Abstract
Environmental factors affect plant physiological processes. Understanding these factors can increase productivity, especially in tropical mountain ecosystems, where they vary with altitude. This study aimed to analyze the physiological variations related to water vapor and gas exchange in Prunus persica L. Batsch according to the altitudinal gradient in North Santander. One plant was selected per altitude, and six leaves were selected per plant and per branch across three phenological stages. Conductance (gs), stomatal resistance (SR), and transpiration (E) were determined using a calibrated portable porometer over two cycles. Linear mixed-effects models with repeated measurements over time, phenological effects, altitude, and light conditions were used. At higher altitudes, gs and E decreased and SR increased, possibly due to higher ultraviolet radiation and lower temperatures with increasing altitude. Maximum values were reached at EF6. gs and E exhibited diurnal patterns, decreasing at the end of the day to minimize water loss during periods of lower solar radiation. The cultivar adjusted its stomatal and water regulation mechanisms according to altitude. These findings provide advanced insights into plant acclimatization strategies in mountain ecosystems and inform the sustainable management practices needed in the face of impending global climate variability.
1. Introduction
1.1. Importance and Development of Prunus persica L. Batsch Cultivation
The peach tree (Prunus persica L. Batsch) is one of the most economically important fruit crops worldwide. With an annual production exceeding 24.6 million tons, it ranks among the top ten most cultivated fruits globally [1]. The fruit sector has experienced rapid growth in recent decades, and peaches now represent the second most widely produced temperate fruit in the Rosaceae family, following apples and pears [2,3,4]. In Colombia, the cultivation of deciduous fruit trees, including peach, constitutes a key agricultural sector [5]. In 2022, the country recorded an average yield of 13.82 t.ha−1 across 2.265 hectares, with the department of Boyacá accounting for 35.92% of the total planted area, largely due to its favorable climatic conditions [6,7].
Traditional peach cultivars typically require 500 to 800 chilling hours during winter to ensure proper dormancy release [8]. However, peach is a genetically dynamic crop, and by the 1990s, more than 500 cultivars had been developed worldwide through breeding programs aimed at improving adaptability and fruit quality [8]. Some of these cultivars exhibit lower chilling requirements [4] and thus are suitable for tropical and subtropical regions, facilitating the crop’s geographic expansion [2,3].
Research in Brazil, such as the study conducted by Anzanello and Lampugnani (2020) [9], proposed the following classification scale for chilling requirements in peach cultivars: low (<200 chilling hours), medium (201–400 chilling hours), and high (>400 chilling hours). This categorization enabled the appropriate regional allocation of cultivars within the state of Rio Grande do Sul. For instance, cultivars such as Flor da Prince, Vanguarda, Mandinho, Cerrito, Libra, Kampai, Granada, and Bonão require fewer than 200 chilling hours; Fascínio, Baby Gold, Atenas, Maciel, Santa Aurea, and Chimarrita require 201–400 h; while Barbosa, Planalto, Della Nona, and Robidoux require over 400 h. Consequently, cultivars with lower chilling requirements are suitable for most regions in the state, whereas those with medium and high requirements are recommended for specific subregions, such as the western border and the Serra Gaúcha highlands, respectively.
The Jarillo Venezuelan Creole peach plant (P. persica) originated from a limited set of genotypes introduced to Venezuela from the United States in the late 19th century. Due to its high degree of self-pollination and narrow genetic base, this cultivar exhibits limited genetic diversity [10]. It is adapted to altitudes ranging between 1800 and 2200 m above sea level (m a.s.l.) [11]. Its low chilling requirement [12,13] enables its cultivation in cold tropical climates under both continuous and forced production systems by suppressing dormancy-inducing factors [11,14,15].
The cultivar is characterized by a delayed onset of fruit production but exhibits high yield potential, with reported averages of 1917 fruit per plant and 135 kg fruit per plant [16]. Fruit development is notably slow [7], with duration exceeding 125 days from full bloom (defined as the point at which 50% of flowers are open) to commercial harvest, and in some cases extending up to 270 days [17]. The fruit is round, with yellow pericarp and trichomes, though susceptible to staining when handled under humid conditions [16]. Morphologically, the fruit is small, with an average weight of 85.6 g, a polar diameter of 4.5 cm, an equatorial diameter of 5.3 cm, and a pulp-to-seed ratio of 7 [18]. They are suitable for both fresh consumption and industrial processing, owing to their small size and post-harvest handling tolerance.
1.2. Ecophysiology of Plants Under Contrasting Conditions
Tropical regions are characterized by relatively uniform temperatures throughout the year, lacking clearly defined thermal seasons. However, temperature decreases progressively with elevation, giving rise to altitudinal thermal “paradises” that enable the cultivation of temperate crops in tropical latitudes [19]. Along with temperature, other environmental variables are also modified with increasing altitude. Notably, precipitation tends to decline, while solar radiation, particularly ultraviolet (UV) radiation, increases markedly [20,21,22,23]. UV radiation rises by approximately 10–12% for every 1000 m gain in elevation due to reduced atmospheric filtering caused by the thinner air column at high altitudes [20].
Light conditions in mountainous environments also fluctuate significantly due to diurnal cycles, cloud cover, and canopy shading [24]. According to [25], during sunrise and sunset -when the sun is at a lower solar angle-solar radiation must traverse a longer atmospheric path, increasing the scattering and absorption of light, and resulting in reduced light intensity. These variations directly influence plant physiological functions such as photosynthesis and stomatal regulation [12,25,26].
Altitude exerts a strong effect on temperature, with an average decrease of 0.6 °C per 100 m of elevation [23], and it also influences water evaporation rates and atmospheric water-holding capacity. Consequently, ecosystems at different altitudes but similar latitudes exhibit distinct thermal and moisture regimes. In addition, the atmospheric pressure of gases such as CO2, O2, and N2—as well as water vapor—declines with elevation, affecting plant gas exchange and metabolism [21].
Moreover, high-altitude tropical ecosystems exhibit pronounced diurnal climate variation, often surpassing seasonal variation, and are increasingly relevant in the context of global climate change due to their sensitivity to environmental fluctuations [23,27].
Studies such as [28] have shown that plants at higher elevations display higher transpiration rates and increased stomatal conductance compared to those growing at lower altitudes. This suggests the existence of specific adaptation strategies along altitudinal gradients. According to [29], species exposed to environmental variability across altitudinal gradients develop broad physiological tolerance via molecular mechanisms that enable acclimation and survival under diverse climatic conditions [30]. However, the specific ecophysiological responses of the Venezuelan Jarillo peach cultivar (P. persica) across altitudinal gradients remain poorly understood.
The department of North Santander, located in the eastern Andean range of Colombia, contains a mountainous physiographic zone classified as the Middle Andean Orobiome (Om-A), ranging between 1800 and 2800 m a.s.l. [31]. This region includes the upper basins of the Orinoco and Catatumbo rivers. Within the upper Orinoco basin, the municipality of Silos (1810 m a.s.l.) records an average temperature of 12 °C and an atmospheric pressure of 817.27 hPa; Cácota (1950 m a.s.l.) reports 14 °C and 815.93 hPa; and Chitagá (2150 m a.s.l.) registers 18 °C and 806.60 hPa. All three sites share a unimodal rainfall pattern with annual precipitation below 1000 mm.
Two additional sites in the upper Catatumbo basin were also selected for this study. Pamplonita (1860 m a.s.l.) exhibits an average temperature of 20 °C, atmospheric pressure of 814.60 hPa, and approximately 900 mm of annual precipitation. Pamplona (2170 m a.s.l.) shows an average temperature of 16 °C, atmospheric pressure of 805.27 hPa, and 1200 mm of annual precipitation. Both municipalities display a bimodal precipitation regime [32].
1.3. Light-Dependent Functions and Integration in Metabolism
Understanding the climatic requirements for the development of a species, ecotype, or crop—and even specific cultivars within a crop—is essential for improving productivity. This is achieved by identifying the physiological mechanisms that mediate organismal responses to environmental cues [33]. In this context, previous research has shown that the adaptation of fruit trees to high-altitude zones (typically ranging between 1600 and 3200 m a.s.l.) is strongly modulated by local climatic factors [27].
Light is not only essential as the energy source for photosynthesis, captured by pigment-protein complexes in the chloroplasts, but it also acts as a central regulator of a wide array of physiological processes. Among these are stomatal dynamics and guard cell metabolism. Blue light, for instance, promotes stomatal opening by activating plasma membrane H+-ATPases and K+ influx through phototropin-mediated signaling pathways [34]. Red light complements this process by enhancing ATP production through local photosynthesis in guard cells [35]. These light responses are coordinated with sugar metabolism and starch degradation, ensuring the osmotic changes necessary for guard cell turgor and sustained stomatal opening [35].
At the ecophysiological level, photoprotection and repair mechanisms are crucial to prevent damage to photosystem II (PSII) caused by excessive light intensity, which is a frequent stressor in low-latitude, high-irradiance environments [36,37]. In P. persica, studies suggest that the PpLhc gene expression is light-harvesting chlorophyll from light, thereby playing a role in drought tolerance by modulating the response to water stress [38]. These mechanisms can significantly influence photosynthetic efficiency and stomatal response to changes in light quality and intensity along altitudinal gradients.
For example, ref. [39] demonstrated that high-altitude accessions of Tibetan peach (P. persica) exhibited reduced stomatal density but increased stomatal size—morphological adaptations that reduce total stomatal conductance and transpiration. These changes are interpreted as adaptations to hypoxic conditions, intense solar radiation, and thermal stress. Furthermore, genetic variation in the PpEPF1 gene family appears to be involved in regulating stomatal patterning in response to environmental gradients, representing a potential evolutionary innovation for optimizing CO2 assimilation while minimizing water loss under abiotic stress.
1.4. Gaseous Exchange, Water Relations, and Photosynthesis Efficiency Under Contrasting Conditions
Key physiological processes in plants—such as gas exchange, water relations, and resource use efficiency—are highly sensitive to environmental conditions, particularly those varying along altitudinal gradients. Stomatal regulation plays a central role in balancing CO2 uptake for photosynthesis with water loss via transpiration. This mechanism is especially susceptible to atmospheric vapor pressure deficit (VPD) and water availability.
According to the ecophysiological optimum model, a reduction in atmospheric pressure at higher altitudes leads to a decreased partial pressure of CO2 and O2, and a higher vapor pressure gradient between leaf and air. This results in increased transpiration per unit of carbon fixed and prompts physiological adjustments such as a decrease in stomatal conductance (gs) and a lower internal-to-external CO2 concentration ratio (χ) [40].
Stomatal movement is governed by a complex network of hydraulic signals, hormonal pathways, and environmental stimuli. Among these, the phytohormone abscisic acid (ABA) plays a pivotal role under water-deficit conditions. ABA triggers the activation of anion channels (e.g., SLAC1), promoting ion efflux (K+, Cl−) from guard cells, which in turn reduces turgor pressure and induces stomatal closure [35,41].
In addition, hydraulic signaling—such as a drop-in leaf or stem water potential (Ψ_leaf, Ψ_stem)—often precedes hormonal responses, particularly in anisohydric species like many Prunus cultivars. This early hydraulic signal enables a rapid physiological adjustment to fluctuating environmental conditions [42]. At the cellular level, vacuolar K+ fluxes mediated by NHX-type exchangers are also essential for maintaining guard cell turgor during stomatal opening and closure [43].
Recent findings also highlight the influence of circadian regulation; transcription factors such as PIFs (Phytochrome-Interacting Factors) initiate stomatal opening at dawn by inducing the expression of the K+ channel gene KAT1, even in the absence of ABA. This underscores the importance of endogenous day-night rhythms in stomatal behavior [44].
In P. persica, water stress conditions that reduce stem water potential below −1.5 MPa have been shown to drastically lower stomatal conductance (gs) and transpiration (E), thereby affecting both vegetative and reproductive development [45]. Other water relations parameters—such as relative water content (RWC) and Ψ_stem—are tightly associated with photosynthetic efficiency under drought conditions [46]. For instance, the exogenous application of lauric acid has been shown to preserve leaf water content and sustain high net CO2 assimilation rates in peach, enhancing water use efficiency (WUE) by improving stomatal function and activating stress-responsive gene expression [46]. Moreover, carbon isotope discrimination (δ13C) has been validated as a reliable long-term integrator of WUE in Prunus hybrids, allowing for the physiological evaluation of drought responses across diverse environments [47].
Globally, it has been observed that, under open-stomata conditions, plant transpiration can double with every 1.000 m increase in altitude due to greater solar radiation and atmospheric vapor diffusivity [48]. Additionally, ref. [49] reported that in productive nectarines, stomatal conductance and transpiration rates decrease under postharvest water stress, and that VPD can become a limiting factor for gas exchange even when stomata remain open.
1.5. Molecular Processes Integrated into Stomatal Physiology
In the context of altitudinal and climatic gradients, it is essential to understand how molecular mechanisms regulating stomatal physiology and light-dependent metabolism are integrated into the adaptive responses of P. persica. Stomatal opening is initiated through light perception by specific photoreceptors—mainly phototropins (responsive to blue light) and phytochromes (responsive to red/far-red light)—which activate a cascade of signaling events in guard cells. These events include the stimulation of plasma membrane H+-ATPases, activation of K+ channels, and the generation of osmotic gradients that facilitate turgor-driven stomatal aperture [35,50].
These light-induced responses are tightly coupled with chloroplastic and mitochondrial metabolism in guard cells. Starch degradation, sugar mobilization, and ATP generation are critical for maintaining osmolyte concentrations and stomatal functionality under favorable light conditions [51]. In parallel, under conditions of high irradiance or water deficit, the accumulation of abscisic acid (ABA) and reactive oxygen species (ROS) triggers the closure of stomata and activates photoprotective mechanisms—including non-photochemical quenching and the regulation of light-harvesting antenna proteins [38,42].
Water stress significantly disrupts plant development and photosynthetic activity by inducing oxidative damage and compromising cellular structures. The application of melatonin has emerged as a promising strategy to enhance plant tolerance by protecting chloroplast membranes, stabilizing chlorophyll content, and modulating stomatal activity. Melatonin functions as an antioxidant by activating enzymatic defense systems that scavenge ROS and mitigate oxidative stress [52].
In mountain environments, where plants are exposed to high UV radiation, low temperatures, reduced oxygen availability, and declining precipitation with elevation [20,21,22,23], these stressors collectively challenge plant homeostasis and photosynthetic efficiency. Under such conditions, ROS accumulation becomes a double-edged sword: at high concentrations, ROS can cause photoinhibition and cellular damage—such as the oxidation of PSII proteins and disruption of photosynthetic electron flow [52,53,54,55]—whereas at moderate levels, ROS act as signaling molecules that mediate stress responses and stomatal behavior [56].
Hence, the capacity of P. persica to modulate stomatal physiology through the integration of environmental, hormonal, and redox signals is a key adaptive feature in altitudinal environments. Understanding these mechanisms is fundamental for developing stress-resilient cultivars and optimizing crop performance in variable climates.
1.6. The Importance of Research in Altitudinal Gradients
Despite the growing interest in understanding the physiological responses of P. persica to varying environmental conditions, significant knowledge gaps remain regarding its ecophysiological performance in high-altitude tropical regions. These zones—characterized by intense solar radiation, marked diurnal thermal fluctuations, and variable water availability—present unique challenges for fruit production and crop adaptation [21,22,23].
Although recent studies have documented physiological and metabolic adaptations in mountain fruit trees [23,57,58], few have explicitly addressed the dynamics of stomatal conductance, resistance, and transpiration in P. persica under productive field conditions in the high-altitude tropics. There is a pressing need for research that examines how these gas exchange and water balance variables respond to natural altitudinal gradients, in order to better understand the mechanisms regulating plant performance in such complex environments.
Furthermore, with the ongoing effects of climate change—particularly rising temperatures and shifts in solar radiation—altitudinal gradients provide natural laboratories to assess how changes in environmental conditions influence plant phenology, physiology, and productivity. These gradients offer an opportunity to anticipate the potential impacts of global climate change on crop systems and to design strategies for climate-resilient agriculture [59].
Given this context, the objective of the present study is to determine the physiological variation of Jarillo or Venezuelan Creole peach plants (P. persica) during the productive phase across different altitudinal gradients in the department of North Santander, Colombia. The results of this research aim to enhance our understanding of how this cultivar responds to varying altitudes and associated microclimatic conditions. Such knowledge is critical for the development of improved management practices and the selection of genotypes better suited to cultivation in mountainous tropical environments.
2. Materials and Methods
2.1. Location
This study was carried out in five Jarillo or Venezuelan Creole peach plants (P. persica) in producing farms in the department of North Santander, Colombia, located between the upper Orinoco basin and Catatumbo, according to the altitudinal gradient. In the upper Orinoco basin, the municipalities Silos (1810 m a.s.l.), Cácota (1950 m a.s.l.), and Chitagá (2150 m a.s.l.) were selected. Two municipalities were selected in the upper Catatumbo basin. The Pamplonita municipality is located at 1860 m a.s.l. The municipality of Pamplona is located at 2170 m a.s.l. In terms of soil type, the municipality of Silos is dominated by Entisol soils, while the rest of the municipalities have Inceptisol soils [60]. Each of these regions is associated with each of the elevations studied [11] (Figure 1).
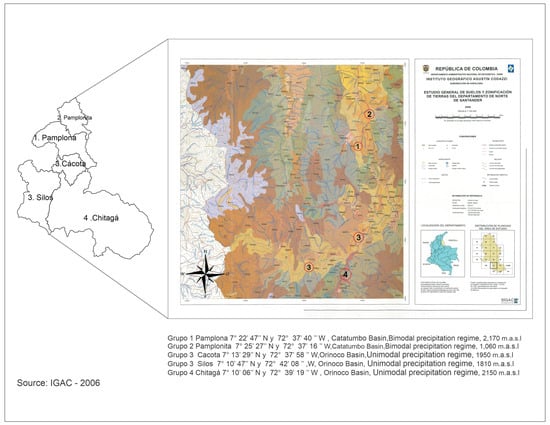
Figure 1.
Geographical location of the study areas according to the altitudinal gradient, in the department of North Santander, Colombia. 1 and 2. Upper Catatumbo Basin (groups 1 and 2), with a bimodal precipitation regime; 1: Municipality of Pamplona (7°22′47″ N and 72°37′40″ W, 2170 m a.s.l.); 2. Municipality of Pamplonita (7°25′27″ N and 72°37′16″ W, 1860 m a.s.l.); 3 and 4: Upper Orinoco Basin (groups 3 and 4), with a unimodal precipitation regime; 3: Municipality of Cacota (7°13′29′′ N and 72°37′58″ W, 1950 m a.s.l.); 3: Municipality of Silos (7°10’47″ N and 72°42′08″ W, 1810 m a.s.l.); 4: Municipality of Chitagá (7°10′06″ N and 72°39′19″ W, 2150 m a.s.l.). Source: [60].
2.2. Plant Material
The Jarillo Venezuelan Creole plant peach (P. persica) was evaluated. This cultivar is highly valued due to its excellent adaptation to local conditions, high productivity, and widespread market acceptance for fresh and processed consumption [16]. In each farm, a ten-year-old individual [61] sexually propagated without grafting (franc) was selected. According to the non-extended BBCH scale [62,63], they were in the main stage 9: stage of senescence and the beginning of vegetative rest at the time of this study. Fruit thinning was carried out between 60 days and 70 days after defoliation, which was managed with the agronomic method of forced production [11,16]. The study plot of Jarillo or Venezuelan Creole peach plants (P. persica) was planted 7 m apart in rows and between rows in a real frame [64], under rainfed conditions with a homogeneous slope and the same agronomic management [11].
2.3. Phenology
Physiological variables were determined in Jarillo or Venezuelan Creole peach plants (P. persica) according to phenological stage, a term used to describe the sequence of morphological and physiological phenomena occurring during the annual growth cycle of plants [65]. From plant defoliation, developmental phenological stages were identified using the non-extended BBCH scale, at main stage 6: flowering, main stage 7: fruit formation, and main stage 8: fruit ripening [62,63]. The variables were recorded monthly during two production cycles. Cycle 1 was from March to December, and Cycle 2 from January to September.
2.4. Variables Evaluated
The conductance (gs) (cm.s−1) and stomatal resistance (SR) (s.cm−1), as well as transpiration (E) (mmol.m−2.s−1) were determined using a diffusion porometer (Delta T AP4, Delta-T Devices, England), calibrated every two hours [66]. For the measurement of the evaluated variables (gs, SR and E), three fully unfolded and photosynthetically active adult leaves were selected from each plant with traditional open-vessel architecture, on identified tertiary branches [28], located in the middle third of the central axis of each plant, in the first (apical part of the branch) and third (basal part of the branch) thirds of the branch (light and shaded conditions, respectively) [16,61]. Three readings per leaf were taken every two hours during the daily course between 8:00 and 16:00 h.
2.5. Plant Sampling
Plant sampling at each altitude was performed using the conditional Latin hypercube [67], and according to the methodology described by [7] for the selection of five plants at random, at the rate of one per altitude of the same age and identified [16]. Sampling was conducted during two production cycles in 2021 and 2022, each lasting 7 months. Between these cycles, there was a rest period of 45 to 60 days, depending on the altitude where the crop was grown [14].
2.6. Experimental Design and Statistical Analysis
The research design was a non-experimental field, observational, repeated measures, with descriptive and explanatory stages. Prior to the statistical analysis, an exploratory data analysis (EDA) was used [68]. This was performed in order to detect the presence of outliers, inconsistencies in the data, and any other anomalies. Box plots were used, and data that might be outside the study population, the scale of measurement of the variables under study, and the distribution of the variables were reviewed.
Six replicates or leaves per plant were used, one plant during three phenological stages (6: flowering; 7; fruit formation; 8: fruit ripening) per altitude (1810; 1860; 1950; 2150 and 2170 m a.s.l.), having a total of 108 leaves with five repeated measurements per leaf (measurement time: 8, 10, 12, 14, 16 h). The cleaned data were initially entered into a Microsoft Excel 2015 spreadsheet prior to statistical analysis. This analysis was performed using SPSS version 27 [69].
To detect departures from normality, the Shapiro–Wilk test, kurtosis and skewness values, and histogram figures were used; and for the verification of homoscedasticity, Levene’s test was used. Due to the distance from normality of the data of the physiological dependent variables, in order to carry out the corresponding statistical hypothesis tests in the analysis of variance (ANOVA), the Neperian logarithm (Nl) transformation was used. The application of which reduces the degree of asymmetry of the distribution of the data. A nonparametric Spearman linear correlation analysis was applied [70].
The intrasubject factor was the time or hour of daily measurement, and the intersubject factors were altitude, phenological state, and their first-order interactions.
For the statistical analysis of the effects of the independent variables on each of the following dependent variables: gs, SR and E, a linear mixed effects model for repeated measures was fitted with the fixed effects structure of altitude, phenological stage, time of day and their first order interactions, where the covariate light-shade effect was introduced; and a residual correlation structure to model the correlations between errors in measurements over time or repeated measures. The selection of the variance-covariance matrix included in the mixed-effects model or repeated measures modelling was made considering the lower values of the Akaike information criteria (AIC), Bayesian information criteria (BIC), and restricted log-likelihood-2, using the maximum likelihood method in the estimation of the parameters. The use of mixed models allows the autocorrelation structure of the residuals to be incorporated into the model and corrects for model heteroscedasticity problems in order to perform reliable hypothesis testing on the significance of the fixed effects parameters. Errors were modeled with the AR (1) or first-order autoregressive covariance matrix.
For the statistical analysis of the fixed effects on the dependent variables of interest, analysis of variance (ANOVA) was used in a linear mixed effects model for repeated measures with an intrasubject factor (time or hour of daily measurement) and the inter-subject factors were altitude, phenological stage, and their first-order interactions. In the fixed factors where the effects were significant (p < 0.05), Bonferroni tests on marginal means were used, and, in the interactions, graphs were constructed for each significant interaction, making an interpretation of each interaction.
3. Results and Discussion
None of the dependent variables in this research presented a data distribution approximating a normal curve, so a logarithmic transformation was used for the variable E; while for the variables gs and SR, the Ln (y + 1) transformation was used, due to the presence of values less than one.
The dimension of the linear mixed effects model used in this research, presented as structuring elements: the fixed effects, intersection, altitude, phenological state, time of measurement of the dependent variables during the day: 8:00 am; 10:00 am; 12:00 m; 2:00 pm; and 4:00 pm, as a repeated measures factor, the interactions; and the light and shade factor as a covariate (without shade, with shade). Errors were modelled with the AR (1) or first-order autoregressive covariance matrix.
For the statistical analysis of the fixed effects on the dependent variables of interest, analysis of variance (ANOVA) was used in a linear mixed effects model for repeated measures with an intrasubject factor (the time or hour of daily measurement) and the inter-subject factors were altitude, phenological state, and their first-order interactions. It can be seen that for Cycle 1 (Table 1), there is a highly significant effect (p < 0.001) of time of measurement, altitude, phenological stage, and their interactions on SR and E.

Table 1.
Summary of the Analysis of Variance for the Physiological Variables of Jarillo or Venezuelan Creole Peach Plants (P. persica) in Productive Phase at Different Altitudes in the Department of North Santander, Colombia.
For gs, there was only a highly significant effect (p < 0.001) of measurement time, and the interaction of measurement time with both altitude and phenological stage. At the same time, the altitude and the interaction measurement time and phenological stage were significant (p < 0.05), without differences for the effect of phenological stage.
In Cycle 2, statistical differences (p < 0.001; p < 0.05) were observed for the simple effects of the study factors on the variables evaluated (Table 1). For the interaction of the factors, only a significant difference (p < 0.05) of altitude by the time of measurement was recorded for the SR. While for the interaction phenological stage and time of measurement, only a significant difference (p < 0.05) was recorded for gs and a highly significant difference (p < 0.001) for SR; no significant differences were observed between the variables evaluated for the interaction altitude and phenological stage.
The variable presence of light and shade was introduced in the model as a covariate, but its effect was significant (p < 0.05) only in the model for the dependent variable E in Cycle 1; while in Cycle 2, it was significant (p < 0.05) for all variables (Table 1).
3.1. Effect of Altitude and Time of Day on Physiological Variables
The effects of the interaction between altitude levels and time of day on the physiological variables under study during the two cycles are presented in Figure 2.
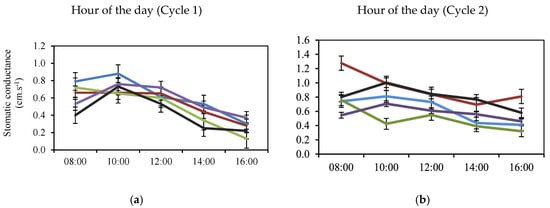
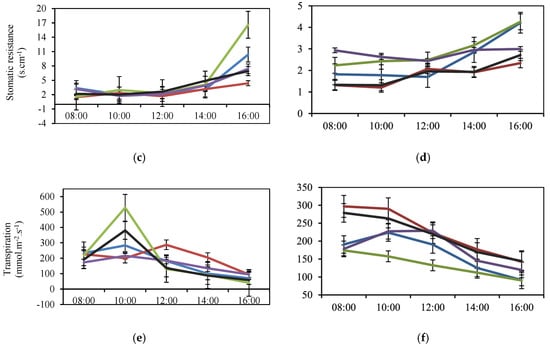
Figure 2.
Diurnal Fluctuation of the Physiological Variables Conductance (a,b) and Stomatic Resistance (c,d) and Transpiration (e,f) of Jarillo or Venezuelan Creole Peach Plants (P. persica) in Productive Phase for the Interaction Altitude (blue: 1810; red: 1860; green: 1950; purple: 2150 and black: 2170 m a.s.l.) and Time during two Cycles (1: March to December and 2: January to September) in the Department of North Santander, Colombia.
In Cycle 1, a slight increase in gs was observed at 10:00 h with the highest mean value recorded for 1810 m a.s.l., after which it decreased over the measurement time, while the lowest value (0.13 cm.s−1) was at 16:00 h for 1950 m a.s.l. (Figure 2a). The higher stomatal conductance (gs) observed at 10:00 h between 1810 and 1950 m above sea level (Figure 2a) may be explained by the atmospheric pressure recorded at each altitude: 817.27 hPa at 1810 m and 815.95 hPa at 1950 m. This pressure difference favors a lower vapor pressure at lower elevations, as previously reported [40].
In Cycle 2, no significant difference (p > 0.05) was observed for the interaction between altitude and time of measurement for gs (Figure 2b).
In relation to the SR, the lowest mean values occurred at 8:00 h, values that increased during the hours of measurement in both cycles (Figure 2c,d), with the highest value recorded at 1950 m a.s.l. (Figure 2c). During the second cycle, greater variability in values was observed from 14:00 h onwards (Figure 2d). E showed a decrease in mean values during the measurement time (Figure 2e,f), with higher variability during Cycle 1 (Figure 2e). The Jarillo or Venezuelan Creole peach plants (P. persica) were established at 1950 m a.s.l. presented statistically higher mean E values (p < 0.001), reaching 527.08 mmol.m−2.s−1 at 10:00 h, from which time they began to decrease until registering the lowest value at 16:00 h (Figure 2e).
In general, the highest mean values of gs and E were recorded at lower altitudes and decreased with increasing altitude, contrary to [4,28], who stated that plants at higher altitudes recorded higher gs and E rates compared to plants located at lower altitudes. The discrepancy with the present study may be related to statements made by several authors that solar radiation, especially ultraviolet (UV) radiation, increases at higher altitudes in tropical high-altitude ecosystems, while temperature and partial pressure of gases decrease [21,22,23,27].
Additionally, it has been shown that field conditions are rarely stable and continuously influence the response of gs during the course of the day, leading to complex kinetic patterns in the diurnal period [24]. To cope with these climatic variations, plants develop specific adaptive strategies [29,31], such as the regulation of stomatal aperture. Hence, the ecophysiological importance of gs, given its relationship with the CO2 partitioning process within the leaf, a process during which carbon acquisition is prioritized over water loss [71].
Additionally, studies conducted by [38] reported that genes of the photosynthetic light-harvesting complex (PpLhc) in P. persica exhibit differential expression patterns, linking light regulation to drought tolerance. These findings suggest that variations in light quality and intensity along an altitudinal gradient may directly modulate photosynthetic efficiency and stomatal responses in this fruit crop.
On the other hand, the increase in SR recorded in the present investigation, with increasing altitude, indicated difficulty for gas exchange, because SR is inversely related to gs, according to [24]. This situation may be associated with the higher incidence of solar radiation, especially ultraviolet (UV) radiation at higher altitudes [21,22,23].
The physiological variables assessed during the day generally followed the same trend (Figure 2), with significant differences between altitudes (Table 1), except during Cycle 2 for the variables gs and E. These differences may be due to the fluctuations typical of tropical high-altitude ecosystems, as reported by [23,27].
3.2. Effect of Phenological Stage and Time of Day on Physiological Variables
The effects of the interaction between phenological stage levels and time of day on the physiological variables under study during the two cycles are presented below (Figure 3).
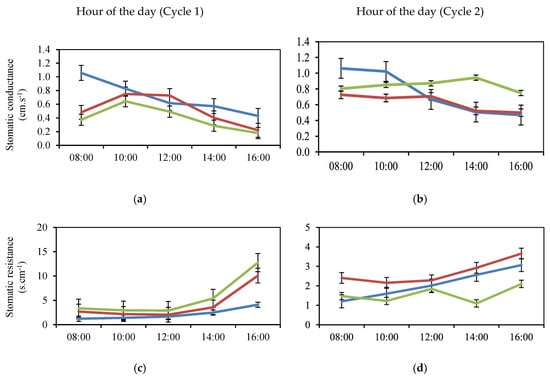
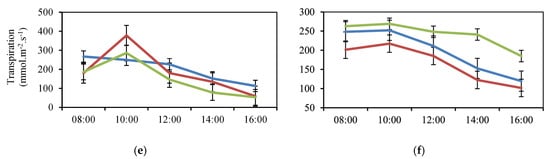
Figure 3.
Diurnal Fluctuation of the Physiological Variables Conductance (a,b) and Stomatic Resistance (c,d) and Transpiration (e,f) of Jarillo or Venezuelan Creole Peach Plants (P. persica) in Productive Phase for the Interaction Phenological State (Stage 6 (flowering): blue; Stage 7 (fruit formation): red; Stage 8 (fruit ripening): green) and Time during two cycles (1: March to December and 2: January to September) in the Department of North Santander, Colombia.
In relation to gs, it can be seen that the means or effects of time of day were different in the three phenological stages of the Jarillo or Venezuelan Creole peach plants (P. persica) (Figure 3a,b); that is, the effect of time of day on gs depended on the phenological stage. In Cycle 1, at 8:00 h, the value of gs at phenological stage 6 was 1.06 cm.s−1, while at phenological stages 7 and 8, it was 0.48 and 0.37 cm.s−1, respectively (Figure 3a).
The trend of gs at phenological stage 6 was to decrease as the time of day passed, while from 8:00 h to 10:00 h, an increase in gs was observed at phenological stages 7 and 8. However, after 12:00 h, there was a trend towards decreasing gs values at all three phenological stages, with the lowest value observed at 16:00 h (0.18 cm.s−1), as noted by [32] who have stated that this behavior occurs even under equal environmental conditions during the morning and at the end of the day. Furthermore, the drop in (gs) and (E) at midday, especially in leaves with high anthocyanin content, confirms that these pigments help filter out harmful radiation, mitigating the overexcitation of photosystem II [72]. It has been reported that plant survival under stressful conditions is due to the regulation of these processes mediated by UVR8 and CRYs [72].
The statistical difference (p ≤ 0.001) observed in gs at 10:00 h between EF6 and EF7 (0.83 and 0.75 cm.s−1, respectively) (Figure 3a) may be associated with the higher demand for photoassimilates required by EF7 (fruit formation), because the fruit constitutes the main sink for photoassimilates during the fruit growth period, as pointed out by [73]. Furthermore, the climatic variations that can occur during the day in high-altitude tropical ecosystems are also important, as stated by [23,27]. These climatic variations during the day affect the physiology of fruit trees [19,26,30] and may be the explanation for the variations observed during the day between the phenological stages evaluated.
During Cycle 2, a decrease in gs was recorded during the measurement time for phenological stage 6 (Figure 3b) with 0.47 cm.s−1 at 16:00 h; the same behavior was observed at phenological stage 7, with a variation at 12:00 h, where a slight increase of 0.03 cm.s−1 occurred compared to 10:00 h. During phenological stage 8, gs increased during the day, including at 14:00 h, and then decreased until reaching the highest value recorded at 16:00 h, compared to phenological stages 6 and 7. These variations in EF8 (Figure 3b) may be associated with the fruit ripening process in which fresh weight gain intensifies until harvest time, as indicated by [74] for P. persica in the Colombian highlands. At this stage, the leaf, by regulating the stomatal aperture, gives priority to carbon gain over water loss, as indicated by [71].
In the same order of ideas, when exploring the significant interaction time of day by phenological stage (Figure 3), in the same way, it could be verified that the means or effects of the time of day on SR were different in the three phenological stages of Jarillo or Venezuelan Creole peach plants (P. persica); that is to say, that the effect of the time of day on SR depended on the phenological stage, this was observed in Figure 3d where it can be appreciated the tendency that presented the means of SR in the three phenological stages for Cycle 1 (Figure 3c), noticing similar values of SR between 1.93 and 2.96 s.cm−1 from 8:00 h to 12:00 h in the three phenological stages. From 14:00 h to 16:00 h, the SR values in the leaves increased, with the highest mean value in phenological stage 8 (12.77 s.cm−1). EF8 comprises the fruit ripening process [62,63].
In Cycle 2 (Figure 3d), the same trend of increasing SR at phenological stage 6 was observed as in Cycle 1, with advancing time of day. Phenological stage 7 also increased with time of day and had the highest values, with stage 8 (fruit ripening) the lowest.
When considering the variable E, the interaction values presented in Figure 3 allow comparing the E trends in the three phenological stages, with a decrease in E at phenological stage 6 during the course of the day in Cycle 1 (Figure 3e). Similarly, in Cycle 1, two mean increases in E were recorded, at 10:00 am, corresponding to values of 377.71 mmol.m−2.s−1 for physiological stage 7 and 285.70 mmol.m−2.s−1 for stage 8. In Cycle 2, no significant difference (p > 0.05) was observed for the interaction between EF altitude and time of measurement for E (Figure 3f).
3.3. Effect of Altitude and Phenological Stage on Physiological Variables
The mean values of the physiological variables of Jarillo or Venezuelan Creole peach plants (P. persica), in the productive phase for the interaction between altitude and phenological stage, are presented in Table 2.

Table 2.
Mean Values of Physiological Variables of Jarillo or Venezuelan Creole Peach Plants (P. persica) in the Production Phase for the Interaction Altitude with Phenological State 6 (flowering), 7 (fruit formation), and 8 (fruit ripening) in the Department of North Santander, Colombia.
The values observed in the variables determined in the plant canopy varied strongly during this study [75] according to phenological stage and altitude. In Cycle 1, stomatal conductance (gs) was highest during phenological stage 6 (flowering) at both 1810 and 2150 m a.s.l., with declines as development progressed into stages 7 and 8 (Table 2), both altitudes in the upper Orinoco basin. Stage 8 (fruit maturation) also had the lowest gs values at 1950 m a.s.l. but values were highest at 2170 m s.a.l.
The lowest values were recorded at 1860 and 2170 m a.s.l. (upper Catatumbo basin) for the same EF. The differences observed may be due to the influence of the environment of the study areas on plant physiology, as they are in different river basins [33].
However, statements made by [31] indicated that the response of gs may be the combination of several internal plant as well as environmental stimuli, hence it is difficult to predict in different ecosystems.
The mean values of SR during flowering (EF6) in Cycle 1 increased with increasing altitude, except at 2150 m a.s.l., where it was lower by 0.74 s.cm−1 when compared to the value recorded at 1950 m a.s.l. (Table 2). In addition, during Cycle 1, a trend towards an increase in mean SR values according to phenological stage was observed in the study areas located in the upper Orinoco basin (1810; 1950 and 2150 m a.s.l.), a trend that was not present in the upper Catatumbo basin (1860 and 2170 m a.s.l.), a difference that may be due to the environmental influence on the plant physiology of the Jarillo or Venezuelan Creole peach plants (P. persica) given that this study was conducted in different hydrographic contexts, as pointed out by [33].
Furthermore, it should be noted that high altitude tropical ecosystems are characterized by climatic variations in a single day [23,27]; conditions that affect Jarillo or Venezuelan Creole peach plants (P. persica) physiological variables, as stated by [21,26]. Among these, gs and SR, as well as E, are important variables that regulate gas exchange and water status of plants [76], events that occur mainly in leaves where stomata play an important role. In turn, stomatal closure is influenced by relative humidity, as well as by stomatal density and size, which close in response to lower relative humidity [25].
In relation to E, the values recorded in the leaves of Jarillo or Venezuelan Creole peach plants (P. persica) during Cycle 1 stood out for being higher than those recorded in Cycle 2 (Table 2). During Cycle 1, Jarillo or Venezuelan Creole peach plants (P. persica) growing at lower altitudes (1810 m a.s.l.) presented statistically higher mean E values (p < 0.001) than those established at higher altitudes, during the flowering phenological stage (EF6), which again indicates the effect of the environment on this response. However, during the phenological stage of growth (EF7) and fruit ripening (EF8), the same behavior was not observed.
In EF7, the mean value of E was statistically higher (p < 0.001) at 1950 m a.s.l.; however, when comparing the mean values recorded at altitudes of 1810, 1860, and 2170 m a.s.l., a slight decrease was observed with increasing altitude. In the absence of any discernible trend in mean E values according to altitude to the EF8, a marked variation was documented, amounting to 203.97%, when contrasting E values across the range of 1810 to 2170 m a.s.l.
During flowering, there was higher transpiration at lower altitudes in Cycle 1 except for 2150 m a.s.l., but there was no such relationship in Cycle 2; the higher E would be associated (r2 = 0.730; p < 0.001) to the higher gs (Table 3), since E is the loss of water, by evaporation, through stomata and leaf cuticle [24]. The behavior of the variables evaluated during the reproductive phase of the Jarillo or Venezuelan Creole peach plants (P. persica) in the study areas supports the findings of [77], who pointed out that the prevailing climatic conditions during their study affected the physicochemical characteristics evaluated in fruit during the process of growth until ripening.

Table 3.
Spearman correlation coefficients between the variables’ stomatal conductance and resistance, and transpiration in the two production cycles.
In addition, plants experience light in a variety of intensities and spectral properties as a result of cloud passage, changes in canopy cover, and self-shading of overlapping leaves [24]. This causes fluctuations in spectral distribution that affect stomatal behavior, carbon gain, and the diurnal course of plant energy efficiency [78,79].
Both the quantity and quality of light available to plants at a given location depend on the sun’s path across the sky. As the sun angle decreases at dawn and dusk, sunlight travels an increasingly longer path through the atmosphere, increasing the absorption and scattering of atmospheric light and reducing the shorter wavelengths of light [24]. Additionally, the contribution of direct compared to diffuse radiation is reduced, often causing a blue light peak [80], affecting light quality and thus plant response. Variations in light quality during the day can influence the response of stomatal dynamics and diurnal behavior and consequently compromise photosynthetic efficiency and water utilization due to diffusion limitations set by gs on the net photosynthetic rate [24,81].
3.4. Covariance Estimates for Conductance and Stomatal Resistance, and Transpiration
The residual variance estimate for the effect of repeated measures on gs was 0.004383; its effect was highly significantly different from zero (p < 0.001), and within-subjects errors were highly significantly correlated, Rho: (0.09; p < 0.001), according to the Wald test. The estimated coefficient value of Rho was 0.09, which is considered low; however, it was significantly positive, indicating that there was a low positive correlation between errors as measurement time elapsed, which was trapped by the selected AR matrix (1).
Similarly, the residual variance estimate for the effect of repeated measures on SR was 0.06468; its effect was highly significant (p < 0.001), and within-subject errors were significantly correlated, Rho: (0.18; p < 0.001), according to the Wald test. The choice of the AR matrix (1) was appropriate in the modelling of the repeated measures. This results in a better estimation of the fixed parameters of the linear mixed-effects model with repeated measures.
On the other hand, in the case of the residual variance estimate for the effect of repeated measures on E, its value was 0.382649, its effect was highly significant (p < 0.001), and the within-subject errors were highly significantly correlated, Rho: (0.32; p < 0.001), according to the Wald test. The linear mixed repeated measures model was able to capture this variation, and the choice of the AR matrix (1) in the repeated measures modelling was appropriate.
The correlation coefficients between the variables evaluated are presented below, highlighting the positive, high and significant relationship between gs and E in the two cycles evaluated, due to the fact that E is the loss of water through the stomata, the opening of which is regulated by gs [24] and they mutually influence each other [82]. Likewise, the high and significant but negative relationship of SR with gs and E stands out in the two cycles evaluated, due to the inverse relationship of SR with gs, a variable that determines the opening of the stomata as indicated above [24] (Table 3).
This study clarified how physiological variables associated with transpiration and gas exchange in Jarillo Venezuelan Creole peach plant (P. persica) change with altitude and phenological stage, employing in situ measurement methodologies together with statistical analyses using repeated measures mixed models. The findings indicated that both stomatal conductance (gs) and transpiration (E) tend to decrease with increasing altitude, while, on the contrary, an increase in stomatal resistance (SR) is observed, which could be attributed to increased exposure to ultraviolet radiation and lower temperatures, as well as reductions in atmospheric pressure in elevated areas. In addition, during the flowering period (EF6), both transpiration and stomatal conductance levels peak and then decline at later stages to efficiently balance respiratory needs against water losses. Diurnal patterns were identified where gs and E showed a decreasing trend towards evening hours, suggesting regulatory adaptations related to hydration and gases within the plant organism. The strong positive correlation between gs and E, together with the inverse link observed between SR and these two variables, offers insights into how plants manage their water-gas exchanges under changing conditions.
It is important to highlight that there is a notable scarcity of published studies providing direct evidence on stomatal conductance (gs), solar radiation (SR), and transpiration (E) in P. persica under altitudinal gradient conditions. This underscores the originality of the present study, which addresses a clear gap in the existing literature.
The study on physiological variation in peach trees along the altitudinal and geographical gradient in North de Santander presents significant findings regarding the impact of altitude on certain physiological characteristics observed in the late yielding, canning, and low chilling requirement cultivar. The main results of the analysis and their possible applications are presented, together with relevant recent references.
Effects of Phenological Stages and Altitude. The research reveals how various elevations and phenological stages (flowering, fruit set, and ripening) affect factors such as stomatal conductance, stomatal resistance, and transpiration. These findings are relevant to identifying optimal conditions that favor peach growth and development, as well as to improving its agronomic management.
Strategies to Adapt to Climate Change. By showing differences in physiological responses to environmental changes, this study points toward potential strategies to select and increase peach cultivars more suitable to different altitudes; these cultivars may show greater resilience to climatic fluctuations.
Implications for Agricultural Practices. The results obtained provide a useful framework for both agronomists and growers to make decisions related to the appropriate planting date or to manage risks at different altitudes, with a view to optimizing both yield and fruit quality.
It is suggested that future research should delve deeper into the molecular and genetic responses of peach to specific altitude-related changes due to climate change; this could facilitate the development of more robust varieties adapted to extreme conditions.
Additionally, to include new cultivars with low cold requirements, early, medium, and late production in order to establish regional trials to obtain valid information on the behavior of physiological processes such as transpiration, and its relationship with agronomic yield under mountain conditions in the high tropics. Potential cultivars come from Peru (Huayco Amarillo, Huayco Rojo, and Azteca Dorado) and from the germplasm bank of P. persica of the University of Pelotas in Brazil.
4. Conclusions
The work demonstrated that variables, such as stomatal conductance (gs), stomatal resistance (SR), and transpiration (E), have a particular pattern of physiological response depending on the altitude-duration interaction of the phenological cycle of the crop. These responses were also observed to change with daily solar radiation and climate. The decrease in gs and E, as well as the increase in SR with increasing altitude, implies an effective regulation of stomata opening by UV radiation, cold temperatures, and lower atmospheric pressure. The identification of these physiological responses, supported by highly sophisticated statistical models, has led to an understanding of how plants regulate their stomatal and E behavior along this altitudinal gradient and, therefore, has advanced the theory in the ecological acclimation of tropical mountain flora.
The phenological cycle of the Jarillo Venezuela Creole peach plant (P. persica) in the high tropics influences the levels of gs and E. In the flowering stage (EF6), an increase in these variables was observed, which implies a greater stomatal opening that favors CO2 uptake, essential for photosynthesis and growth during this period. In later phenological phases, both gs and E tended to decrease, indicating a physiological regulatory mechanism that helps reduce water loss under adverse environmental conditions, such as high ultraviolet radiation and low temperatures, typical of the high tropics in the Pamplona province at altitudes of 1800 to 2200 m a.s.l. In addition, a diurnal tendency towards a decrease at the end of the day was identified, suggesting an adaptive strategy to optimize water use and maintain photosynthetic efficiency as a function of light availability. These findings reinforce the idea that phenology, as part of crop development, activates crucial physiological responses that ensure both survival and productivity of the Jarillo Venezuela Creole peach plant (P. persica) in the Santander mountains, by adequately regulating stomatal aperture according to phenological stage and environmental conditions.
This result not only confirms the ecological plasticity and water regulation strategies of mountain fruit trees but also provides a mechanistic basis for future work on upland agricultural management. Considering the climatic and radiation variations that will impact crops, it allows practical recommendations to be made for such sites.
Under the changes posed by global warming, this study innovatively concludes that the regulation of stomatal performance of Jarillo or Venezuelan Creole peach plants (P. persica) at different altitudes is crucial for both the productivity of these trees and their compatibility. A better understanding of this mechanism allows for much more effective planning in orchard management in the high tropics.
In summary, this work expands our knowledge of the theoretical application of plant physiology in sustainable agriculture for the highland tropics with solid results.
Author Contributions
Conceptualization, E.Q.-G. and J.A.C.-L.; methodology, E.Q.-G.; software, E.Q.-G.; validation, E.Q.-G. and J.A.C.-L.; formal analysis, E.Q.-G. and J.A.C.-L.; investigation, E.Q.-G.; resources, E.Q.-G.; data curation, E.Q.-G. and J.A.C.-L.; writing, E.Q.-G., J.A.C.-L. and J.d.L.; supervision, E.Q.-G., J.A.C.-L. and J.d.L.; project administration, E.Q.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Food and Agriculture Organization of the United Nations. Available online: https://faostat.fao.org (accessed on 31 July 2025).
- Manganaris, G.A.; Sansavini, S.; Gradziel, T.M.; Bassi, D.; Crisosto, C.H. Peach: A Introduction. In Peach; Manganaris, G., Costa, G., Crisosto, C.H., Eds.; CABI: Wallingford, UK, 2023; pp. 1–16. [Google Scholar]
- Micheloud, N.G.; Giovannelli, C.; Flaviani, M.I.; Buyatti, M.A.; Gariglio, N.F. Evaluation of low-chill peach and nectarine cultivars in a temperate-subtropical climate transition zone of central-eastern Argentina. Acta Physiol. Plant 2021, 43, 94. [Google Scholar] [CrossRef]
- Li, X.; Gao, P.; Zhang, C.; Xiao, X.; Chen, C.; Song, F. Aroma of peach fruit: A review on aroma volatile compounds and underlying regulatory mechanisms. Int. J. Food Sci. Technol. 2023, 58, 4965–4979. [Google Scholar] [CrossRef]
- Cancino, S.E.; Cancino-Escalante, G.O.; Quevedo-García, E. Factores determinantes de la rentabilidad económica del cultivo de durazno en la Provincia de Pamplona, Norte de Santander, Colombia. Rev. Espacios. 2019, 40, 18. Available online: https://www.revistaespacios.com/a19v40n13/19401318.html (accessed on 7 June 2025).
- Red de Información y Comunicación del Sector Agropecuario Colombiano. Resultado de la Evaluación Agrícola Municipal del año 2022; AGRONET: Bogotá, Colombia, 2022. Available online: https://www.agronet.gov.co/estadistica/Paginas/home.aspx?cod=3 (accessed on 8 July 2025).
- Quevedo-García, E.; Murcia, M.A.; Escalante, G.O.C. Growth of peaches at three altitudes in the Santander mountains of northeastern Colombia. Bioagro 2024, 36, 299–310. [Google Scholar] [CrossRef]
- Scorza, R.L.; Okie, W.R. Peaches (Prunus). Acta Hortic. 1991, 290, 177–231. [Google Scholar] [CrossRef]
- Anzanello, R.; Lampugnani, C.S. Requerimento de frio de cultivares de pessegueiro e recomendação de cultivo no Rio Grande do Sul. Pesqui. Agropec. Gaúch. 2020, 26, 18–28. [Google Scholar] [CrossRef][Green Version]
- Jana, B.R. Scientific cultivation of low chill peach [Prunus persica (L) Batsch.] in North Eastern Plateau and Hill Regions. Biot. Res. Today 2021, 3, 687–690. [Google Scholar][Green Version]
- Darghan, A.E.; Quevedo García, E.; Gamboa Muñoz, S.E.; Rivera Moreno, C.A. Growth rates of morphometric variables in approximately linear sections using lines in R3. Int. J. Agron. 2022, 2022, 8249268. [Google Scholar] [CrossRef]
- Sangronis, J.; Hernández, A.; Aular, J.; Torres, J.; Cásares, M. Variabilidad genética en durazneros cultivados en el peñón de Gabante, estado Aragua, Venezuela. Bioagro 2017, 29, 219–224. Available online: https://www.redalyc.org/pdf/857/85752807007.pdf (accessed on 8 June 2025).
- Parejo-Farnés, C.; Aparicio, A.; Albaladejo, R.G. Una aproximación a la ecología epigenética en plantas. Ecosistemas 2019, 28, 69–74. [Google Scholar] [CrossRef]
- Quevedo-García, E. Modelación de los Factores Biológicos y Edafoclimáticos en la Producción de Durazno Jarillo (Prunus persica L. Batsch.) en un Gradiente Altitudinal en la Provincia de Pamplona, Colombia. Doctoral Thesis, Universidad Nacional, Heredia, Costa Rica, 2020. [Google Scholar]
- Pinzón, E.H.; Cruz Morillo, A.; Fischer, G. Aspectos fisiológicos del duraznero Prunus persica L. Batsch en el trópico alto. Una revisión. Rev. UDCA Act. Div. Cient. 2014, 17, 401–411. Available online: https://repository.udca.edu.co/server/api/core/bitstreams/d2088db5-1faa-49fb-b82a-84b170315804/content (accessed on 8 June 2025).
- Quevedo-García, E.; Murcia-Rodríguez, M.A.; Ochoa-Reyes, M.P. Modelos de regresión para predecir la cosecha con variables asociadas a la calidad del fruto, el tiempo de defoliación y la altitud del durazno Jarillo. Rev. UDCA Act. Div. Cient. 2023, 26, e2235. [Google Scholar] [CrossRef]
- Elsadr, H.; Sherif, S.; Banks, T.; Somers, D.; Jayasankar, S. Refining the genomic region containing a major locus controlling fruit maturity in peach. Sci. Rep. 2019, 9, 7522. [Google Scholar] [CrossRef]
- Aular, J.; Cásares, M. Características de frutos de durazneros provenientes de ‘El Peñom de Gabante’, estado Aragua, Venezuela. Bioagro 2020, 31, 113–122. Available online: https://revistas.uclave.org/index.php/bioagro/article/view/2632 (accessed on 8 June 2025).
- Fischer, G.; Orduz-Rodríguez, J.O. Ecofisiología en frutales. In Manual Para el Cultivo de Frutales en el Trópico; Fischer, G., Ed.; Produmedios: Bogotá, Colombia, 2012; pp. 54–72. [Google Scholar]
- Benavides, H.O.; Simbaqueva, O.; Zapata, H.J. Atlas de Radiación Solar, Ultravioleta y Ozono de Colombia; IDEAM y UPME: Bogotá, Colombia, 2017. Available online: https://www.andi.com.co//Uploads/RADIACION.compressed.pdf (accessed on 7 June 2025).
- Fischer, G.; Orduz-Rodríguez, J.O.; Amarante, C.V.T. Sunburn disorder in tropical and subtropical fruits. A review. Rev. Colomb. Cienc. Hortíc. 2022, 16, e15703. [Google Scholar] [CrossRef]
- Fischer, G.; Parra-Coronado, A.; Balaguera-López, H.E. Altitude as a determinant of fruit quality with emphasis on the Andean tropics of Colombia. A review. Agron. Colomb. 2022, 40, 212–227. [Google Scholar] [CrossRef]
- Körner, C. The use of altitude in ecological research. Trends. Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.S.A.; Vialet-Chabrand, S.; Lawson, T. Role of blue and red light in stomatal dynamic behaviour. J. Exp. Bot. 2020, 71, 2253–2269. [Google Scholar] [CrossRef]
- Driesen, E.; Van den Ende, W.; De Proft, M.; Saeys, W. Influence of environmental factors light, CO2, temperature, and relative humidity on stomatal opening and development: A review. Agronomy 2020, 10, 1975. [Google Scholar] [CrossRef]
- Fischer, G.; Melgarejo, L. The ecophysiology of cape gooseberry (Physalis peruviana L.)—An Andean fruit crop. A review. Rev. Colomb. Cienc. Hortíc. 2020, 14, 76–89. [Google Scholar] [CrossRef]
- Flórez-Velasco, N.; Fischer, G.; Balaguera-López, H.E. Photosynthesis in fruit crops of the high tropical Andes: A systematic review. Agron. Colomb. 2024, 42, 1–18. [Google Scholar] [CrossRef]
- Rajsnerová, P.; Klem, K.; Holub, P.; Novotná, K.; Večeřová, K.; Kozáčiková, M.; Rivas-Ubach, A.; Sardans, J.; Marek, M.V.; Peñuelas, J.; et al. Morphological, biochemical and physiological traits of upper and lower canopy leaves of European beech tend to converge with increasing altitude. Tree Physiol. 2015, 35, 47–60. [Google Scholar] [CrossRef]
- Mehta, N.; Chawla, A. Eco-physiological trait variation in widely occurring species of Western Himalaya along elevational gradients reveals their high adaptive potential in stressful conditions. Photosynth. Res. 2024, 159, 29–59. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Instituto de Hidrología, Meteorología y Estudios Ambientales (IDEAM). Atlas Climatológico de Colombia; IDEAM: Bogotá, Colombia, 2018. Available online: https://www.ideam.gov.co (accessed on 7 June 2025).
- Corporación Autónoma Regional de la Frontera (Corponor). Plan Estratégico Ambiental Regional, 2016–2035. Available online: https://corponor.gov.co/es/index.php/politicas-planes-y-lineas-estrategicas (accessed on 27 July 2025).
- Restrepo-Diaz, H.; Sánchez-Reinoso, A.D. Ecophysiology of fruit crops: A glance at its impact on fruit crop productivity. In Fruit Crops; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 59–66. [Google Scholar] [CrossRef]
- Yang, J.; Li, C.; Kong, D.; Guo, F.; Wei, H. Light-mediated signaling and metabolic changes coordinate stomatal opening and closure. Front. Plant Sci. 2020, 11, 601478. [Google Scholar] [CrossRef]
- Daloso, D.M.; Medeiros, D.B.; dos Anjos, L.; Yoshida, T.; Araújo, W.L.; Fernie, A.R. Metabolism within the specialized guard cells of plants. New Phytol. 2017, 216, 1018–1033. [Google Scholar] [CrossRef]
- Khan, I.; Zaman, S.; Li, G.; Fu, M. Adaptive responses of plants to light stress: Mechanisms of photoprotection and acclimation. A review. Front. Plant Sci. 2025, 16, 1550125. [Google Scholar] [CrossRef]
- Didaran, F.; Kordrostami, M.; Ghasemi-soloklui, A.A.; Pashkovskiy, P.; Kreslavski, V.; Kuznetsov, V.; Allakhverdiev, S.I. The mechanisms of photoinhibition and repair in plants under high light conditions and interplay with abiotic stressors. J. Photochem. Photobiol. B Biol. 2024, 259, 113004. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, J.; Shi, X.; Qian, W.; Mehmood, J.; Yin, Y.; Jia, H. Identification of the light–harvesting chlorophyll a/b binding protein gene family in peach (Prunus persica L.) and their expression under drought stress. Genes 2023, 14, 1475. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, S.; Zuo, H.; Zheng, W.; Zhang, S.; Huang, Y.; Pingcuo, G.; Yin, H.; Zhao, F.; Li, Y.; et al. Genomic basis of high-altitude adaptation in Tibetan Prunus fruit trees. Curr. Biol. 2021, 31, 3848–3860. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Prentice, I.C.; Davis, T.W.; Keenan, T.F.; Wright, I.J.; Peng, C. Photosynthetic responses to altitude: An explanation based on optimality principles. New Phytol. 2017, 213, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Casson, S. Connecting stomatal development and physiology. New Phytol. 2014, 201, 1079–1082. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.M.; Cochard, H.; Delzon, S.; Boivin, T.; Burlett, R.; Cailleret, M.; Corso, D.; Delmas, C.E.L.; De Caceres, M.; Diaz-Espejo, A.; et al. Plant hydraulics at the heart of plant, crops and ecosystem functions in the face of climate change. New Phytol. 2024, 241, 984–999. [Google Scholar] [CrossRef]
- Andrés, Z.; Pérez-Hormaeche, J.; Leidi, E.O.; Schlücking, K.; Steinhorst, L.; McLachlan, D.H.; Schumacher, K.; Hetherington, A.M.; Kudla, J.; Cubero, B.; et al. Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc. Nati. Acad. Sci. USA 2014, 111, E1806–E1814. [Google Scholar] [CrossRef]
- Rovira, A.; Veciana, N.; Basté-Miquel, A.; Quevedo, M.; Locascio, A.; Yenush, L.; Toledo-Ortiz, G.; Leivar, P.; Monte, E. PIF transcriptional regulators are required for rhythmic stomatal movements. Nat. Commun. 2024, 15, 4540. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Davarynejad, G.H.; Génard, M.; Bannayan, M.; Azizi, M.; Vercambre, G. Peach water relations, gas exchange, growth and shoot mortality under water deficit in semi-arid weather conditions. PLoS ONE 2015, 10, e0120246. [Google Scholar] [CrossRef]
- Zhang, B.; Du, H.; Yang, S.; Wu, X.; Liu, W.; Guo, J.; Xiao, Y.; Peng, F. Physiological and transcriptomic analyses of the effects of exogenous lauric acid on drought resistance in peach (Prunus persica (L.) Batsch). Plants 2023, 12, 1492. [Google Scholar] [CrossRef] [PubMed]
- Mininni, A.N.; Tuzio, A.C.; Brugnoli, E.; Dichio, B.; Sofo, A. Carbon isotope discrimination and water use efficiency in interspecific Prunus hybrids subjected to drought stress. Plant Physiol. Biochem. 2022, 175, 33–43. [Google Scholar] [CrossRef]
- Gale, J. Plants and altitude-revisited. Ann. Bot. 2004, 94, 199–420. [Google Scholar] [CrossRef] [PubMed]
- Conesa, M.R.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M.C. Effects of postharvest water deficits on the physiological behavior of early-maturing nectarine trees. Plants 2020, 9, 1104. [Google Scholar] [CrossRef]
- Inoue, S.I.; Kinoshita, T. Blue light regulation of stomatal opening and the plasma membrane H+-ATPase. Plant Physiol. 2017, 174, 531–538. [Google Scholar] [CrossRef]
- Santelia, D.; Lawson, T. Rethinking guard cell metabolism. Plant Physiol. 2016, 172, 1371–1392. [Google Scholar] [CrossRef]
- Jiang, S.; Lan, Z.; Zhang, Y.; Kang, X.; Zhao, L.; Wu, X.; Gao, H. Mechanisms by which exogenous substances enhance plant salt tolerance through the modulation of ion membrane transport and reactive oxygen species metabolism. Antioxidants 2024, 13, 1050. [Google Scholar] [CrossRef]
- Pech, R.; Volná, A.; Hunt, L.; Bartas, M.; Červeň, J.; Pečinka, P.; Špunda, V.; Nezval, J. Regulation of phenolic compound production by light varying in spectral quality and total irradiance. Int. J. Mol. Sci. 2022, 23, 6533. [Google Scholar] [CrossRef] [PubMed]
- Kakuszi, A.; Sárvári, É.; Solti, Á.; Czégény, G.; Hideg, É.; Hunyadi-Gulyás, É.; Bóka, K.; Böddi, B. Light piping driven photosynthesis in the soil: Low-light adapted active photosynthetic apparatus in the under-soil hypocotyl segments of bean (Phaseolus vulgaris). J. Photochem. Photobiol. B Biol. 2016, 161, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Swanson, S.; Gilroy, S. ROS in plant development. Physiol. Plant. 2010, 138, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yu, S.; Zhang, W.; Zhang, S.; Fu, J.; Ying, H.; Pingcuo, G.; Liu, S.; Zhao, F.; Wu, Q.; et al. The content and diversity of carotenoids associated with high-altitude adaptation in Tibetan peach fruit. Food Chem. 2023, 398, 133909. [Google Scholar] [CrossRef]
- Karagiannis, E.; Tanou, G.; Samiotaki, M.; Michailidis, M.; Diamantidis, G.; Minas, I.S.; Molassiotis, A. Comparative physiological and proteomic analysis reveal distinct regulation of peach skin quality traits by altitude. Front. Plant Sci. 2016, 7, 1689. [Google Scholar] [CrossRef]
- Ramírez, F.; Kallarackal, J. Tree Pollination Under Global Climate Change; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Instituto Geográfico Agustín Codazzi IGAC. Estudio General de Suelos y Zonificación de Tierras: Del Departamento de Norte de Santander, 2nd ed.; IGAC: Bogotá, Colombia, 2011; 359p.
- Poirier-Pocovi, M.M.; Lothier, J.; Buck-Sorlin, G. Modelling temporal variation of parameters used in two photosynthesis models: Influence of fruit load and girdling on leaf photosynthesis in fruit-bearing branches of apple. Ann. Bot. 2018, 121, 821–832. [Google Scholar] [CrossRef]
- Meier, U.; Bleiholder, H.; Buhr, L.; Feller, C.; Hack, H.; Hes, M.; Lancashire, P.D.; Schnock, U.; Staus, R.; van den Boom, T.; et al. The BBCH system to coding the phenological growth stages of plants–history and publications. J. für. Kult. 2009, 61, 41–52. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20093092784 (accessed on 8 June 2025).
- Lisandru, T.; Füstös, A.; Miter, V.; Dumitras, A. Sweet cherry (Prunus avium L.) and peach (Prunus persica L.) phenological growth stages according to BBCH scale. Bull. UASVM Hortic. 2017, 74, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Campos, T.J. Especies y variedades de hoja caduca en Colombia. In Los Frutales Caducifolios en Colombia—Situación Actual, Sistemas de Cultivo y Plan de Desarrollo; Miranda, D., Fischer, G., Carranza, C., Eds.; Sociedad Colombiana de Ciencias Hortícolas: Bogotá, Colombia, 2013; pp. 47–64. [Google Scholar]
- Grossman, J.J. Phenological physiology: Seasonal patterns of plant stress tolerance in a changing climate. New Phytol. 2023, 237, 1508–1524. [Google Scholar] [CrossRef]
- Aniorte, E.R. Protocolo de Uso del Porómetro AP4 (Delta-T); Universidad de Alicante: Alicante, Spain, 2005; 15p. [Google Scholar]
- Minasny, B.; Malone, B.P.; Mcbratney, A.B. Digital Soil Assessment and Beyond; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Hernández-Sampieri, R.; Fernández-Collado, C.; Baptista-Lucio, M.D.P. Metodología de la Investigación-Sampieri; McGraw-Hill: México City, Mexico, 2014. [Google Scholar]
- SPSS Statistics [Software], version 25.0; IBM Corporation: Armonk, NY, USA, 2017.
- Gómez-Degraves, A.; Gómez-Marquina, K. Diseño y Análisis de Experimentos Agrícolas con SPSS; Amazon: Madrid, Spain, 2018. [Google Scholar]
- Lawson, L.; Leakey, A.D.B. Stomata: Custodians of leaf gaseous exchange. J. Exp. Bot. 2024, 75, 6677–6682. [Google Scholar] [CrossRef]
- Morales, L.O.; Shapiguzov, A.; Rai, N.; Aphalo, P.J.; Brosché, M. Protection of Photosynthesis by UVR8 and Cryptochromes in Arabidopsis Under Blue and UV Radiation. Plant Cell Environ. 2025, 48, 6321–6335. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Fan, P.; Liang, Z.; Wang, Y.; Niu, N.; Li, W.; Li, S. Accumulation of end products in source leaves affects photosynthetic rate in peach via alteration of stomatal conductance and photosynthetic efficiency. J. Am. Soc. Hortic. Sci. 2009, 134, 667–676. [Google Scholar] [CrossRef]
- Casierra-Posada, F.; Barreto, V.E.; Fonseca, O.L. Crecimiento de frutos y ramas de duraznero (Prunus persica L. Batsch, cv. ’Conservero’) en los altiplanos colombianos. Agron. Colomb. 2004, 22, 40–45. [Google Scholar]
- Millan, M.; Simonneau, T.; Coupe-Ledru, A.; Boulord, R.; Christophe, A.; Pallas, B. Relationships between leaf temperature, stomatal conductance and architecture: Potential impact on leaf burning among a range of genotypes in grapevine. OENO One 2023, 57, 345–359. [Google Scholar] [CrossRef]
- Márquez, D.; Gardner, A.; Busch, F. Navigating challenges in interpreting plant physiology responses through gas exchange results in stressed plants. Plant Ecophysiol. 2025, 1, 2. [Google Scholar] [CrossRef]
- Quevedo-García, E.; Casierra-Posada, F.; Darghan, C.A.E. Quality of peach fruits Jarillo cv. (Prunus persica L.) in Pamplona, Colombia. Rev. Bras. Frutic. 2018, 40, e-040. [Google Scholar] [CrossRef]
- Matthews, J.S.A.; Vialet-Chabrand, S.R.M.; Lawson, T. Diurnal variation in gas exchange: The balance between carbon fixation and water loss. Plant Physiol. 2017, 174, 614–623. [Google Scholar] [CrossRef]
- Matthews, J.S.A.; Vialet-Chabrand, S.R.M.; Lawson, T. Acclimation to fluctuating light impacts the rapidity of response and diurnal rhythm of stomatal conductance. Plant Physiol. 2018, 176, 1939–1951. [Google Scholar] [CrossRef]
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal conductance increases with rising temperature. Plant Signal. Behav. 2017, 12, e1356534. [Google Scholar] [CrossRef] [PubMed]
- Aasamaa, K.; Sõber, A. Responses of stomatal conductance to simultaneous changes in two environmental factors. Tree Physiol. 2011, 31, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Cowan, I.; Farquhar, G. Stomatal conductance correlates with photosynthetic capacity. Nature 1979, 282, 424–426. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).