Use of Amino Acids and Slow-Release Urea-Based Biostimulants to Enhance Yield and Grain Quality in Durum Wheat Under No-Tillage Conditions in Semi-Arid Region
Abstract
1. Introduction
2. Material and Methods
2.1. Localization, Experimental Design and Treatments
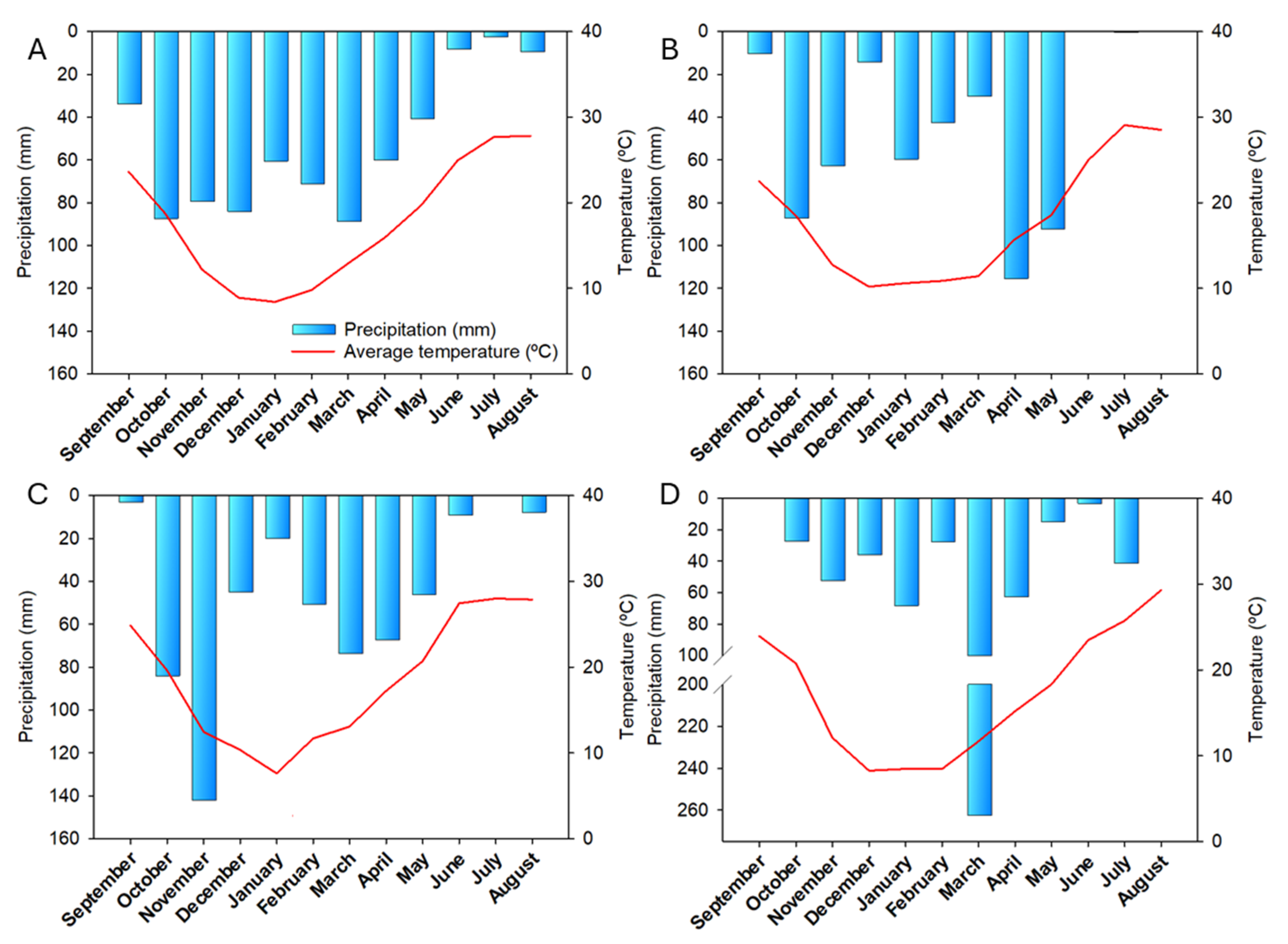
2.2. Climate Conditions
2.3. Soil Sampling and Analysis
2.4. Plant Sampling and Analysis
2.5. Statistical Analyses
3. Results
3.1. Spike Number
3.2. Straw and Grain Yield
3.3. Harvest Index
3.4. Protein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Penuelas, J.; Coello, F.; Sardans, J. A Better Use of Fertilizers Is Needed for Global Food Security and Environmental Sustainability. Agric. Food Secur. 2023, 12, 5. [Google Scholar] [CrossRef]
- Agbodjato, N.A.; Adoko, M.Y.; Babalola, O.O.; Amogou, O.; Badé, F.T.; Noumavo, P.A.; Adjanohoun, A.; Baba-Moussa, L. Efficacy of Biostimulants Formulated with Pseudomonas Putida and Clay, Peat, Clay-Peat Binders on Maize Productivity in a Farming Environment in Southern Benin. Front. Sustain. Food Syst. 2021, 5, 666718. [Google Scholar] [CrossRef]
- Omomowo, O.I.; Babalola, O.O. Genomic Insights into Two Endophytic Strains: Stenotrophomonas Geniculata NWUBe21 and Pseudomonas Carnis NWUBe30 from Cowpea with Plant Growth-Stimulating Attributes. Appl. Sci. 2022, 12, 12953. [Google Scholar] [CrossRef]
- Zhen, S.; Deng, X.; Li, M.; Zhu, D.; Yan, Y. 2D-DIGE Comparative Proteomic Analysis of Developing Wheat Grains under High-Nitrogen Fertilization Revealed Key Differentially Accumulated Proteins That Promote Storage Protein and Starch Biosyntheses. Anal. Bioanal. Chem. 2018, 410, 6219–6235. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Napoli, M.; Mancini, M.; Masella, P.; Cappelli, A.; Parenti, A.; Orlandini, S. Wheat Grain Composition, Dough Rheology and Bread Quality as Affected by Nitrogen and Sulfur Fertilization and Seeding Density. Agronomy 2020, 10, 233. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as Biostimulant: Exploiting the Multilevel Properties of a Plant Beneficial Fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Krol, D.J.; Forrestal, P.J.; Wall, D.; Lanigan, G.J.; Sanz-Gomez, J.; Richards, K.G. Nitrogen Fertilisers with Urease Inhibitors Reduce Nitrous Oxide and Ammonia Losses, While Retaining Yield in Temperate Grassland. Sci. Total Environ. 2020, 725, 138329. [Google Scholar] [CrossRef]
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Anglade, J.; Garnier, J. 50 Year Trends in Nitrogen Use Efficiency of World Cropping Systems: The Relationship between Yield and Nitrogen Input to Cropland. Environ. Res. Lett. 2014, 9, 105011. [Google Scholar] [CrossRef]
- Ramanantenasoa, M.M.J.; Génermont, S.; Gilliot, J.-M.; Bedos, C.; Makowski, D. Meta-Modeling Methods for Estimating Ammonia Volatilization from Nitrogen Fertilizer and Manure Applications. J. Environ. Manag. 2019, 236, 195–205. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, X.; Sha, Z.; Li, S.; Chen, X.; Chen, Y.; Liu, X. Mitigation of Reactive Nitrogen Loss from Arable Soils through Microbial Inoculant Application: A Meta-Analysis. Soil Tillage Res. 2024, 235, 105883. [Google Scholar] [CrossRef]
- Maignan, V.; Bernay, B.; Géliot, P.; Avice, J.-C. Biostimulant Impacts of Glutacetine® and Derived Formulations (VNT1 and VNT4) on the Bread Wheat Grain Proteome. J. Proteom. 2021, 244, 104265. [Google Scholar] [CrossRef] [PubMed]
- Tejada, M.; Rodríguez-Morgado, B.; Paneque, P.; Parrado, J. Effects of Foliar Fertilization of a Biostimulant Obtained from Chicken Feathers on Maize Yield. Eur. J. Agron. 2018, 96, 54–59. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced Systemic Resistance and Plant Responses to Fungal Biocontrol Agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Laurent, E.-A.; Ahmed, N.; Durieu, C.; Grieu, P.; Lamaze, T. Marine and Fungal Biostimulants Improve Grain Yield, Nitrogen Absorption and Allocation in Durum Wheat Plants. J. Agric. Sci. 2020, 158, 279–287. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-Based Biostimulants Modulate Rhizosphere Microbial Populations and Improve N Uptake Efficiency, Yield, and Nutritional Quality of Leafy Vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; González-López, J.; Vallejo, A.; Bedmar, E.J. Effect of Urease and Nitrification Inhibitors on Ammonia Volatilization and Abundance of N-cycling Genes in an Agricultural Soil. J. Plant Nutr. Soil Sci. 2020, 183, 99–109. [Google Scholar] [CrossRef]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving Nitrogen Use Efficiency in Crops for Sustainable Agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Ottaiano, L.; Di Mola, I.; Cozzolino, E.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Biostimulant Application under Different Nitrogen Fertilization Levels: Assessment of Yield, Leaf Quality, and Nitrogen Metabolism of Tunnel-Grown Lettuce. Agronomy 2021, 11, 1613. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Caruso, G.; Cozzolino, E.; De Pascale, S.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Stand-Alone and Combinatorial Effects of Plant-Based Biostimulants on the Production and Leaf Quality of Perennial Wall Rocket. Plants 2020, 9, 922. [Google Scholar] [CrossRef]
- Popko, M.; Michalak, I.; Wilk, R.; Gramza, M.; Chojnacka, K.; Górecki, H. Effect of the New Plant Growth Biostimulants Based on Amino Acids on Yield and Grain Quality of Winter Wheat. Molecules 2018, 23, 470. [Google Scholar] [CrossRef]
- Pichereaux, C.; Laurent, E.-A.; Gargaros, A.; Viudes, S.; Durieu, C.; Lamaze, T.; Grieu, P.; Burlet-Schiltz, O. Analysis of Durum Wheat Proteome Changes under Marine and Fungal Biostimulant Treatments Using Large-Scale Quantitative Proteomics: A Useful Dataset of Durum Wheat Proteins. J. Proteom. 2019, 200, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, T.; Li, Y.; Hu, X.; Wang, Z.; Liu, J.; Qin, W.; Ashraf, U. Deep Fertilization Improves Rice Productivity and Reduces Ammonia Emissions from Rice Fields in China; a Meta-Analysis. Field Crops Res. 2022, 289, 108704. [Google Scholar] [CrossRef]
- Walkley, A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Van Wesemael, J. Den Haag: Stichting Uitgeverij Sigma Chemie. Chemisch Weekblad 1951, 35–36, 596. [Google Scholar]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Mabesa, R.L.; Impa, S.M.; Grewal, D.; Johnson-Beebout, S.E. Contrasting Grain-Zn Response of Biofortification Rice (Oryza sativa L.) Breeding Lines to Foliar Zn Application. Field Crops Res. 2013, 149, 223–233. [Google Scholar] [CrossRef]
- Manzeke, M.G.; Mtambanengwe, F.; Nezomba, H.; Watts, M.J.; Broadley, M.R.; Mapfumo, P. Zinc Fertilization Increases Productivity and Grain Nutritional Quality of Cowpea (Vigna unguiculata [L.] Walp.) under Integrated Soil Fertility Management. Field Crops Res. 2017, 213, 231–244. [Google Scholar] [CrossRef]
- Simoglou, K.B.; Dordas, C. Effect of Foliar Applied Boron, Manganese and Zinc on Tan Spot in Winter Durum Wheat. Crop Prot. 2006, 25, 657–663. [Google Scholar] [CrossRef]
- Ghoname, A.A.; El-Bassiouny, A.M.; Abdel-Mawgoud, A.M.R.; El-Tohamy, W.A.; Gruda, N. Growth, Yield and Blossom-End Rot Incidence in Bell Pepper as Affected by Phosphorus Level and Amino Acid Applications. Gesunde Pflanz. 2012, 64, 29–37. [Google Scholar] [CrossRef]
- Kumar Sootahar, M.; Zeng, X.; Wang, Y.; Su, S.; Soothar, P.; Bai, L.; Kumar, M.; Zhang, Y.; Mustafa, A.; Ye, N. The Short-Term Effects of Mineral- and Plant-Derived Fulvic Acids on Some Selected Soil Properties: Improvement in the Growth, Yield, and Mineral Nutritional Status of Wheat (Triticum aestivum L.) under Soils of Contrasting Textures. Plants 2020, 9, 205. [Google Scholar] [CrossRef]
- Yadav, R.; Ror, P.; Rathore, P.; Ramakrishna, W. Bacteria from Native Soil in Combination with Arbuscular Mycorrhizal Fungi Augment Wheat Yield and Biofortification. Plant Physiol. Biochem. 2020, 150, 222–233. [Google Scholar] [CrossRef]
- Jin, L.; Cui, H.; Li, B.; Zhang, J.; Dong, S.; Liu, P. Effects of Integrated Agronomic Management Practices on Yield and Nitrogen Efficiency of Summer Maize in North China. Field Crops Res. 2012, 134, 30–35. [Google Scholar] [CrossRef]
- Ma, B.L.; Subedi, K.D.; Liu, A. Variations in Grain Nitrogen Removal Associated with Management Practices in Maize Production. Nutr. Cycl. Agroecosyst. 2006, 76, 67–80. [Google Scholar] [CrossRef]
- Arslan, E.; Agar, G.; Aydin, M. Humic Acid as a Biostimulant in Improving Drought Tolerance in Wheat: The Expression Patterns of Drought-Related Genes. Plant Mol. Biol. Rep. 2021, 39, 508–519. [Google Scholar] [CrossRef]
- Hammad, S.A.R.; Ali, O.A.M. Physiological and Biochemical Studies on Drought Tolerance of Wheat Plants by Application of Amino Acids and Yeast Extract. Ann. Agric. Sci. 2014, 59, 133–145. [Google Scholar] [CrossRef]
- Cañasveras, J.C.; Sánchez-Rodríguez, A.R.; Del Campillo, M.C.; Barrón, V.; Torrent, J. Lowering Iron Chlorosis of Olive by Soil Application of Iron Sulfate or Siderite. Agron. Sustain. Dev. 2013, 34, 677–684. [Google Scholar] [CrossRef]
- González-Caballo, P.; Barrón, V.; Torrent, J.; Del Campillo, M.C.; Sánchez-Rodríguez, A.R. Wheat and Maize Grown on Two Contrasting Zinc-Deficient Calcareous Soils Respond Differently to Soil and Foliar Application of Zinc. J. Soil Sci. Plant Nutr. 2022, 22, 1718–1731. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, A.R.; Rey, M.-D.; Nechate-Drif, H.; Castillejo, M.Á.; Jorrín-Novo, J.V.; Torrent, J.; Del Campillo, M.C.; Sacristán, D. Combining P and Zn Fertilization to Enhance Yield and Grain Quality in Maize Grown on Mediterranean Soils. Sci. Rep. 2021, 11, 7427. [Google Scholar] [CrossRef]
- Farias, R.M.D.; Grangeiro, L.C.; Sousa, V.D.F.L.D.; Morais, É.G.; Oliveira, R.R.T.; Pereira, D.D.F.; Souza, B.D.P.; Carmo, L.H.D.A.; Paiva, L.G.D.; Medeiros, G.B.F.D.; et al. Physiology, Biochemistry and Yield of Melon in a Semi-Arid Region with the Application of Biostimulants. Rev. Bras. Eng. Agríc. Ambient. 2025, 29, e283055. [Google Scholar] [CrossRef]
- Montesinos, C.; Benito, P.; Porcel, R.; Bellón, J.; González-Guzmán, M.; Arbona, V.; Yenush, L.; Mulet, J.M. Field Evaluation and Characterization of a Novel Biostimulant for Broccoli (Brassica oleracea Var. Italica) Cultivation under Drought and Salt Stress Which Increases Antioxidant, Glucosinolate and Phytohormone Content. Sci. Hortic. 2024, 338, 113584. [Google Scholar] [CrossRef]




| Treatment | Name | Origin | Rate (L ha−1) | Composition (Weight %) | Composition (kg ha−1) |
|---|---|---|---|---|---|
| T1 | Control (C) | - | - | - | - |
| T2 | Biostimulants | Enzymatic hydrolysis of plant extracts | 2 | 2.7% organic N (amino acids), 4.8% inorganic N | 0.15 kg N ha−1 |
| T3 | Slow-release urea | Synthetic | 10 | 28.5% N (11.5% ureic N and 17%-urea formaldehyde) | 2.85 kg N ha−1 |
| T4 | Biostimulants plus slow-release urea | Same as T2 and T3 | 10 (T2 and 80% of T3) | See T2 and T3 | 2.43 kg N ha−1 |
| T5 | Mg and micronutrients | Synthetic | 3 | 3.9% Ureic N, 9.1% Mg, 9.1% Mn, 4.9% Zn, 3% Cu | 0.12 kg N ha−1, 0.27 kg Mg ha−1, 0.27 kg Mn ha−1, 0.15 kg Zn ha−1, 0.09 kg Cu ha−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Moraga, A.; Sánchez-Rodríguez, A.R.; González-Sánchez, E.J.; Márquez-García, F. Use of Amino Acids and Slow-Release Urea-Based Biostimulants to Enhance Yield and Grain Quality in Durum Wheat Under No-Tillage Conditions in Semi-Arid Region. Agronomy 2025, 15, 2150. https://doi.org/10.3390/agronomy15092150
Moreno-Moraga A, Sánchez-Rodríguez AR, González-Sánchez EJ, Márquez-García F. Use of Amino Acids and Slow-Release Urea-Based Biostimulants to Enhance Yield and Grain Quality in Durum Wheat Under No-Tillage Conditions in Semi-Arid Region. Agronomy. 2025; 15(9):2150. https://doi.org/10.3390/agronomy15092150
Chicago/Turabian StyleMoreno-Moraga, Alfonso, Antonio Rafael Sánchez-Rodríguez, Emilio J. González-Sánchez, and Francisco Márquez-García. 2025. "Use of Amino Acids and Slow-Release Urea-Based Biostimulants to Enhance Yield and Grain Quality in Durum Wheat Under No-Tillage Conditions in Semi-Arid Region" Agronomy 15, no. 9: 2150. https://doi.org/10.3390/agronomy15092150
APA StyleMoreno-Moraga, A., Sánchez-Rodríguez, A. R., González-Sánchez, E. J., & Márquez-García, F. (2025). Use of Amino Acids and Slow-Release Urea-Based Biostimulants to Enhance Yield and Grain Quality in Durum Wheat Under No-Tillage Conditions in Semi-Arid Region. Agronomy, 15(9), 2150. https://doi.org/10.3390/agronomy15092150







