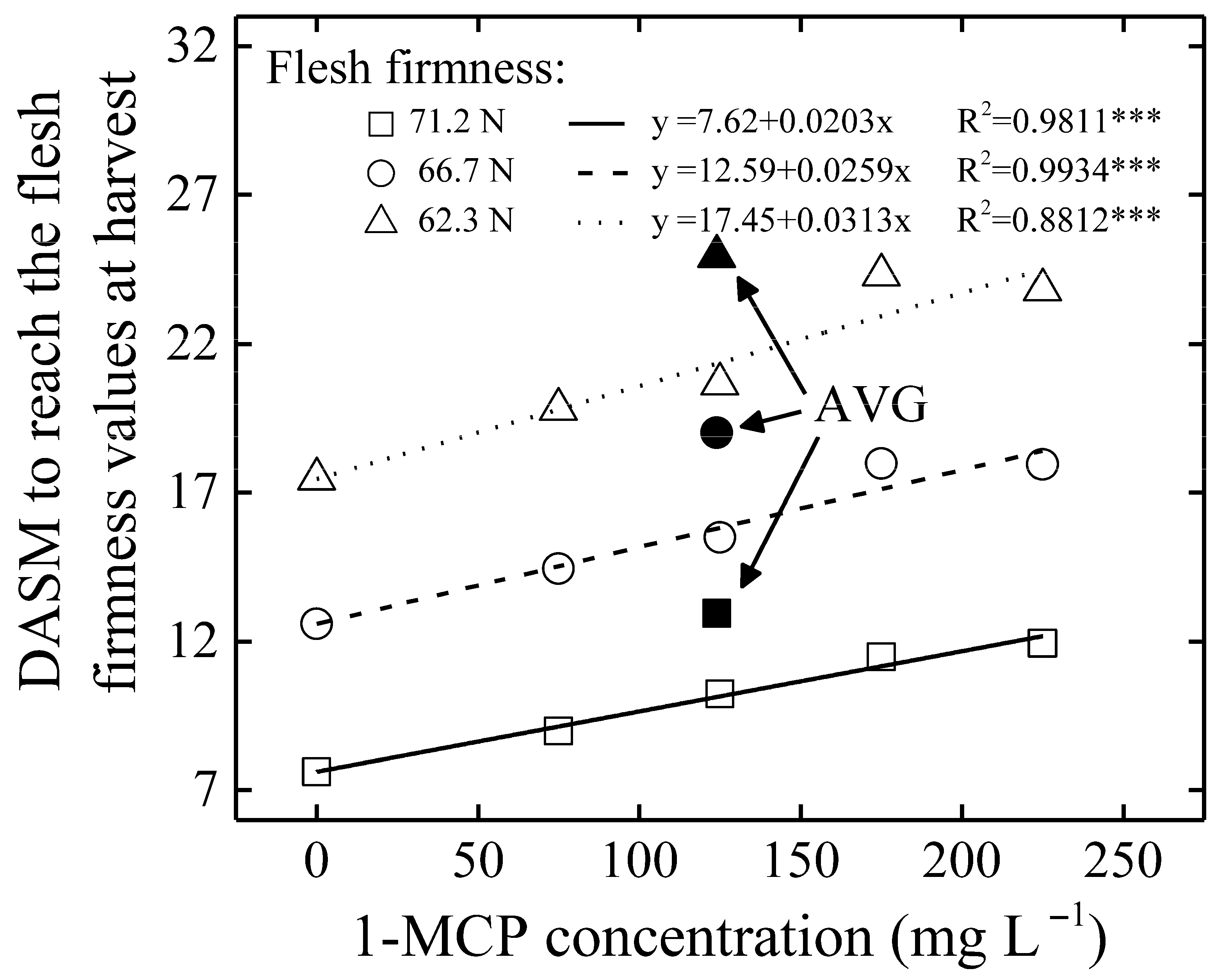
3.3. General Considerations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | 1-aminocyclopropane-1-carboxylate |
| a.i. | Active ingredient |
| AVG | Aminoethoxyvinylglycine |
| CA | Controlled atmosphere |
| DASM | Days after spraying 1-MCP |
| DBAH | Days before the anticipated harvest time |
| 1-MCP | 1-methylcyclopropene |
| NAA | Naphthalene acetic acid |
| RH | Relative humidity |
| SS | Soluble solid |
| TA | Titratable acidity |
References
- Petri, J.L.; Leite, G.B.; Couto, M.; Francescatto, P. Avanços na cultura da macieira no Brasil. Rev. Bras. Frutic. 2011, 39, 48–56. [Google Scholar] [CrossRef]
- Kist, B.B. Anuário Brasileiro da maçã 2019. Gazeta Santa Cruz, Santa Cruz do Sul (Brazilian Apple Year Book). Available online: https://www.editoragazeta.com.br/wp-content/uploads/2019/06/MA%C3%A7%C3%A2_2019_DUPLA.pdf (accessed on 12 August 2025).
- Lin, H.H.; Walsh, C.S. Studies of the “tree factor” and its role in the maturation and ripening of ‘Gala’ and ‘Fuji’ apples. HortScience 2008, 43, 184–188. [Google Scholar] [CrossRef]
- Argenta, L.C.; Scolaro, A.M.T.; do Amarante, C.V.T.; Vieira, M.J.; Werner, S.S. Preharvest treatment of ‘Gala’ apples with 1-MCP and AVG—I: Effects on fruit maturation on the tree. Acta Hortic. 2018, 1194, 113–119. [Google Scholar] [CrossRef]
- Amarante, C.V.T.; Simioni, A.; Megguer, C.A.; Blum, L.E.B. Effect of aminoethoxyvinilglycine (AVG) on preharvest fruit drop and maturity of apples. Rev. Bras. Frutic. 2002, 24, 661–664. [Google Scholar] [CrossRef]
- Petri, J.L.; Leite, G.B.; Hawerroth, F.J. Maturação, qualidade e queda pré-colheita de maçãs ‘Imperial Gala’ em função da aplicação de aminoetoxivinilglicina. Bragantia 2010, 69, 99–608. [Google Scholar] [CrossRef]
- Arseneault, M.H.; Cline, J.A. A review of apple preharvest fruit drop and practices for horticultural management. Sci. Hortic. 2016, 211, 40–52. [Google Scholar] [CrossRef]
- Bangerth, F. The effect of a substituted amino acid on ethylene biosynthesis, respiration, ripening and pre-harvest drop of apple fruits. J. Am. Soc. Hortic. Sci. 1978, 103, 401–404. [Google Scholar] [CrossRef]
- Autio, W.R.; Bramlage, W.J. Effects of AVG on maturation, ripening, and storage of apples. J. Am. Soc. Hortic. Sci. 1982, 107, 1074–1077. [Google Scholar] [CrossRef]
- Schupp, J.R.; Greene, D.W. Effect of aminoethoxyvinylglycine (AVG) on preharvest drop, fruit quality, and maturation of ‘McIntosh’ apples. I. Concentration and timing of dilute applications of AVG. HortScience 2004, 39, 1030–1035. [Google Scholar] [CrossRef]
- Byers, R.E.; Carbaugh, D.H.; Combs, L.D. Ethylene inhibitors delay fruit drop, maturity, and increase fruit size of ‘Arlet’ apples. HortScience 2005, 40, 2061–2065. [Google Scholar] [CrossRef]
- Yuan, R.; Carbaugh, D.H. Effects of NAA, AVG, and 1-MCP on ethylene biosynthesis, preharvest fruit drop, fruit maturity, and quality of ‘Golden Supreme’ and ‘Golden Delicious’ apples. HortScience 2007, 42, 101–105. [Google Scholar] [CrossRef]
- Liu, J.; Islam, M.T.; Sherif, S.M. Effects of aminoethoxyvinylglycine (AVG) and 1-methylcyclopropene (1-MCP) on the pre-harvest drop rate, fruit quality, and stem-end splitting in ‘Gala’ apples. Horticulturae 2022, 8, 1100. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.; Dong, C.; Terry, L.A.; Watkins, C.B.; Yu, Z.; Cheng, Z.-M. Meta-analysis of the effects of 1-methylcyclopropene (1-MCP) treatment on climacteric fruit ripening. Hortic. Res. 2020, 7, 208. [Google Scholar] [CrossRef]
- McArtney, S.J.; Obermiller, J.D.; Schupp, J.R.; Parker, M.L.; Edgington, T.B. Preharvest 1-methylcyclopropene delays fruit maturity and reduces softening and superficial scald of apples during long-term storage. HortScience 2008, 43, 366–371. [Google Scholar] [CrossRef]
- Yuan, R.; Li, J. Effect of sprayable 1-MCP, AVG, and NAA on ethylene biosynthesis, preharvest fruit drop, fruit maturity, and quality of ‘Delicious’ apples. HortScience 2008, 43, 1454–1460. [Google Scholar] [CrossRef]
- Varanasi, V.; Shin, S.; Johnson, F.; Mattheis, J.P.; Zhu, Y. Differential suppression of ethylene biosynthesis and receptor genes in ‘Golden Delicious’ apple by preharvest and postharvest 1-MCP treatments. J. Plant Growth Regul. 2013, 32, 585–595. [Google Scholar] [CrossRef]
- Amarante, C.V.T.; Argenta, L.C.; de Freitas, S.T.; Steffens, C.A. Efficiency of pre-harvest application of 1-MCP (Harvista™ 1.3 SC) to delay maturation of ‘Cripps Pink’ apple fruit. Sci. Hortic. 2022, 293, 110715. [Google Scholar] [CrossRef]
- Doerflinger, F.C.; Shoffe, Y.A.; Sutanto, G.; Nock, J.F.; Watkins, C.B. Preharvest 1-methylcyclopropene (1-MCP) treatment effects on quality of spot and strip picked ‘Gala’ apples at harvest and after storage as affected by postharvest 1-MCP and temperature conditioning treatments. Sci. Hortic. 2024, 325, 112682. [Google Scholar] [CrossRef]
- Elfving, D.C.; Drake, S.R.; Reed, A.N.; Visser, D.B. Preharvest applications of sprayable 1-methylcyclopropene in the orchard for management of apple harvest and postharvest condition. HortScience 2007, 42, 1192–1199. [Google Scholar] [CrossRef]
- McArtney, S.J.; Obermiller, J.D.; Hoyt, T.; Parker, M.L. ‘Law Rome’ and ‘Golden Delicious’ apples differ in their response to preharvest and postharvest 1-methylcyclopropene treatment combinations. HortScience 2009, 44, 1632–1636. [Google Scholar] [CrossRef]
- DeEll, J.R.; Ehsani-Moghaddam, B. Preharvest 1-methylcyclopropene treatment reduces soft scald in ‘Honeycrisp’ apples during storage. HortScience 2010, 45, 414–417. [Google Scholar] [CrossRef]
- Watkins, C.B.; James, H.; Nock, J.F.; Reed, N.; Oakes, R.L. Preharvest application of 1-methylcyclopropene (1-MCP) to control fruit drop of apples, and its effects on postharvest quality. Acta Hortic. 2010, 877, 365–374. [Google Scholar] [CrossRef]
- Robinson, T.; Hoying, S.; Iungerman, K.; Kviklys, D. AVG combined with NAA control pre-harvest drop of ‘McIntosh’ apples better than either chemical alone. Acta Hortic. 2010, 884, 343–350. [Google Scholar] [CrossRef]
- Yildiz, K.; Ozturk, B.; Ozkan, Y. Effects of aminoethoxyvinylglycine (AVG) on preharvest fruit drop, fruit maturity, and quality of ‘Red Chief’ apple. Sci. Hortic. 2012, 144, 121–124. [Google Scholar] [CrossRef]
- Sakaldas, M.; Gundogdu, M.A. The effects of preharvest 1-methylcyclopropene (Harvista) treatments on harvest maturity of ‘Golden Delicious’ apple cultivar. Acta Hortic. 2016, 1139, 601–607. [Google Scholar] [CrossRef]
- Dal Cin, V.; Rizzini, F.M.; Botton, A.; Tonutti, P. The ethylene biosynthetic and signal transduction pathways are differently affected by 1-MCP in apple and peach fruit. Postharvest Biol. Technol. 2006, 42, 125–133. [Google Scholar] [CrossRef]
- Scolaro, A.M.T.; Argenta, L.C.; Amarante, C.V.T.; Petri, J.L.; Hawerroth, F.J. Preharvest control of ‘Royal Gala’ apple fruit maturation by the inhibition of ethylene action or synthesis. Rev. Bras. Frutic. 2015, 37, 38–47. [Google Scholar] [CrossRef]
- Doerflinger, F.C.; Nock, J.F.; Miller, W.B.; Watkins, C.B. Preharvest aminoethoxyvinylglycine (AVG) and 1-methylcyclopropene (1-MCP) effects on ethylene and starch concentrations of ‘Empire’ and ‘McIntosh’ apples. Sci. Hortic. 2019, 244, 134–140. [Google Scholar] [CrossRef]
- Algul, B.E.; Shoffe, Y.A.; Park, D.; Cheng, L.; Watkins, C.B. Preharvest 1-methylcyclopropene and aminoethoxyvinylglycine treatment effects on ‘NY2’ (RubyFrost®) apple fruit quality and postharvest watercore dissipation at different temperatures. Postharvest Biol. Technol. 2025, 220, 113301. [Google Scholar] [CrossRef]
- Harker, F.R.; Kupferman, E.M.; Marin, A.B.; Gunson, F.A.; Triggs, C.M. Eating quality standards for apples based on consumer preferences. Postharvest Biol. Technol. 2008, 50, 70–78. [Google Scholar] [CrossRef]
- MAPA. Regulamento Técnico de Identidade e Qualidade da Maçã; Instrução Normativa, 5; Ministério da Agricultura, Pecuária e Abastecimento (MAPA): Brasília, Brazil, 2006.
| Treatments | Adjusted Models | R2 | Statistical Significance |
|---|---|---|---|
| Ethylene (nmol C2H4 kg−1 h−1) | |||
| 1-MCP (mg L−1): | y = 39.80 + 10.37 × e(x − 7)/6.87 | 0.9991 | *** |
| 75 | y = 40.69 + 5.08 × e(x − 7)/5.77 | 0.9780 | *** |
| 125 | y = 34.55 + 7.21 × e(x − 7)/6.51 | 0.9670 | *** |
| 175 | y = 36.88 + 0.37 × e(x − 7)/3.65 | 0.9426 | *** |
| 225 | y = 36.12 + 0.20 × e(x − 7)/3.34 | 0.9012 | *** |
| AVG (mg L−1): 124 | y = 16.55 + 1.55 × e(x − 7)/5.57 | 0.9993 | *** |
| Respiration (µmol CO2 kg−1 h−1) | |||
| 1-MCP (mg L−1): | y = 410.5 + 2.98x | 0.4878 | * |
| 75 | y = 362.2 + 4.39x | 0.8568 | ** |
| 125 | y = 337.1 + 5.55x | 0.8468 | ** |
| 175 | y = 325.8 + 4.42x | 0.9416 | *** |
| 225 | y = 304.3 + 6.10x | 0.8857 | ** |
| AVG (mg L−1): 124 | y = 352.2 + 1.17x | 0.8579 | ** |
| Flesh firmness (N) | |||
| 1-MCP (mg L−1): | y = 78.08 − 0.9035x | 0.9866 | *** |
| 75 | y = 78.59 − 0.8226x | 0.9878 | *** |
| 125 | y = 79.94 − 0.8541x | 0.9983 | *** |
| 175 | y = 79.14 − 0.6921x | 0.9690 | *** |
| 225 | y = 80.15 − 0.7494x | 0.9661 | *** |
| AVG (mg L−1): 124 | y = 80.80 − 0.7415x | 0.9647 | *** |
| Skin red color (%) | |||
| 1-MCP (mg L−1): | y = 56.32 + 0.8718x | 0.9847 | *** |
| 75 | y = 42.58 + 1.2255x | 0.9471 | ** |
| 125 | y = 49.54 + 0.8851x | 0.9280 | ** |
| 175 | y = 43.21 + 1.1974x | 0.9031 | ** |
| 225 | y = 38.87 + 1.3027x | 0.9221 | ** |
| AVG (mg L−1): 124 | y = 35.67 + 1.3825x | 0.9916 | *** |
| Skin background color (1–5) | |||
| 1-MCP (mg L−1): | y = 1.640 + 0.103x | 0.9634 | *** |
| 75 | y = 1.412 + 0.100x | 0.9402 | ** |
| 125 | y = 1.785 + 0.084x | 9632 | *** |
| 175 | y = 1.622 + 0.081x | 0.8993 | ** |
| 225 | y = 1.455 + 0.088x | 0.9391 | ** |
| AVG (mg L−1): 124 | y = 1.635 + 0.089x | 0.9724 | *** |
| Starch-iodine index (1–9) | |||
| 1-MCP (mg L−1): | y = 6.570 + 0.0908x | 0.9042 | ** |
| 75 | y = 5.172 + 0.1230x | 0.9980 | *** |
| 125 | y = 4.371 + 0.1510x | 0.9763 | *** |
| 175 | y = 4.531 + 0.1228x | 0.8558 | ** |
| 225 | y = 3.358 + 0.1677x | 0.9185 | ** |
| AVG (mg L−1): 124 | y = 1.969 + 0.2405x | 0.9764 | *** |
| Soluble solids (%) | |||
| 1-MCP (mg L−1): 0 | y = 10.67 + 0.0923x | 0.9963 | *** |
| 75 | y = 10.38 + 0.0950x | 0.9915 | *** |
| 125 | y = 10.70 + 0.0827x | 0.9719 | *** |
| 175 | y = 10.36 + 0.0951x | 0.9959 | *** |
| 225 | y = 10.47 + 0.0821x | 0.9797 | *** |
| AVG (mg L−1): 124 | y = 10.31 + 0.0892x | 0.9935 | *** |
| Titratable acidity (%) | |||
| 1-MCP (mg L−1): | y = 0.3076 − 0.0028x | 0.9119 | ** |
| 75 | y = 0.3103 − 0.0031x | 0.8493 | ** |
| 125 | y = 0.3182 − 0.0031x | 0.9534 | ** |
| 175 | y = 0.3082 − 0.0027x | 0.9235 | ** |
| 225 | y = 0.3352 − 0.0040x | 0.9575 | ** |
| AVG (mg L−1): 124 | y = 0.3151 − 0.0032x | 0.8680 | ** |
| Treatments | Adjusted Models | R2 | Statistical Significance |
|---|---|---|---|
| Flesh firmness (N) | |||
| 1-MCP (mg L−1): 0 | y = 57.10 − 0.6736x | 0.8746 | * |
| 75 | y = 80.73 − 1.3408x | 0.9091 | ** |
| 125 | y = 76.29 − 1.1136x | 0.7630 | * |
| 175 | y = 77.34 − 0.9897x | 0.9943 | *** |
| 225 | y = 79.29 − 1.0342x | 0.8787 | * |
| AVG (mg L−1): 124 | y = 73.28 − 1.0977x | 0.7711 | * |
| Soluble solids (%) | |||
| 1-MCP (mg L−1): 0 | y = 11.62 + 0.0375x | 0.7291 | * |
| 75 | y = 11.89 + 0.0236x | 0.7100 | * |
| 125 | y = 11.80 + 0.0040x | 0.8189 | * |
| 175 | y = 11.97 + 0.0111x | 0.7700 | * |
| 225 | y = 12.05 + 0.0018x | 0.4701 | ns |
| AVG (mg L−1): 124 | y = 12.09 + 0.0057x | 0.2506 | ns |
| Titratable acidity (%) | |||
| 1-MCP (mg L−1): 0 | y = 0.2130 − 0.0001x | 0.8291 | * |
| 75 | y = 0.2146 − 0.0001x | 0.0535 | ns |
| 125 | y = 0.2299 − 0.0010x | 0.0336 | ns |
| 175 | y = 0.2121 − 0.0001x | 0.4157 | ns |
| 225 | y = 0.2487 − 0.0020x | 0.9714 | ** |
| AVG (mg L−1): 124 | y = 0.2346 − 0.0018x | 0.9737 | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Amarante, C.V.T.; Argenta, L.C.; de Freitas, S.T.; Steffens, C.A. Preharvest Application of 1-Methylcyclopropene (1-MCP) to Schedule the Harvest and Maintain the Storage Quality of ‘Maxi Gala’ Apples. Agronomy 2025, 15, 2151. https://doi.org/10.3390/agronomy15092151
do Amarante CVT, Argenta LC, de Freitas ST, Steffens CA. Preharvest Application of 1-Methylcyclopropene (1-MCP) to Schedule the Harvest and Maintain the Storage Quality of ‘Maxi Gala’ Apples. Agronomy. 2025; 15(9):2151. https://doi.org/10.3390/agronomy15092151
Chicago/Turabian Styledo Amarante, Cassandro Vidal Talamini, Luiz Carlos Argenta, Sergio Tonetto de Freitas, and Cristiano André Steffens. 2025. "Preharvest Application of 1-Methylcyclopropene (1-MCP) to Schedule the Harvest and Maintain the Storage Quality of ‘Maxi Gala’ Apples" Agronomy 15, no. 9: 2151. https://doi.org/10.3390/agronomy15092151
APA Styledo Amarante, C. V. T., Argenta, L. C., de Freitas, S. T., & Steffens, C. A. (2025). Preharvest Application of 1-Methylcyclopropene (1-MCP) to Schedule the Harvest and Maintain the Storage Quality of ‘Maxi Gala’ Apples. Agronomy, 15(9), 2151. https://doi.org/10.3390/agronomy15092151






