Abstract
Sugarcane smut, caused by Sporisorium scitamineum, is a globally prevalent disease that severely impacts sugarcane yield and quality. The most cost-effective and sustainable approach to disease control is breeding for smut-resistant varieties. In this study, we conducted a genome-wide association study (GWAS) using a panel of core sugarcane parents and their derived lines to elucidate the genetic basis of smut resistance across seven different environments. We identified 68 new loci significantly associated with smut resistance across all the chromosomes. Based on functional annotations and genomic positions, 164 candidate genes were identified, many of which are related to enzymatic systems, resistance genes, transcription factors, and other pathways implicated in smut defense. Using resistance ratings and associated SNPs, we further selected ten elite parents and derivatives as potential donors for marker-assisted selection (MAS). This study provides a valuable reservoir of genetic resources for improving smut resistance in sugarcane.
1. Introduction
The sugarcane smut, caused by the fungus Sporisorium scitamineum, is one of the most destructive sugarcane diseases worldwide. Infected plants exhibit premature germination, slender leaves, stunted stems, excessive tillering, and the emergence of characteristic smut whips, all of which substantially reduce yield and sucrose content [1,2]. Reported yield losses typically range from 10% to 30% but can reach up to 50–70% in severe outbreaks, with significant declines in sugar content [3]. Breeding for disease resistance is the most economical and effective strategy for managing smut. However, due to sugarcane’s complex genomic structure—being an aneuploid heteropolyploid with a genome exceeding 10 Gb—relatively few resistance genes have been successfully utilized in breeding programs. Thus, identifying quantitative trait loci (QTLs) or resistance genes and integrating them through marker-assisted selection (MAS) is crucial for enhancing smut resistance.
Genome-wide association studies (GWAS) are a robust method for dissecting the genetic basis of complex traits. By analyzing associations between phenotypes and high-density genome-wide markers such as SNPs, GWAS enables the identification of loci linked to trait variation. Originally applied to human diseases such as age-related macular degeneration [4], GWAS has since been widely adopted in plant genetics. It has been instrumental in mapping resistance loci for various crop diseases, including maize gray leaf spot [5], barley net blotch [6], and apple brown leaf spot [7]. These studies have revealed numerous resistance QTLs that have been successfully integrated into breeding pipelines [8,9,10].
Despite the growing application of GWAS in crop genetics, progress in sugarcane has lagged due to its polyploidy and genomic complexity. Previous sugarcane GWAS efforts have focused primarily on agronomic traits such as yield, sugar content, and fiber percentage [11,12,13,14]. Only a few studies have explored disease resistance. Dijoux et al. (2024) [15] conducted a GWAS on a population of interspecific hybrids, identifying six QTLs associated with orange rust resistance using SNP markers. Similarly, Gouy et al. (2015) [11] evaluated smut and brown rust resistance using AFLP, DArT, and SSR markers with sugarcane accessions, identifying ten markers for smut resistance and nine for brown rust. McCord et al. (2019) [16] detected nine SNPs markers associated with yellow rust resistance in a big sugarcane population, providing valuable loci for resistance breeding.
China possesses rich sugarcane germplasm resources. The Hainan Breeding Station maintains over 2400 accessions from five genera (Succharum L., Erianthus Michx, Miscanthus Anderss., Narenga Bor., and Pennisetum) and 14 species globally. Among them, more than 300 are breeding parents, which have contributed to the development of elite cultivars such as Xin Taitang 22, Guiliu 05-136, and Guitang 42. These parents exhibit substantial genetic diversity and have undergone long-term environmental selection, making them valuable reservoirs of smut resistance genes for breeding.
Therefore, the present study aimed to identify smut resistance loci and candidate genes in a natural population of 216 Chinese core parents and their derivatives. The specific objectives were (i) to evaluate smut resistance in this panel across multiple environments; (ii) to identify significant loci associated with resistance using GWAS; and (iii) to annotate these loci to uncover potential candidate genes, thereby providing a foundation for MAS and resistance improvement in sugarcane.
2. Materials and Methods
2.1. Plant Materials
A natural population of 216 sugarcane accessions was selected from over 300 core parents and their derivatives preserved at the Hainan Breeding Station, China (Table S1). These genotypes originated from multiple major sugarcane-producing countries, including China (150), the United States (32), Australia (10), India (4), Cuba (7), Brazil (2), France (1), the Philippines (4), Mauritius (2), South Africa (2), Thailand (1), and Indonesia (1). The selected accessions were chosen based on superior agronomic performance, including yield, sugar content, and disease resistance, and collectively represent a key genetic background of China [17].
2.2. Sugarcane Smut Inoculation and Evaluation
To avoid introducing novel S. scitamineum races to the experimental sites, smut spores were sourced from two locations in Guangdong Province: the Institute of Nanfan & Seed Industry Bases in Zhanjiang (110.24° E, 21.39° N) and Wengyuan (114.13° E, 24.36° N). Spore suspensions were diluted to a concentration of 106 spores/mL. For each accession, 30–60 single-budded setts were used. Bud regions were pricked using insect needles at five points before soaking in the spore suspensions from both sites for 10 min. Inoculated setts were incubated at 28 °C in a moist chamber for 24 h, then planted in a randomized complete block design with 2–3 replicates at each location. Standard field management practices were followed.
Smut infection was monitored at regular intervals, and infected plants exhibiting smut whips were removed immediately to prevent secondary transmission. Surveys were conducted every 15 days during the early infection phase. After 30 days, cumulative counts of infected plants were recorded, and the incidence rate was calculated for each accession. Resistance was scored on a standardized 1–9 scale: 1 = 0–3% incidence, 2 = 4–6%, 3 = 7–9%, 4 = 10–12%, 5 = 13–25%, 6 = 26–35%, 7 = 36–50%, 8 = 51–75%, and 9 = 76–100% [18].
2.3. Genome Resequencing and Genotyping
Whole-genome resequencing of the 216 sugarcane accessions was performed by Beijing Nuohe Zhiyuan Bioinformatics Technology Co., Ltd. (Guangzhou, China), using the Illumina HiSeq 2500 platform at 5× coverage. Raw reads were filtered to remove adapters, sequences with >10% ambiguous bases (N), and reads with <10 bp or >50% low-quality bases. Clean reads were aligned to the reference genome of Xin Taitang 22 using BWA-MEM (v.0.7.17) [19]. PCR duplicates were marked by GATK (v4.1.9.0). SNPs were called for each sample using the HaplotypeCaller module of GATK (4.1.9.0) in GVCF mode, followed by joint genotyping using the GATK CombineGVCFs function. SNPs with the following parameters: QD < 2.0 || FS > 60.0 || MQRankSum < −12.5 || ReadPosRankSum < −8.0 || SOR > 3.0 || MQ < 40.0 || DP > 30 || DP < 3, a minor allele frequency (MAF) ≥ 5%, and a missing rate ≤ 20% were retained for downstream analysis [17].
2.4. Linkage Disequilibrium Analysis
Linkage disequilibrium (LD) was assessed using PLINK (v1.90b3.42) with SNPs filtered for MAF > 0.05 and missing rate < 20%. The following parameters were used: --ld-window-r2 0 --ld-window-kb 500 [20]. The LD coefficient (r2) was calculated genome-wide, where r2 = 0 indicates linkage equilibrium (no LD), and r2 = 1 represents complete linkage. LD decay was evaluated by calculating the average r2 between SNP pairs within 500 kb, and decay distance was defined as the physical distance at which r2 dropped to 0.1.
2.5. Genome-Wide Association Study (GWAS)
SNP imputation was performed using Beagle software (v5.5) with default settings [19,21]. Population structure was inferred using Admixture (v1.3.0; http://software.genetics.ucla.edu/admixture/ (accessed on 22 April 2025)), and the resulting Q-values were incorporated as fixed effects in a linear mixed model, with kinship (calculated by zzlab.net/GAPIT) included as a random effect, the BLUP values for each material using LME4 packge [22]. Association mapping was performed by combining SNP data with phenotype scores via the GAPIT package with parameter model = “MLM”. A genome-wide significance threshold was set at −log10(p) ≥ 1/1509012 based on the number of independent SNPs identified by PLINK with the parameter “--indep-pairwise 100 50 0.2.”. Manhattan plots of GWAS results were generated using the R package ggplot2.
2.6. Candidate Gene Identification for Associated SNPs
Significant SNPs were clustered into LD blocks using a cutoff of r2 ≥ 0.2. A confidence interval of ±20 kb around each LD block was defined, and genes within these regions were considered candidate genes. Gene function was annotated using InterProScan (v5.39-77.0) with default settings, based on Xin Taitang 22 protein sequences [19,23]. To predict biological functions and regulatory networks, Gene Ontology (GO) analysis was conducted using the PANTHER database (https://geneontology.org/ (accessed on 10 May 2025)).
3. Results
3.1. Phenotypic Analysis of Smut Resistance in the Sugarcane Natural Population
Following artificial inoculation with S. scitamineum spores, typical smut symptoms—particularly the emergence of black whips—began to appear at the seedling stage (Figure 1). Disease responses varied substantially among the 216 sugarcane core parents and their derivatives (Figure 2; Table S1). Disease ratings ranged from 1 to 9 across the different environments tested. As shown in Figure 2, the distribution of disease scores was bimodal, with one peak at grade 1 and another at grade 5. Inoculations conducted in Wengyuan during 2020 and 2024 showed unusually high resistance frequencies, likely due to unfavorable weather conditions for disease development; 192 accessions (97.46%) were resistant in 2020 and 164 accessions (87.23%) in 2024.

Figure 1.
Typical symptoms of sugarcane smut after inoculation with smut spores at the seedling stage.
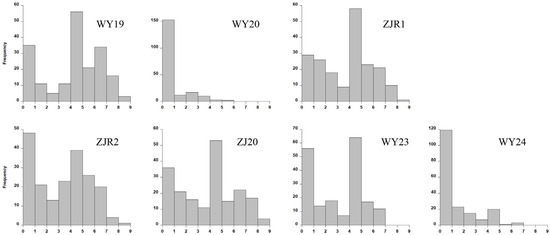
Figure 2.
Frequency distribution of sugarcane smut resistance in natural population. WY19 and WY20 indicate disease reaction with local inoculum in Wengyuan in 2019 and 2020, respectively; ZJR1 and ZJR2 indicate two environments of disease reaction with local inoculum in Zhanjiang in 2019; ZJ20 indicates disease reaction with local inoculum in Zhanjiang in 2020; and WY23 and WY24 indicate disease reaction with local inoculum in Wengyuan in 2023 and 2024, respectively.
Across all environments, an average of 58.29% of accessions were resistant, while 41.71% were susceptible, indicating a diverse spectrum of smut resistance alleles within the population. Pearson correlation analysis revealed strong positive correlations among experiments conducted in Zhanjiang during 2019 and 2020, with correlation coefficients ranging from 0.53 to 0.88. Moderate correlations were observed between trials in Zhanjiang and Wengyuan in 2019, with coefficients ranging from 0.39 to 0.52 (Figure 3), suggesting an environmental influence on disease expression while confirming overall consistency in resistance phenotyping.
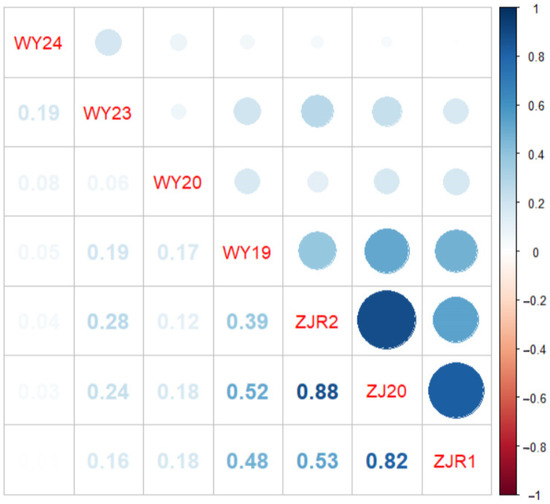
Figure 3.
Pearson’s correlation matrix for sugarcane smut resistance in natural population. The shaded scale refers to the strength of correlation. In Pearson’s correlation, abs r = 0.5 to 1 means a greater correlation, abs r = 0.3 to 0.5 means medium correlation, abs r = 0.1 to 0.3 means lesser correlation, and abs r = 0 to 0.1 suggests no correlation. WY24, WY23, WY20, and WY19 indicate disease reaction with local inoculum in Wengyuan in 2024, 2023, 2020, and 2019, respectively. ZJR2 and ZJR1 indicate two environments of disease reaction with local inoculum in Zhanjiang in 2019; and ZJ20 indicates disease reaction with local inoculum in Zhanjiang in 2020.
3.2. Genome-Wide Association Study of Smut Resistance
Genome-wide association analysis identified 68 new loci significantly associated with smut resistance (−log10(p) ≥ 6.63) across seven phenotypic datasets. Of these, 36 and 5 loci were identified from Wengyuan trials in 2020 (WY20) and 2024 (WY24), respectively, while 1 and 2 loci were associated with Zhanjiang replicates ZJR1 and ZJR2, respectively. An additional 24 loci were identified using the BLUP values, integrating all phenotypic datasets (Figure 4 and Figure S1; Table S2). These SNPs were distributed across 43 distinct linkage groups spanning the entire sugarcane genome, indicating that resistance to smut is a complex, polygenic trait with loci widely dispersed throughout the genome.
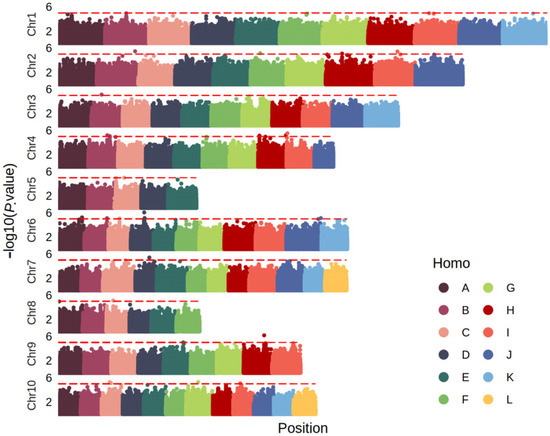
Figure 4.
Manhattan plot of sugarcane smut resistance in natural population (BLUP values). The Y-axis represents the −log10(p value) of each SNP, and the X-axis represents the chromosome and genomic location of the SNP. Homo A–L represent homologs.
3.3. Candidate Gene Analysis
Candidate genes were predicted based on a 20 kb LD decay window flanking each significant SNP (−log10(p) peak). Gene predictions considered both functional annotations and physical locations in the reference genome. As summarized in Table S3, 164 candidate genes were identified across the 68 significant loci. These genes encode a range of resistance-related proteins, including catalase (from Saccharum hybrid cultivar R570), leucine-rich repeat receptor-like kinases (LRR-RLKs) from Sorghum bicolor and Miscanthus lutarioriparius, ribosomal proteins from Zea mays, and serine/threonine protein kinases from Miscanthus lutarioriparius. Several transcription factors were also among the candidates. Collectively, these genes may function either directly or indirectly in sugarcane defense responses against smut infection.
3.4. Core Parents and Their Derivatives with SNP-Tagged Smut Resistance
Ten germplasm resources exhibiting consistent resistance (≤Grade 2) across all tested environments were selected for further analysis (Table 1). These accessions carried favorable alleles at key SNP loci, with most maintaining heterozygosity at those markers, except for HOCP07-612 and GT-03-1453. For instance, C88-380, Fu_nong-07-14, CP84-1198, and Q208 were associated with Snp3 (S4I_2508239); CP75-1632 with Snp3 (S4I_2508239) and Snp4 (S5A_68155075); Fu_nong-91-23 with Snp3 and Snp8 (S10I_26152541); CP72-2086 with Snp1 (S2B_118242947); and Yunzhe-89-151 with Snp4.

Table 1.
The elite core parents and their derivatives with smut resistance and the nearest markers.
These accessions, tagged with elite resistance alleles, provide valuable resources for both fine mapping of resistance loci and use in MAS. Their stable phenotypic resistance and associated SNP markers support their immediate application in breeding programs aimed at improving sugarcane smut resistance.
4. Discussion
Sugarcane smut, caused by S. scitamineum, is a significant threat to global sugarcane production, as it significantly reduces yield, compromises quality, and impairs root development [1,2]. The pathogen typically infects sugarcane via buds, suggesting that bud morphology may be an important factor in smut resistance [24,25]. Orellana et al. (1987) [26] found that bud scales can physically protect sugarcane buds from infection. In this study, we evaluated 216 diverse sugarcane accessions with varying bud structures and observed a wide range of resistance levels, reinforcing the importance of morphological diversity in smut resistance.
In addition to host-related factors, pathogenic variation also influences resistance. S. scitamineum exhibits physiological race differentiation, and at least three distinct races with varying pathogenicity have been reported in different sugarcane-growing regions of mainland China [27]. Strains from Zhanjiang and Wengyuan, two regions in Guangdong Province, have also been shown to differ in virulence [28,29]. Consequently, correlation between smut resistance phenotypes across locations was generally low (r < 0.28), with only moderate correlation observed in 2019 (r = 0.39–0.52). This geographic and race-based variation likely contributed to the absence of shared resistance loci across environments in our GWAS.
A total of 68 new loci were significantly associated with smut resistance across seven environments, yet no overlapping loci were detected across all trials. This result suggests significant genotype × environment × pathogen race interactions. Disease expression may be affected by subtle interactions between host genotype, pathogen race, and environmental conditions. In some cases, disease evaluation is based entirely on obvious smut whip incidence, which may overlook latent infections or understate partial resistance, thereby biasing the GWAS findings. To obtain more reliable loci, more functional verification on each marker related to smut resistance needs to be further validated.
During pathogen attack, plants generate large amounts of reactive oxygen species (ROS) as part of their defense response. Excess ROS, if not efficiently scavenged, can damage plant cells and trigger programmed cell death. Plants rely on enzymatic antioxidant systems, including catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), and superoxide dismutase (SOD), to maintain ROS homeostasis and minimize oxidative damage [30,31]. From the 68 significant SNP loci identified in our GWAS, 164 candidate genes were predicted. Among them, three are involved in ROS scavenging: Sh_So04I0214004 (encoding CAT in Saccharum hybrid R570), Sh_Ss10I0021315 (encoding POD in Sorghum bicolor), and Sh_Ss10I0021178 (encoding APX in Miscanthus lutarioriparius). These enzymes decompose hydrogen peroxide (H2O2), a major ROS, into water and oxygen, reducing oxidative stress.
ROS-scavenging enzymes have been implicated in disease resistance across multiple crops, including rice blast, bacterial blight, maize leaf spot, and soybean Phytophthora [32,33,34,35]. In sugarcane, catalase activity in the resistant cultivar Yacheng 05-179 consistently exceeds that of the susceptible Liucheng 03-182, suggesting a positive correlation between CAT activity and smut resistance [36]. Several antioxidant genes, including S-CAT, SsCAT-1, EaCAT-1a, ScPOD02, and ScAPX1, have been cloned from sugarcane cultivars and wild relatives and shown to be strongly associated with smut resistance [36,37,38,39,40,41,42]. Functional characterization of these genes and their regulatory networks is therefore crucial for understanding resistance mechanisms.
Plant disease resistance genes (R genes) are central components of the immune system and are generally classified based on conserved domains, such as nucleotide-binding site leucine-rich repeat (NLR), serine/threonine protein kinase (STK), leucine-rich repeat transmembrane (LRR-TM), and wall-associated kinase (WAK), among others [43,44]. In our study, three candidate genes (Sh_So08C0301280, Sh_So08C0301282, and Sh_So08C0301283) were associated with NLRs. NLRs are the largest class of R genes and function as pattern recognition receptors (PRRs) that detect pathogen effectors and activate immune responses. Many NLRs form receptor complexes or act directly as sensors to trigger defense signaling cascades [45]. For instance, LRR-RLKs, a common feature of R genes, initiate downstream responses upon detection of pathogen-associated molecular patterns, enhancing resistance to a wide range of diseases, including rice blast, sheath blight, tomato early blight, and eggplant bacterial wilt [46,47,48].
Additionally, two candidate genes (Sh_Ss02B0100883 and Sh_Ss06A0234530) were linked to STK domains. STKs play essential roles in transmitting immune signals via phosphorylation cascades. Several resistance genes, such as Lr10 (wheat leaf rust), Pm21 (wheat powdery mildew), and OsCPK4 (rice blast), contain STK domains and are crucial in host defense [49,50,51]. We also identified a WAK-associated gene (Sh_Ss05A0221703), which may play a role in sugarcane smut resistance. WAKs are known to participate in cell wall integrity sensing and pathogen response, and SsWAK genes are constitutively expressed in sugarcane tissues and upregulated in response to smut infection [52]. The exact mechanisms of WAK-mediated resistance in sugarcane, however, remain to be elucidated.
Transcription factors (TFs) are key regulators of plant immunity, modulating defense gene expression through complex signaling networks. Major TF families involved in disease resistance include WRKY, MYB, and bHLH. In this study, we identified several TF-related candidate genes. For example, Sh_So08C0301281 encodes a WRKY transcription factor, which has been extensively linked to plant immune responses. WRKYs function as positive or negative regulators of defense genes and are key targets for disease-resistance engineering. Wang et al. (2025) [2] reported that ScWRKY2 negatively regulates smut resistance in sugarcane by inhibiting ScLRR-RLK expression and interacting with chloroplast protein ScPsbP, thereby suppressing ROS-scavenging gene expression. Similarly, ScWRKY3 was shown to be stably expressed in resistant varieties but downregulated in susceptible ones [53].
We also identified Sh_Ss02E0111101, a candidate gene encoding a MYB transcription factor (TFs). MYB proteins regulate plant defense by modulating hormone signaling, secondary metabolism, and stress-responsive gene expression [54,55]. Que et al. (2014) [56] showed that expression of 25 MYB, 18 WRKY, and 18 ERF genes was altered in sugarcane infected by Fusarium, suggesting their involvement in pathogen response. More recently, Agisha et al. (2022) [57] demonstrated that NAC, ERF, MYB, and WRKY transcription factors are active participants in sugarcane–smut interactions.
Despite these advances, candidate gene identification was based on a single reference genome (Xin Taitang 22), which may have overlooked structural changes between different sugarcane backgrounds. The precise roles and molecular mechanisms of these TFs and resistance genes in sugarcane smut defense require further functional validation. Continued research into their expression, protein interactions, and regulatory networks will be critical for leveraging these genes in breeding and genetic engineering for enhanced smut resistance.
5. Conclusions
In this study, we identified 68 new loci significantly associated with sugarcane smut resistance across seven environments by GWAS, encompassing 164 candidate genes, several linked to disease defense. These include three encoding antioxidant enzymes (CAT, POD, APX), three associated with NLR resistance genes, two with STK genes, one with a WAK gene, and one each from the WRKY and MYB transcription factor families. The exact roles and molecular mechanisms of these resistance genes and TFs in sugarcane smut defense require further functional validation. Based on disease grading and favorable alleles at key loci, ten core parents and their derivatives tagged with SNPs were identified as elite smut resistance donors for MAS. These results provide a rich genetic resource for improving smut resistance in sugarcane.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15092111/s1: Figure S1: Manhattan plot of sugarcane smut resistance in natural population; Table S1: A natural population of 216 sugarcane accessions and their disease reaction; Table S2: 68 loci associated with sugarcane smut resistance; Table S3: 164 candidate genes identified across the 68 loci.
Author Contributions
X.C., J.W. and N.Z. conceived the project and designed the experiments; E.C. and J.F. conducted the inoculation and collected the original data; X.L. organized and analyzed the original data; X.C. drafted the manuscript; X.F. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research is financially supported by Guangdong Provincial Natural Science Foundation for Basic and Applied Basic Research 2022A1515011249; GDAS’ Project of Science and Technology Development (2022GDASZH-2022010201-05); the special project of Guangdong Academy of Sciences (2019GDASYL-0103034); the Guangdong Basic and Applied Basic Research Foundation (2023A1515012024); CARS (CARS-17); The Innovative Team Construction Project of the Modern Agricultural Industry Technology System in Guangdong Province by Agricultural Product Units (Sugarcane and Sisal Industry Technology System) (2024CXTD03-03); and the 2024 Guangdong Rural Revitalization Strategy Special Fund Seed Industry Project (First Batch) (Yue Cai Nong [2024] No. 83).
Data Availability Statement
Data are attached in Table S1.
Acknowledgments
We thank Phillip A. Jackson for reviewing the manuscript with excellent editorial comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yan, M.; Zhu, G.; Lin, S.; Zhang, L.H. The mating-type locus b of the sugarcane smut Sporisorium scitamineum is essential for mating, filamentous growth and pathogenicity. Fungal Genet. Biol. 2015, 86, 1–8. [Google Scholar] [CrossRef]
- Wang, D.; Gou, Y.; Yi, C.; Li, Z.; Wang, W.; Lin, P.; Wang, W.; Sun, T.; Wang, T.; Zhao, W.; et al. ScWRKY2: A key regulator for smut resistance in sugarcane. Plant Biotechnol. J. 2025, 1–15. [Google Scholar] [CrossRef]
- Jing, Y.; Zhou, H.; Liu, X.; Tan, F.; Zhang, X.; Zhang, R.; Song, X.; Li, Y.; Yan, M.; Lei, J.; et al. Smut Resistant Identification and Analysis of New Sugarcane Clones of Guitang. Chin. J. Trop. Crops 2020, 41, 333–338. (In Chinese) [Google Scholar]
- Edwards, A.; Ritter, R.; Abel, K.; Manning, A.; Panhuysen, C.; Farrer, L.A. Complement factor polymorphism and age-related macular degeneration. Science 2005, 308, 421–424. [Google Scholar] [CrossRef]
- Hu, C.; Kuang, T.; Shaw, R.K.; Zhang, Y.; Fan, J.; Bi, Y.; Jiang, F.; Guo, R.; Fan, X. Genetic dissection of resistance to gray leaf spot by genome-wide association study in a multi-parent maize population. BMC Plant Biol. 2024, 24, 10. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Steffenson, B.; Smith, K.; Smith, M.; Dill-Macky, R. Identification of quantitative trait loci for net form net blotch resistance in contemporary barley breeding germplasm from the USA using genome-wide association mapping. Theor. Appl. Genet. 2020, 133, 1019–1037. [Google Scholar] [CrossRef]
- Noh, J.; Do, S.; Kim, G.; Choi, C. A genome-wide association study for the detection of genes related to apple Marssonina Blotch disease resistance in apples. Sci. Hortic. 2020, 262, 108986. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, Q.; Xing, Y.; Wei, L.; Chao, Q.; Zuo, W.; Lubberstedt, T.; Xu, M. Marker-assisted introgression of QhSR1 to improve maize resistance to head smut. Mol. Breed. 2012, 30, 1077–1088. [Google Scholar] [CrossRef]
- Chen, X.; Jia, Y.; Jia, H.; Pinson, R.; Wang, X.; Wu, B. Functional Interactions between Major Rice Blast Resistance Genes Pi-ta and Pi-b, and Minor Blast Resistance Quantitative Trait Loci. Phytopathology 2018, 108, 1095–1103. [Google Scholar] [CrossRef]
- Xu, Z.; Hua, J.; Wang, F.; Cheng, Z.; Meng, Q.; Chen, Y.; Han, X.; Tie, S.; Liu, C.; Li, X.; et al. Marker-assisted selection of qMrdd8 to improve maize resistance to rough dwarf disease. Breed. Sci. 2020, 70, 183–192. [Google Scholar] [CrossRef]
- Gouy, M.; Rousselle, Y.; Thong, A.; Anglade, A.; Royaert, S.; Nibouche, S.; Costet, L. Genome wide association mapping of agro-morphological and disease resistance traits in sugarcane. Euphytica 2015, 202, 269–284. [Google Scholar] [CrossRef]
- Barreto, F.; Rosa, J.; Balsalobre, T.; Pastina, M.; Silva, R.; Hoffmann, H.; Souza, A.; Garcia, A.; Carneiro, M. A genome-wide association study identified loci for yield component traits in sugarcane (Saccharum spp.). PLoS ONE 2019, 14, e0219843. [Google Scholar] [CrossRef]
- Fickett, N.; Gutierrez, A.; Verma, M.; Pontif, M.; Hale, A.; Kimbeng, C.; Baisakh, N. Genome-wide association mapping identifies markers associated with cane yield components and sucrose traits in the Louisiana sugarcane core collection. Genomics 2019, 111, 1794–1801. [Google Scholar] [CrossRef]
- Yang, X.; Luo, Z.; Todd, J.; Sood, S.; Wang, J. Genome-wide association study of multiple yield traits in a diversity panel of polyploid sugarcane (Saccharum spp.). Plant Genome 2020, 13, e20006. [Google Scholar] [CrossRef]
- Dijoux, J.; Rio, S.; Hervouet, C.; Garsmeur, O.; Barau, L.; Dumont, T.; Rott, P.; D’Hont, A.; Hoarau, J. Unveiling the predominance of Saccharum spontaneum alleles for resistance to orange rust in sugarcane using genome-wide association. Theor. Appl. Genet. 2024, 137, 81. [Google Scholar] [CrossRef] [PubMed]
- Mccord, P.; Glynn, N.; Comstock, J. Identifying markers for resistance to sugarcane orange rust (Puccinia kuehnii) via selective genotyping and capture sequencing. Euphytica 2019, 215, 150. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Z.; Fu, D.; Fang, J.; Feng, X.; Xie, J.; Wu, B.; Luo, Y.; Zhu, M.; Qi, Y. Identification of Genetic Loci for Sugarcane Leaf Angle at Different Developmental Stages by Genome-Wide Association Study. Front. Plant Sci. 2022, 13, 841693. [Google Scholar] [CrossRef]
- Cai, Q.; Fan, Y. Descriptors and Data Standard for Sugarcane (Saccharum officinarum L.); China Agricultural Publishing House: Beijing, China, 2006; pp. 45–46. (In Chinese) [Google Scholar]
- Zhang, J.; Qi, Y.; Hua, X.; Wang, Y.; Wang, B.; Qi, Y.; Huang, Y.; Yu, Z.; Gao, R.; Zhang, Y.; et al. The highly allo-autopolyploid modern sugarcane genome and very recent allopolyploidization in Saccharum. Nat. Genet. 2025, 57, 242–253. [Google Scholar] [CrossRef]
- Sadowski, M.; Kraft, A.; Szalaj, P.; Wlasnowolski, M.; Tang, Z.; Ruan, Y.; Plewczynski, D. Spatial chromatin architecture alteration by structural variations in human genomes at the population scale. Genome Biol. 2019, 20, 148. [Google Scholar] [CrossRef]
- Khvorykh, G.V.; Khrunin, A.V. Imputeqc: An R package for assessing imputation quality of genotypes and optimizing imputation parameters. BMC Bioinform. 2020, 21, 304. [Google Scholar] [CrossRef] [PubMed]
- Pino, D.C.D.; Lozano, R.; Wolfe, M.D.; Jannink, J.L. Genome-Wide Association Studies and Heritability Estimation in the Functional Genomics Era. In Population Genomics; Springer: Cham, Switzerland, 2018; pp. 361–425. [Google Scholar]
- Chen, S.; Feng, X.; Zhang, Z.; Hua, X.; Zhang, Q.; Chen, C.; Li, J.; Liu, X.; Weng, C.; Chen, B.; et al. ScDB: A comprehensive database dedicated to Saccharum, facilitating functional genomics and molecular biology studies in sugarcane. Plant Biotechnol. J. 2024, 22, 14457. [Google Scholar] [CrossRef]
- Bhuiyan, S.; Croft, B.J. Incidence versus severity—What difference would it make to smut screening. In Proceedings of the 33rd Annual Conference of the Australian Society of Sugar Cane Technologists 2011, Mackay, QLD, Australia, 4–6 May 2011; pp. 118–125. [Google Scholar]
- Marques, J.R.P.; Hoy, W.J.; Beatriz, A.; Viveros, F.G.; Vieira, M.L.C.; Baisakh, N. Sugarcane Cell Wall-Associated Defense Responses to Infection by Sporisorium scitamineum. Front. Plant Sci. 2018, 9, 9698. [Google Scholar] [CrossRef] [PubMed]
- Orellana, P.P.A.; Perez, P.J. Evaluation of the resistance in sugar cane plantlets (Saccharum spp. hybrids) against Ustilago scitaminea Syd. Jpn. J. Appl. Phys. 1987, 17, 733–734. [Google Scholar]
- Deng, Q.Q.; Xu, G.H.; Dou, Z.M.; Shen, W.K. Identification of three Sporisorium scitamineum pathogenic races in mainland China. Int. J. Agric. Biol. 2018, 20, 799–802. [Google Scholar]
- Shen, W.; Deng, H. Analysis of Results from Smut Resistant Identification in Sugarcane Varieties Introduced. Chin. Agric. Sci. Bull. 2011, 27, 234–238. (In Chinese) [Google Scholar]
- Zhang, Y.Y.; Huang, N.; Xiao, X.H.; Huang, L.; Liu, F.; Su, W.H.; Que, Y.X. Molecular variation of Sporisorium scitamineum in Mainland China revealed by internal transcribed spacers. Genet. Mol. Res. GMR 2015, 14, 7894–7909. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Wang, Y.; Tang, J.; Xue, P.; Li, C.; Liu, L.; Hu, B.; Yang, F.; Loake, G.; Chu, C. Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol. 2012, 158, 451–464. [Google Scholar] [CrossRef]
- Giulietti, S.; Bigini, V.; Savatin, D.V. ROS and RNS production, subcellular localization and signaling triggered by immunogenic danger signals. J. Exp. Bot. 2024, 75, 4512–4534. [Google Scholar] [CrossRef]
- Jiang, G.; Yin, D.; Zhao, J.; Chen, H.; Guo, L.; Zhu, L.; Zhai, W. The rice thylakoid membranebound ascorbate peroxidase OsAPX8 functions in tolerance to bacterial blight. Sci. Rep. 2016, 6, 26104. [Google Scholar]
- Zhang, J.; Jia, X.; Wang, G.; Ma, S.; Wang, S.; Yang, Q.; Chen, X.; Zhang, Y.; Lyu, Y.; Wang, X. Ascorbate peroxidase 1 confers resistance to southern corn leaf blight in maize. J. Integr. Plant Biol. 2022, 64, 1196–1211. [Google Scholar] [CrossRef]
- You, X.; Zhang, F.; Liu, Z.; Wang, M.; Xu, X.; He, F.; Wang, D.; Wang, R.; Wang, Y.; Wang, G.; et al. Rice catalase OsCATC is degraded by E3 ligase APIP6 to negatively regulate immunity. Plant Physiol. 2022, 190, 1095–1099. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, L.; Zhu, R.; Zhou, X.; Zhang, J.; Li, D.; He, S.; Qiao, Y. Phytophthora sojae effector PsAvh113 associates with the soybean transcription factor GmDPB to inhibit catalasemediated immunity. Plant Biotechnol. J. 2023, 21, 1393–1407. [Google Scholar] [CrossRef]
- Su, Y.; Guo, J.; Ling, H.; Chen, S.; Wang, S.; Xu, L.; Allan, A.C.; Que, Y. Isolation of a Novel Peroxisomal Catalase Gene from Sugarcane, Which is Responsive to Biotic and Abiotic Stresses. PLoS ONE 2014, 9, e84426. [Google Scholar] [CrossRef]
- Casu, R.E.; Dimmock, C.M.; Chapman, S.C.; Grof, C.P.L.; McIntyre, C.L.; Bonnett, G.D.; Manners, J.M. Identification of differentially expressed transcripts frommaturing stem of sugarcane by in silico analysis of stem expressed sequence tags and gene expression profiling. Plant Mol. Biol. 2004, 54, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, J.; Li, G.; Que, Y.; Xu, L. Electronic cloning and characterization of CAT gene from Saccharum officinarum using bioinformatics tool. Chin. J. Bioinform. 2012, 10, 65–70. (In Chinese) [Google Scholar]
- Liu, Y.; Yao, Y.; Hu, X.; Xu, L.; Xing, S.; Zhang, S. Isolation and Characterization of Catalase (SsCAT-1) Gene in Saccharum spontaneum L. Mol. Plant Breed. 2014, 12, 1251–1258. (In Chinese) [Google Scholar]
- Liu, Y.; Yao, Y.; Hu, X.; Xu, L.; Xing, S.; Zhang, S. Isolation and Characterization of Catalase Gene (EaCAT-1a) in Erianthus arundinaceus (Retz.) Jesw. Southwest China J. Agric. Sci. 2015, 4, 1535–1541. (In Chinese) [Google Scholar]
- Su, Y.; Wang, Z.; Li, Z.; Liu, F.; Xu, L.; Que, Y.; Dai, M.; Chen, Y. Molecular Cloning and Functional Identification of Peroxidase Gene ScPOD02 in Sugarcane. ACTA Agron. Sin. 2017, 43, 510–521. [Google Scholar] [CrossRef]
- Zhang, B.; Shao, M.; Huang, Y.; Huang, X.; Song, X.; Chen, H.; Wang, S.; Tan, Q.; Yang, L.; Li, Y. Cloning and Expression Analysis of Peroxidase Gene (ScAPX1) from Sugarcane. Biotechnol. Bull. 2019, 35, 31–37. (In Chinese) [Google Scholar]
- Dangl, J.L.; Jones, J.D. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef]
- Sun, B.Y.; Yang, L.Y.; Dong, S.M. Advances in regulatory mechanisms of plant resistance genes. Chin. Bull. Life Sci. 2025, 37, 477–489. (In Chinese) [Google Scholar]
- Liang, X.; Zhou, J.M. Receptor-Like Cytoplasmic Kinases: Central Players in Plant Receptor Kinase-Mediated Signaling. Annu. Rev. Plant Biol. 2018, 69, 267–299. [Google Scholar] [CrossRef]
- Reddy, A.C.; Venkat, S.; Sing, T.H.; Aswath, C.; Reddy, K.M.; Reddy, D.C.L. Isolation, characterization and evolution of NBS-LRR encoding disease-resistance gene analogs in eggplant against bacterial wilt. Eur. J. Plant Pathol. 2015, 143, 417–426. [Google Scholar] [CrossRef]
- Xu, Y.J.; Liu, F.; Zhu, S.W.; Li, X.Y. The maize NBS-LRR gene ZmNBS25 enhances disease resistance in rice and Arabidopsis. Front. Plant Sci. 2018, 9, 1033. [Google Scholar] [CrossRef]
- Hassan, Z.; Rahim, M.A.; Jung, H.J.; Park, J.I.; Kim, H.T.; Nou, I.S. Genome-wide characterization of NBS-encoding genes in watermelon and their potential association with gummy stem blight resistance. Int. J. Mol. Sci. 2019, 20, 902. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.C.; Chen, G.P.; Zhao, B.C.; Shen, Y.Z.; Huang, Z.J. Cloning and functional characterization of a wheat serine/threonine kinase gene (TaSTK) related to salt-resistance. Plant Sci. 2007, 173, 55–60. [Google Scholar] [CrossRef]
- Gao, L.; Wang, S.; Li, X.Y.; Wei, X.J.; Zhang, Y.J.; Wang, H.Y.; Liu, D.Q. Expression and functional analysis of a pathogenesis-related protein 1 gene, TcLr19PR1, involved in wheat resistance against leaf rust fungus. Plant Mol. Biol. Report. 2015, 33, 797–805. [Google Scholar] [CrossRef]
- Bundo, M.; Coca, M. Enhancing blast disease resistance by overexpression of the calcium-dependent protein kinase OsCPK4 in rice. Plant Biotechnol. J. 2016, 14, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.J.; Qin, L.O.; Wu, M.X.; Zou, W.H.; Zang, S.J.; Zhao, Z.N.; Lin, P.X.; Guo, J.L.; Wang, H.B.; Que, Y.X. Identification and characterization of WAK gene family in Saccharum and the negative roles of ScWAK1 under the pathogen stress. Int. J. Biol. Macromol. 2023, 224, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, F.; Zhang, X.; Wang, W.; Sun, T.; Chen, Y.; Dai, M.; Yu, S.; Xu, L.; Su, Y.; et al. Expression characteristics and functional analysis of the ScWRKY3 gene from sugarcane. Int. J. Mol. Sci. 2018, 19, 4059. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Yuan, D.; Duan, M.; Liu, Y.; Shen, Z.; Yang, C.; Qiu, Z.; Liu, D.; Wen, P.; et al. An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc. Natl. Acad. Sci. USA 2020, 117, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, X.; He, Q.; Guo, D.; Liu, C.; Cao, J.; Wu, Z.; Kang, Z.; Wang, X. TaMYB29: A novel R2R3-MYB transcription factor involved in wheat defense against stripe rust. Front. Plant Sci. 2021, 12, 783388. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.; Su, Y.; Guo, J.; Wu, Q.; Xu, L. A global view of transcriptome dynamics during Sporisorium scitamineum challenge in sugarcane by RNA-seq. PLoS ONE 2014, 9, e106476. [Google Scholar] [CrossRef]
- Agisha, V.N.; Ashwin, N.M.R.; Vinodhini, R.T.; Nalayeni, K.; Ramesh, S.A.; Malathi, P.; Viswanathan, R. Transcriptome analysis of sugarcane reveals differential switching of major defense signaling pathways in response to Sporisorium scitamineum isolates with varying virulent attributes. Front. Plant Sci. 2022, 13, 969826. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).