Long-Term Fertilization Mediates Microbial Keystone Taxa to Regulate Straw-Derived 13C Incorporation in Soil Aggregates
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. In Situ Field Experiment and Soil Sampling
2.3. Soil Aggregate Fractionation
2.4. Analysis of the Contents of Organic C and Straw-Derived C of Aggregates
2.5. High-Throughput Sequencing
2.6. Bioinformatics Analysis
2.7. Structural Equation Model Analysis
3. Results
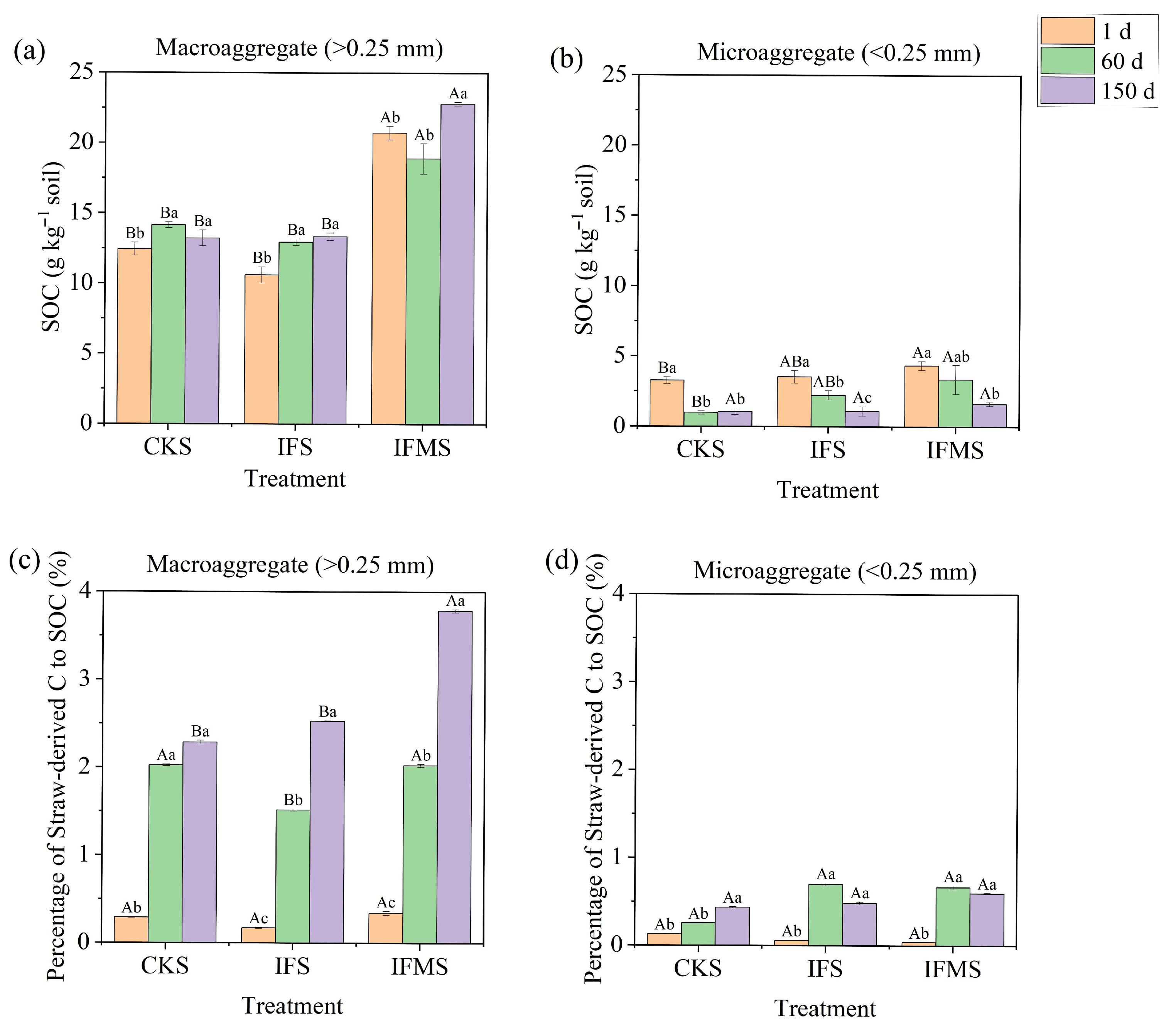
3.1. Organic C and Straw-Derived C Content in Soil Aggregates
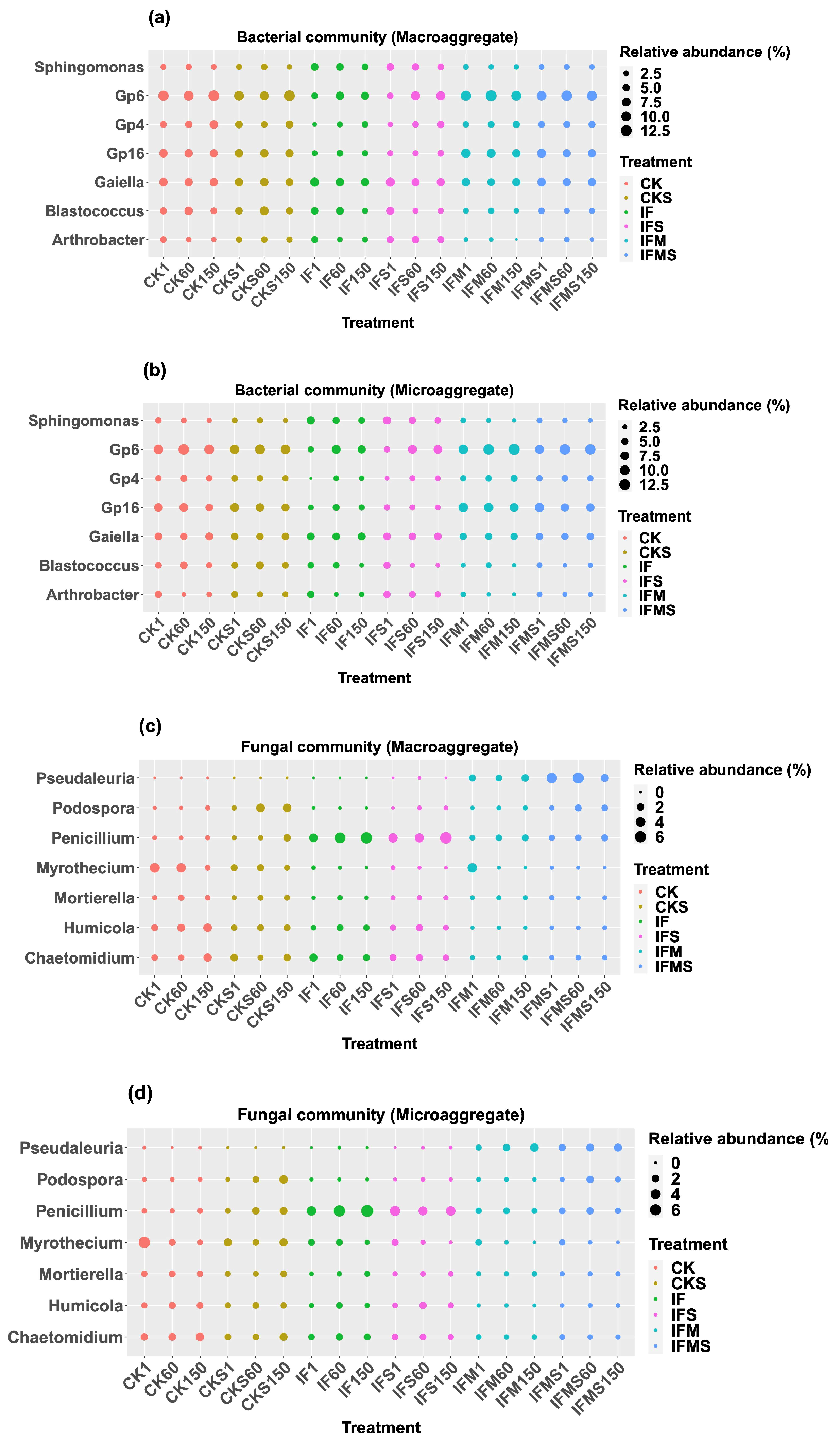
3.2. Bacterial and Fungal Community Composition in Soil Aggregates
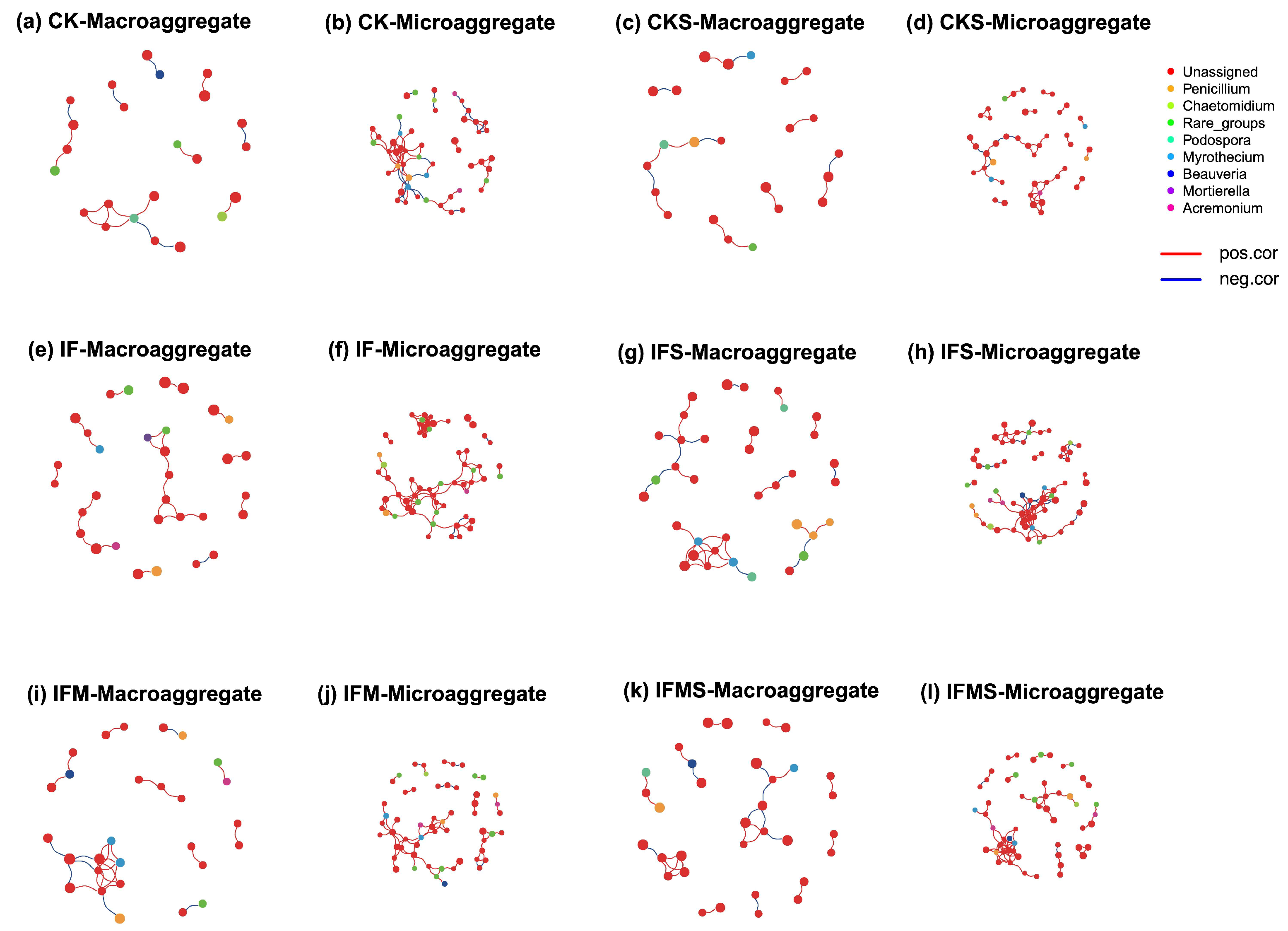
3.3. Bacterial and Fungal Co-Occurrence Network in Soil Aggregates
3.4. Bacterial and Fungal Keystone Taxa in Soil Aggregates
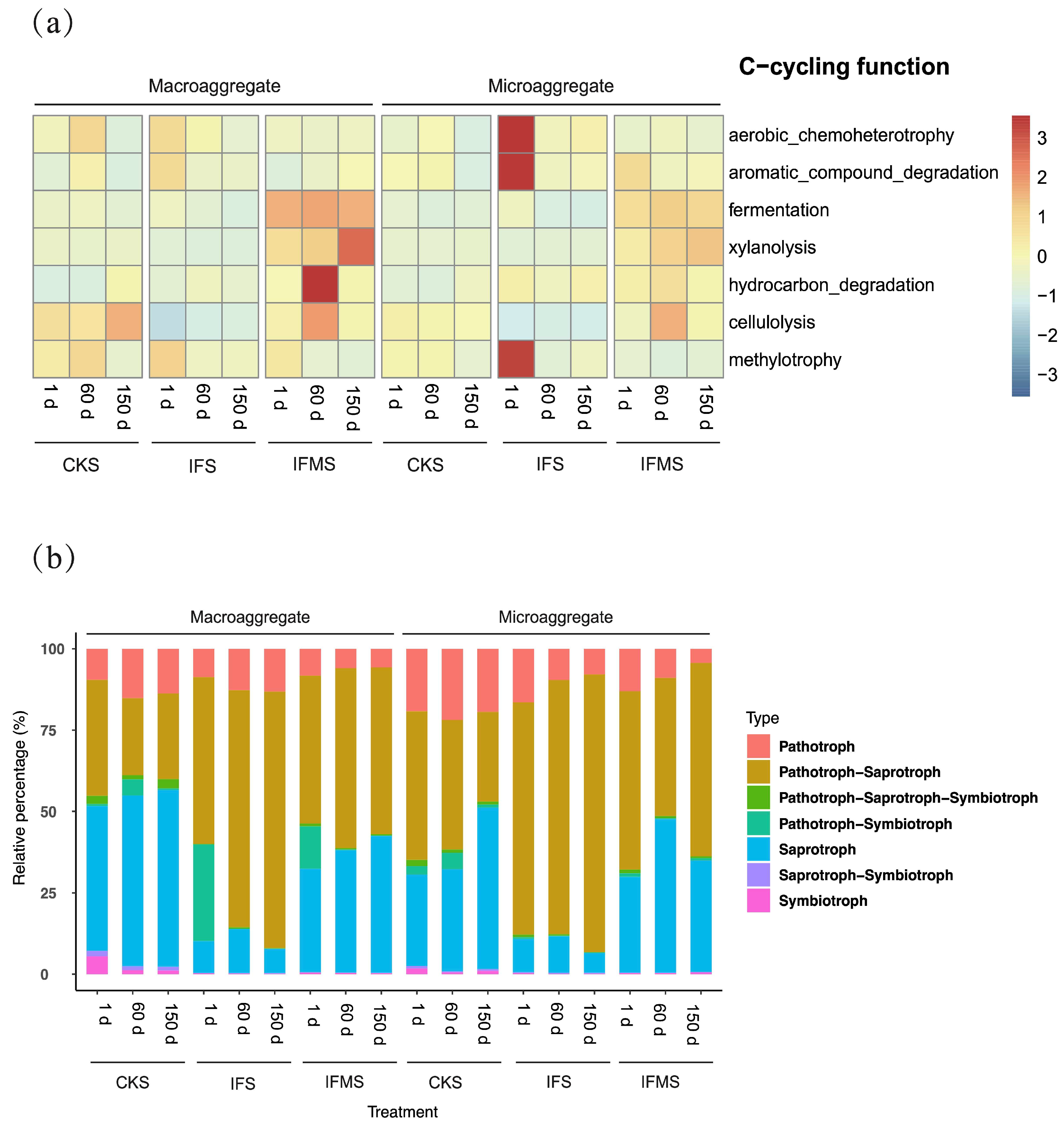
3.5. Bacterial and Fungal Functional Characteristics in Soil Aggregates
3.6. Fertilization and Microbes Affect Straw-Derived C in Soil Aggregates
4. Discussion
4.1. Fertilization Effects
4.2. Microbial Composition Changes in Bacterial and Fungal Communities
4.3. Straw Decomposition and Aggregate Network Complexities
4.4. Microbial Keystone Taxa Exhibit Different Patterns Within Aggregates
4.5. Microbial Function Shifted Within Aggregates During Straw Decomposition
4.6. Straw-Derived C Within Aggregates Was Regulated by Fertilization and Microbial Keystone Taxa
4.7. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Wei, D.; Zhou, B.; Zhang, L.; Hao, X.; Zhao, S.; Xu, X.; He, P.; Zhao, Y.; Qiu, S.; et al. Responses of soil aggregation and aggregate-associated carbon and nitrogen in black soil to different long-term fertilization regimes. Soil Till. Res. 2021, 213, 105157. [Google Scholar] [CrossRef]
- Gu, Z.; Xie, Y.; Gao, Y.; Ren, X.; Cheng, C.; Wang, S. Quantitative assessment of soil productivity and predicted impacts of water erosion in the black soil region of northeastern China. Sci. Total Environ. 2018, 637–638, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bao, X.; Xie, H.; He, H.; Zhang, X.; Shao, P.; Zhu, X.; Jiang, Y.; Liang, C. Frequent stover mulching builds healthy soil and sustainable agriculture in Mollisols. Agric. Ecosyst. Environ. 2022, 326, 107815. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Wang, L.; Zheng, M.; Wang, Z.; Song, K. Effects of cropland reclamation on soil organic carbon in China’s black soil region over the past 35 years. Glob. Change Biol. 2023, 29, 5460–5477. [Google Scholar] [CrossRef] [PubMed]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Till. Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Wilpiszeski, R.L.; Aufrecht, J.A.; Retterer, S.T.; Sullivan, M.B.; Graham, D.E.; Pierce, E.M.; Zablocki, O.D.; Palumbo, A.V.; Elias, D.A. Soil aggregate microbial communities: Towards understanding microbiome interactions at biologically relevant scales. Appl. Environ. Microbiol. 2019, 85, e00324-19. [Google Scholar] [CrossRef]
- Gupta, V.V.S.R.; Germida, J.J. Soil aggregation: Influence on microbial biomass and implications for biological processes. Soil Biol. Biochem. 2015, 80, A3–A9. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Trivedi, P.; Delgado-Baquerizo, M.; Jeffries, T.C.; Trivedi, C.; Anderson, I.C.; Lai, K.; McNee, M.; Flower, K.; Pal Singh, B.; Minkey, D.; et al. Soil aggregation and associated microbial communities modify the impact of agricultural management on carbon content. Environ. Microbiol. 2017, 19, 3070–3086. [Google Scholar] [CrossRef]
- Upton, R.N.; Bach, E.M.; Hofmockel, K.S. Spatio-temporal microbial community dynamics within soil aggregates. Soil Biol. Biochem. 2019, 132, 58–68. [Google Scholar] [CrossRef]
- Lehmann, A.; Zheng, W.; Rillig, M.C. Soil biota contributions to soil aggregation. Nat. Ecol. Evol. 2017, 1, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Muller, L.A.; Lehmann, A. Soil aggregates as massively concurrent evolutionary incubators. ISME J. 2017, 11, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, J.-Y.; Zhang, X.-Z.; Li, S.-S.; Latif Virk, A.; Zhao, X.; Xiao, X.-P.; Zhang, H.-L. Effects of tillage and residue management on soil aggregates and associated carbon storage in a double paddy cropping system. Soil Till. Res. 2019, 194, 104339. [Google Scholar] [CrossRef]
- Huang, T.; Yang, H.; Huang, C.; Ju, X. Effects of nitrogen management and straw return on soil organic carbon sequestration and aggregate-associated carbon. Eur. J. Soil Sci. 2018, 69, 913–923. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Louis, B.P.; Maron, P.-A.; Viaud, V.; Leterme, P.; Menasseri-Aubry, S. Soil C and N models that integrate microbial diversity. Environ. Chem. Lett. 2016, 14, 331–344. [Google Scholar] [CrossRef]
- Juarez, S.; Nunan, N.; Duday, A.-C.; Pouteau, V.; Chenu, C. Soil carbon mineralisation responses to alterations of microbial diversity and soil structure. Biol. Fertil. Soils 2013, 49, 939–948. [Google Scholar] [CrossRef]
- Smith, A.P.; Marin-Spiotta, E.; de Graaff, M.A.; Balser, T.C. Microbial community structure varies across soil organic matter aggregate pools during tropical land cover change. Soil Biol. Biochem. 2014, 77, 292–303. [Google Scholar] [CrossRef]
- Wang, X.; Bian, Q.; Jiang, Y.; Zhu, L.; Chen, Y.; Liang, Y.; Sun, B. Organic amendments drive shifts in microbial community structure and keystone taxa which increase C mineralization across aggregate size classes. Soil Biol. Biochem. 2021, 153, 108062. [Google Scholar] [CrossRef]
- Duan, Y.; Xu, M.; Gao, S.; Yang, X.; Huang, S.; Liu, H.; Wang, B. Nitrogen use efficiency in a wheat–corn cropping system from 15 years of manure and fertilizer applications. Field Crops Res. 2014, 157, 47–56. [Google Scholar] [CrossRef]
- Li, T.T.; Zhang, Y.L.; Bei, S.K.; Li, X.L.; Reinsch, S.; Zhang, H.Y.; Zhang, J.L. Contrasting impacts of manure and inorganic fertilizer applications for nine years on soil organic carbon and its labile fractions in bulk soil and soil aggregates. Catena 2020, 194, 104739. [Google Scholar] [CrossRef]
- Trivedi, P.; Rochester, I.J.; Trivedi, C.; Van Nostrand, J.D.; Zhou, J.; Karunaratne, S.; Anderson, I.C.; Singh, B.K. Soil aggregate size mediates the impacts of cropping regimes on soil carbon and microbial communities. Soil Biol. Biochem. 2015, 91, 169–181. [Google Scholar] [CrossRef]
- Tian, S.; Zhu, B.; Yin, R.; Wang, M.; Jiang, Y.; Zhang, C.; Li, D.; Chen, X.; Kardol, P.; Liu, M. Organic fertilization promotes crop productivity through changes in soil aggregation. Soil Biol. Biochem. 2022, 165, 108533. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, Y.; Zuo, Q.; Du, B.; Chen, W.; Wei, D.; Huang, Q. Contrasting responses of bacterial and fungal communities to aggregate-size fractions and long-term fertilizations in soils of northeastern China. Sci. Total Environ. 2018, 635, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.P.; Schaeffer, S.M. Microbial control over carbon cycling in soil. Front. Microbiol. 2012, 3, 348. [Google Scholar] [CrossRef]
- Banerjee, S.; Kirkby, C.A.; Schmutter, D.; Bissett, A.; Kirkegaard, J.A.; Richardson, A.E. Network analysis reveals functional redundancy and keystone taxa amongst bacterial and fungal communities during organic matter decomposition in an arable soil. Soil Biol. Biochem. 2016, 97, 188–198. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Sun, D.; Lin, Q.; Angst, G.; Huang, L.; Anikó, C.; Emsens, W.-J.; Diggelen, R.v.; Vicena, J.; Cajthaml, T.; Frouz, J. Microbial communities in soil macro-aggregates with less connected networks respire less across successional and geographic gradients. Eur. J. Soil Biol. 2022, 108, 103378. [Google Scholar] [CrossRef]
- Song, Z.; Gao, H.; Zhu, P.; Peng, C.; Deng, A.; Zheng, C.; Mannaf, M.A.; Islam, M.N.; Zhang, W. Organic amendments increase corn yield by enhancing soil resilience to climate change. Crop J. 2015, 3, 110–117. [Google Scholar] [CrossRef]
- Xie, H.; Li, J.; Zhu, P.; Peng, C.; Wang, J.; He, H.; Zhang, X. Long-term manure amendments enhance neutral sugar accumulation in bulk soil and particulate organic matter in a Mollisol. Soil Biol. Biochem. 2014, 78, 45–53. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Ding, F.; Xu, Y.; Zhu, P.; Ding, X.; Wang, J. Dynamics of maize straw residue 13C incorporation into aggregates of a Mollisol as affected by long-term fertilization. J. Soils Sediments 2018, 19, 1151–1160. [Google Scholar] [CrossRef]
- Dou, X.L.; Cheng, X.L.; He, P.; Zhu, P.; Zhou, W.; Wang, L.G. Dynamics of physically-separated soil organic carbon pools assessed from delta 13C changes under 25 years of cropping systems. Soil Till. Res. 2017, 174, 6–13. [Google Scholar] [CrossRef]
- Ge, Z.; Li, S.Y.; Bol, R.; Zhu, P.; Peng, C.; An, T.T.; Cheng, N.; Liu, X.; Li, T.Y.; Xu, Z.Q.; et al. Differential long-term fertilization alters residue-derived labile organic carbon fractions and microbial community during straw residue decomposition. Soil Till. Res. 2021, 213, 105120. [Google Scholar] [CrossRef]
- An, T.; Schaeffer, S.; Li, S.; Fu, S.; Pei, J.; Li, H.; Zhuang, J.; Radosevich, M.; Wang, J. Carbon fluxes from plants to soil and dynamics of microbial immobilization under plastic film mulching and fertilizer application using 13C pulse-labeling. Soil Biol. Biochem. 2015, 80, 53–61. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Zhang, S.; Xing, Y.; Wang, R.; Liang, W. Organic amendment effects on aggregate-associated organic C, microbial biomass C and glomalin in agricultural soils. Catena 2014, 123, 188–194. [Google Scholar] [CrossRef]
- Gao, X.; Wang, R.; Hu, Y.; Li, W.; Du, L.; Guo, S.; Wu, S.; Huang, P. Effects of fractionation methods on soil aggregate microbial community composition: Settling vs. Wet. Sieving. J. Soil Sci. Plant Nutr. 2024, 24, 1160–1171. [Google Scholar] [CrossRef]
- Wang, S.Q.; Li, T.X.; Zheng, Z.C. Distribution of microbial biomass and activity within soil aggregates as affected by tea plantation age. Catena 2017, 153, 1–8. [Google Scholar] [CrossRef]
- Zhang, S.X.; Li, Q.; Lu, Y.; Zhang, X.P.; Liang, W.J. Contributions of soil biota to C sequestration varied with aggregate fractions under different tillage systems. Soil Biol. Biochem. 2013, 62, 147–156. [Google Scholar] [CrossRef]
- Gartzia-Bengoetxea, N.; González-Arias, A.; Merino, A.; Martínez de Arano, I. Soil organic matter in soil physical fractions in adjacent semi-natural and cultivated stands in temperate Atlantic forests. Soil Biol. Biochem. 2009, 41, 1674–1683. [Google Scholar] [CrossRef]
- De Troyer, I.; Amery, F.; Van Moorleghem, C.; Smolders, E.; Merckx, R. Tracing the source and fate of dissolved organic matter in soil after incorporation of a 13C labelled residue: A batch incubation study. Soil Biol. Biochem. 2011, 43, 513–519. [Google Scholar] [CrossRef]
- Blaud, A.; Lerch, T.Z.; Chevallier, T.; Nunan, N.; Chenu, C.; Brauman, A. Dynamics of bacterial communities in relation to soil aggregate formation during the decomposition of 13C-labelled rice straw. Appl. Soil Ecol. 2012, 53, 1–9. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2018, 47, D259–D264. [Google Scholar] [CrossRef]
- Rottjers, L.; Faust, K. From hairballs to hypotheses-biological insights from microbial networks. FEMS Microbiol. Rev. 2018, 42, 761–780. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Wang, J.; Chapman, S.J.; Yao, H. Incorporation of 13C-labelled rice rhizodeposition into soil microbial communities under different fertilizer applications. Appl. Soil Ecol. 2016, 101, 11–19. [Google Scholar] [CrossRef]
- Xiong, B.; Skitmore, M.; Xia, B. A critical review of structural equation modeling applications in construction research. Autom. Constr. 2015, 49, 59–70. [Google Scholar] [CrossRef]
- Xu, X.; Bi, R.; Song, M.; Dong, Y.; Jiao, Y.; Wang, B.; Xiong, Z. Organic substitutions enhanced soil carbon stabilization and reduced carbon footprint in a vegetable farm. Soil Till. Res. 2024, 236, 105955. [Google Scholar] [CrossRef]
- Mo, F.; Zhang, Y.-Y.; Liu, Y.; Liao, Y.-C. Microbial carbon-use efficiency and straw-induced priming effect within soil aggregates are regulated by tillage history and balanced nutrient supply. Biol. Fertil. Soils 2021, 57, 409–420. [Google Scholar] [CrossRef]
- Bimuller, C.; Kreyling, O.; Kolbl, A.; von Lutzow, M.; Kogel-Knabner, I. Carbon and nitrogen mineralization in hierarchically structured aggregates of different size. Soil Till. Res. 2016, 160, 23–33. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef]
- Abdalla, K.; Sun, Y.; Zarebanadkouki, M.; Gaiser, T.; Seidel, S.; Pausch, J. Long-term continuous farmyard manure application increases soil carbon when combined with mineral fertilizers due to lower priming effects. Geoderma 2022, 428, 116216. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Purahong, W.; Buscot, F.; Reitz, T. Reinoculation elucidates mechanisms of bacterial community assembly in soil and reveals undetected microbes. Biol. Fertil. Soils 2016, 52, 1073–1083. [Google Scholar] [CrossRef]
- Tian, J.; Dungait, J.A.J.; Lu, X.; Yang, Y.; Hartley, I.P.; Zhang, W.; Mo, J.; Yu, G.; Zhou, J.; Kuzyakov, Y. Long-term nitrogen addition modifies microbial composition and functions for slow carbon cycling and increased sequestration in tropical forest soil. Glob. Change Biol. 2019, 25, 3267–3281. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Awasthi, S.K.; Liu, T.; Verma, S.; Wang, Q.; Chen, H.; Ren, X.; Zhang, Z.; Awasthi, M.K. Positive impact of biochar alone and combined with bacterial consortium amendment on improvement of bacterial community during cow manure composting. Bioresour. Technol. 2019, 280, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Urzi, C.; Salamone, P.; Schumann, P.; Rohde, M.; Stackebrandt, E. Blastococcus saxobsidens sp. nov., and emended descriptions of the genus Blastococcus Ahrens and Moll 1970 and Blastococcus aggregatus Ahrens and Moll 1970. Int. J. Syst. Evol. Microbiol. 2004, 54, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, P.; Padmanabhan, S.; DeRito, C.; Gray, A.; Gannon, D.; Snape, J.R.; Tsai, C.S.; Park, W.; Jeon, C.; Madsen, E.L. Respiration of 13C-labeled substrates added to soil in the field and subsequent 16S rRNA gene analysis of 13C-labeled soil DNA. Appl. Environ. Microbiol. 2003, 69, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Banerjee, S.; Baah-Acheamfour, M.; Carlyle, C.N.; Bissett, A.; Richardson, A.E.; Siddique, T.; Bork, E.W.; Chang, S.X. Determinants of bacterial communities in Canadian agroforestry systems. Environ. Microbiol. 2016, 18, 1805–1816. [Google Scholar] [CrossRef]
- Ning, Q.; Chen, L.; Jia, Z.; Zhang, C.; Ma, D.; Li, F.; Zhang, J.; Li, D.; Han, X.; Cai, Z.; et al. Multiple long-term observations reveal a strategy for soil pH-dependent fertilization and fungal communities in support of agricultural production. Agric. Ecosyst. Environ. 2020, 293, 106837. [Google Scholar] [CrossRef]
- Xu, L.; Ravnskov, S.; Larsen, J.; Nilsson, R.H.; Nicolaisen, M. Soil fungal community structure along a soil health gradient in pea fields examined using deep amplicon sequencing. Soil Biol. Biochem. 2012, 46, 26–32. [Google Scholar] [CrossRef]
- Hansen, K.; Perry, B.A.; Dranginis, A.W.; Pfister, D.H. A phylogeny of the highly diverse cup-fungus family Pyronemataceae (Pezizomycetes, Ascomycota) clarifies relationships and evolution of selected life history traits. Mol. Phylogenet Evol. 2013, 67, 311–335. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Z.; Gong, Q.; Zhai, B.; Li, Z. Responses of fungal–bacterial community and network to organic inputs vary among different spatial habitats in soil. Soil Biol. Biochem. 2018, 125, 54–63. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2023, 22, 226–239. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2022, 4, 4–18. [Google Scholar] [CrossRef]
- Ma, P.; Nan, S.; Yang, X.; Qin, Y.; Ma, T.; Li, X.; Yu, Y.; Bodner, G. Macroaggregation is promoted more effectively by organic than inorganic fertilizers in farmland ecosystems of China—A meta-analysis. Soil Till. Res. 2022, 221, 105394. [Google Scholar] [CrossRef]
- Liao, H.; Hao, X.; Zhang, Y.; Qin, F.; Xu, M.; Cai, P.; Chen, W.; Huang, Q. Soil aggregate modulates microbial ecological adaptations and community assemblies in agricultural soils. Soil Biol. Biochem. 2022, 172, 108769. [Google Scholar] [CrossRef]
- Henriksen, T.M.; Breland, T.A. Nitrogen availability effects on carbon mineralization, fungal and bacterial growth, and enzyme activities during decomposition of wheat straw in soil. Soil Biol. Biochem. 1999, 31, 1121–1134. [Google Scholar] [CrossRef]
- Guhra, T.; Stolze, K.; Totsche, K.U. Pathways of biogenically excreted organic matter into soil aggregates. Soil Biol. Biochem. 2022, 164, 108483. [Google Scholar] [CrossRef]
- Zhang, X.F.; Xin, X.L.; Zhu, A.N.; Yang, W.L.; Zhang, J.B.; Ding, S.J.; Mu, L.; Shao, L.L. Linking macroaggregation to soil microbial community and organic carbon accumulation under different tillage and residue managements. Soil Till. Res. 2018, 178, 99–107. [Google Scholar] [CrossRef]
- Guan, X.-K.; Wei, L.; Turner, N.C.; Ma, S.-C.; Yang, M.-D.; Wang, T.-C. Improved straw management practices promote in situ straw decomposition and nutrient release, and increase crop production. J. Clean. Prod. 2020, 250, 119514. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, Y.C.; Wang, K.; Hao, X.L.; Chen, W.L.; Huang, Q.Y. Complexity of bacterial and fungal network increases with soil aggregate size in an agricultural Inceptisol. Appl. Soil Ecol. 2020, 154, 103640. [Google Scholar] [CrossRef]
- Zhao, Y.; Weng, Q.; Hu, B. Microbial interaction promote the degradation rate of organic matter in thermophilic period. Waste Manag. 2022, 144, 11–18. [Google Scholar] [CrossRef]
- Machado, D.; Maistrenko, O.M.; Andrejev, S.; Kim, Y.; Bork, P.; Patil, K.R.; Patil, K.R. Polarization of microbial communities between competitive and cooperative metabolism. Nat. Ecol. Evol. 2021, 5, 195–203. [Google Scholar] [CrossRef]
- Liu, X.; Fan, X.; Zhang, M.; Zhang, H.; Yue, Y.; Wu, J.; Teng, W.; Mu, N.; Teng, K.; Wen, H. Insights into the interlinkages between rhizosphere soil extracellular enzymes and microbiome assemblages across soil profiles in grasslands. Appl. Soil Ecol. 2025, 211, 106139. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, H.; Zhao, X.; Liu, P.; Wang, L.; Wang, W. A functional metagenomics study of soil carbon and nitrogen degradation networks and limiting factors on the Tibetan plateau. Front. Microbiol. 2023, 14, 2023. [Google Scholar] [CrossRef]
- Xun, W.; Liu, Y.; Li, W.; Ren, Y.; Xiong, W.; Xu, Z.; Zhang, N.; Miao, Y.; Shen, Q.; Zhang, R. Specialized metabolic functions of keystone taxa sustain soil microbiome stability. Microbiome 2021, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liu, X.; Yang, N.; Li, Y.; Zhang, J. The Contribution of Microbial- and Plant-Derived Carbon to Soil Organic Carbon Fractions and Stability Under Manure Application Combined with Straw Incorporation. Agronomy 2025, 15, 1424. [Google Scholar] [CrossRef]
- Su, Y.; He, Z.; Yang, Y.; Jia, S.; Yu, M.; Chen, X.; Shen, A. Linking soil microbial community dynamics to straw-carbon distribution in soil organic carbon. Sci. Rep. 2020, 10, 5526. [Google Scholar] [CrossRef]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberan, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M.; et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef]
- Faust, S.; Heinze, S.; Ngosong, C.; Sradnick, A.; Oltmanns, M.; Raupp, J.; Geisseler, D.; Joergensen, R.G. Effect of biodynamic soil amendments on microbial communities in comparison with inorganic fertilization. Appl. Soil Ecol. 2017, 114, 82–89. [Google Scholar] [CrossRef]
- Wang, S.; Bao, X.; Feng, K.; Deng, Y.; Zhou, W.; Shao, P.; Zheng, T.; Yao, F.; Yang, S.; Liu, S.; et al. Warming-driven migration of core microbiota indicates soil property changes at continental scale. Sci. Bull. 2021, 66, 2025–2035. [Google Scholar] [CrossRef]
- Zhou, Y.; He, S.; Gong, G.; Zhang, S.; Chang, X.; Liu, N.; Sun, X.; Qi, X.; Ye, K.; Wang, Y. Soil fungal diversity in three nature reserves of Jiuzhaigou County, Sichuan Province, China. Ann. Microbiol. 2014, 64, 1275–1290. [Google Scholar] [CrossRef]
- Shinde, R.; Shahi, D.K.; Mahapatra, P.; Naik, S.K.; Thombare, N.; Singh, A.K. Potential of lignocellulose degrading microorganisms for agricultural residue decomposition in soil: A review. J. Environ. Manag. 2022, 320, 115843. [Google Scholar] [CrossRef]
- Osono, T. Colonization and succession of fungi during decomposition of Swida controversa leaf litter. Mycologia 2005, 97, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Powlson, D.S.; Riche, A.B.; Coleman, K.; Glendining, M.J.; Whitmore, A.P. Carbon sequestration in European soils through straw incorporation: Limitations and alternatives. Waste Manag. 2008, 28, 741–746. [Google Scholar] [CrossRef] [PubMed]
- McKinley, K.R.; Wetzel, R.G. Photolithotrophy, photoheterotrophy, and chemoheterotrophy: Patterns of resource utilization on an annual and a diurnal basis within a pelagic microbial community. Microb. Ecol. 1979, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Zhao, B.; Zhou, G.; Ruan, L. Bacterial community structure in maize stubble-amended soils with different moisture levels estimated by bar-coded pyrosequencing. Appl. Soil Ecol. 2015, 86, 62–70. [Google Scholar] [CrossRef]
- Schmidt, R.; Mitchell, J.; Scow, K. Cover cropping and no-till increase diversity and symbiotroph:saprotroph ratios of soil fungal communities. Soil Biol. Biochem. 2019, 129, 99–109. [Google Scholar] [CrossRef]
- Wang, J.; Rhodes, G.; Huang, Q.; Shen, Q. Plant growth stages and fertilization regimes drive soil fungal community compositions in a wheat-rice rotation system. Biol. Fertil. Soils 2018, 54, 731–742. [Google Scholar] [CrossRef]
- Yin, Y.; Yuan, Y.; Zhang, X.; Huhe; Cheng, Y.; Borjigin, S.; Cuomo Christina, A. Comparison of the responses of soil fungal community to straw, inorganic fertilizer, and compost in a farmland in the Loess Plateau. Microbiol. Spectr. 2022, 10, e02230-21. [Google Scholar] [CrossRef]
- Lin, Y.X.; Ye, G.P.; Kuzyakov, Y.; Liu, D.Y.; Fan, J.B.; Ding, W.X. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Ho, A.; Di Lonardo, D.P.; Bodelier, P.L. Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol. 2017, 93, fix006. [Google Scholar] [CrossRef]
- Ranjard, L.; Poly, F.; Combrisson, J.; Richaume, A.; Gourbière, F.; Thioulouse, J.; Nazaret, S. Heterogeneous Cell Density and Genetic Structure of Bacterial Pools Associated with Various Soil Microenvironments as Determined by Enumeration and DNA Fingerprinting Approach (RISA). Microb. Ecol. 2000, 39, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Qiu, S.; Xu, X.; Ciampitti, I.A.; Zhang, S.; He, P. Change in straw decomposition rate and soil microbial community composition after straw addition in different long-term fertilization soils. Appl. Soil Ecol. 2019, 138, 123–133. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.; Wei, W.; He, Z.; Kuzyakov, Y.; Bol, R.; Hu, R. Soil organic matter priming and carbon balance after straw addition is regulated by long-term fertilization. Soil Biol. Biochem. 2019, 135, 383–391. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.N.; Parra-Saldivar, R. Soil carbon sequestration—An interplay between soil microbial community and soil organic matter dynamics. Sci. Total Environ. 2022, 815, 152928. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Ren, D.; Liu, C.; Zhang, Y.; Wang, S.; Li, Z.; Zhang, M. Interlinkages between soil properties and keystone taxa under different tillage practices on the North China Plain. Appl. Soil Ecol. 2022, 178, 104551. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, Y.; Wang, Q.; Van Zwieten, L.; Liang, C.; Xu, J.; Guggenberger, G.; Luo, Y. Deciphering the microbial players driving straw decomposition and accumulation in soil components of particulate and mineral-associated organic matter. Soil Biol. Biochem. 2025, 209, 109871. [Google Scholar] [CrossRef]
- Shi, A.; Chakrawal, A.; Manzoni, S.; Fischer, B.M.C.; Nunan, N.; Herrmann, A.M. Substrate spatial heterogeneity reduces soil microbial activity. Soil Biol. Biochem. 2021, 152, 108068. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Hu, X.; Liu, J.; Liang, A.; Gu, H.; Liu, Z.; Jin, J.; Wang, G. Soil metagenomics reveals reduced tillage improves soil functional profiles of carbon, nitrogen, and phosphorus cycling in bulk and rhizosphere soils. Agric. Ecosyst. Environ. 2025, 379, 109371. [Google Scholar] [CrossRef]
- Peng, J.; Zhou, X.; Rensing, C.; Liesack, W.; Zhu, Y.-G. Soil microbial ecology through the lens of metatranscriptomics. Soil Ecol. Lett. 2023, 6, 230217. [Google Scholar] [CrossRef]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Wu, Y.; Zhu, M.; Yu, W.; Yao, H.; Zhu, Y.-G.; Chu, H. Suppressed N fixation and diazotrophs after four decades of fertilization. Microbiome 2019, 7, 143. [Google Scholar] [CrossRef]


| Treatment | Fertilization Regime | Fertilizer Rates (kg ha−1 yr−1) | Organic Amendment | Straw Addition |
|---|---|---|---|---|
| CK | No fertilization (control) | None | None | No |
| IF | Inorganic fertilizer | 165 N, 82.5 P2O5, 82.5 K2O | None | No |
| IFM | Inorganic fertilizer + manure | 50 N, 82.5 P2O5, 82.5 K2O | 115 N from pig manure | No |
| CKS | No fertilization (control) | None | None | Yes |
| IFS | Inorganic fertilizer | 165 N, 82.5 P2O5, 82.5 K2O | None | Yes |
| IFMS | Inorganic fertilizer + manure | 50 N, 82.5 P2O5, 82.5 K2O | 115 N from pig manure | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Z.; Bol, R.; Wang, T.; Zhu, P.; An, T.; Li, S.; Wang, J. Long-Term Fertilization Mediates Microbial Keystone Taxa to Regulate Straw-Derived 13C Incorporation in Soil Aggregates. Agronomy 2025, 15, 2116. https://doi.org/10.3390/agronomy15092116
Ge Z, Bol R, Wang T, Zhu P, An T, Li S, Wang J. Long-Term Fertilization Mediates Microbial Keystone Taxa to Regulate Straw-Derived 13C Incorporation in Soil Aggregates. Agronomy. 2025; 15(9):2116. https://doi.org/10.3390/agronomy15092116
Chicago/Turabian StyleGe, Zhuang, Roland Bol, Tianhao Wang, Ping Zhu, Tingting An, Shuangyi Li, and Jingkuan Wang. 2025. "Long-Term Fertilization Mediates Microbial Keystone Taxa to Regulate Straw-Derived 13C Incorporation in Soil Aggregates" Agronomy 15, no. 9: 2116. https://doi.org/10.3390/agronomy15092116
APA StyleGe, Z., Bol, R., Wang, T., Zhu, P., An, T., Li, S., & Wang, J. (2025). Long-Term Fertilization Mediates Microbial Keystone Taxa to Regulate Straw-Derived 13C Incorporation in Soil Aggregates. Agronomy, 15(9), 2116. https://doi.org/10.3390/agronomy15092116







