Monitoring of Harmful Noctuid Pests with Synthetic Sex Pheromones and Semisynthetic Bisexual Lures (SBL): Benefits and Limitations of Separate, Parallel and Combined Use of the Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Tests
2.2. Traps
2.3. Baits
2.4. Field Experiment Design
2.5. Sampling and Data Analysis
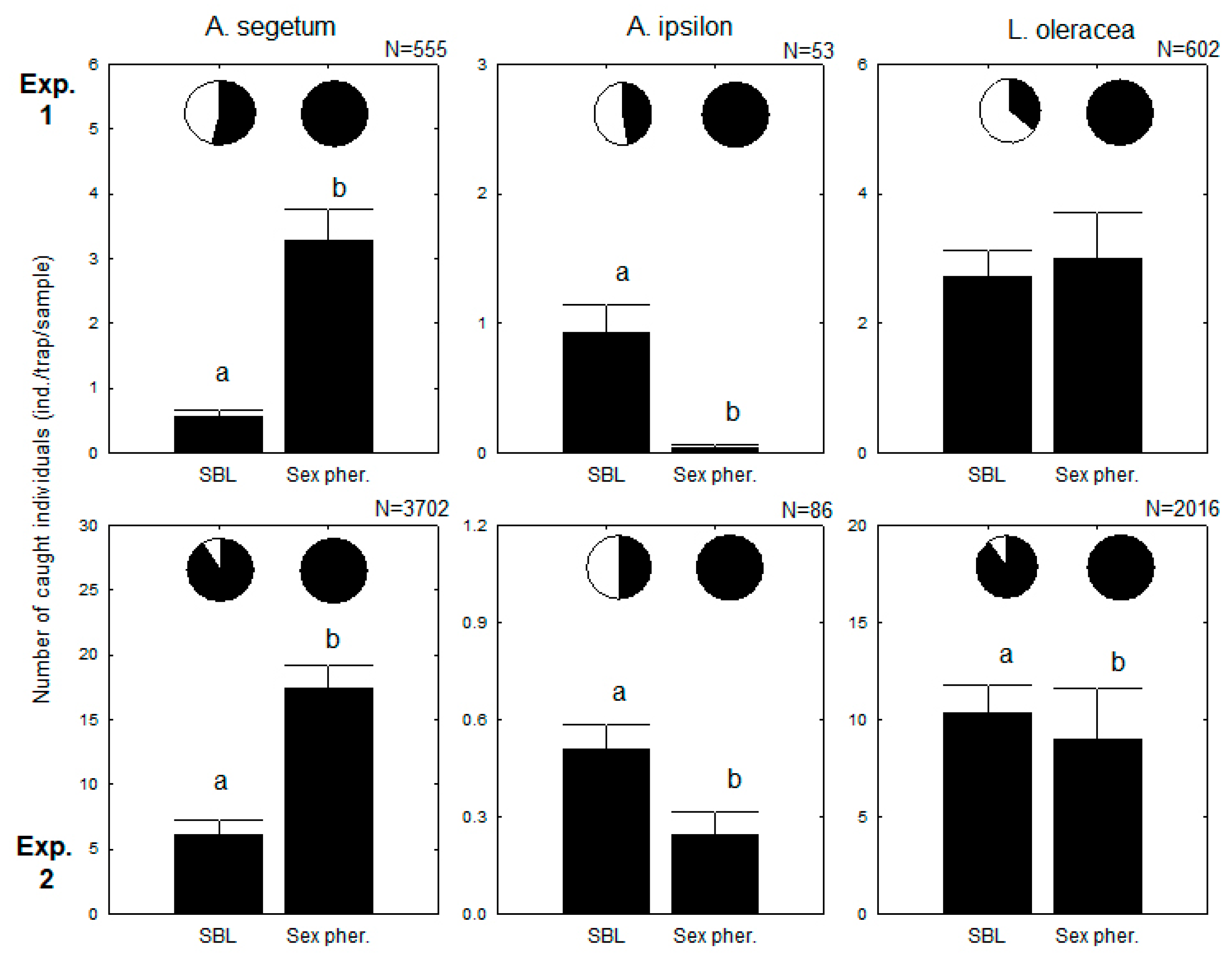
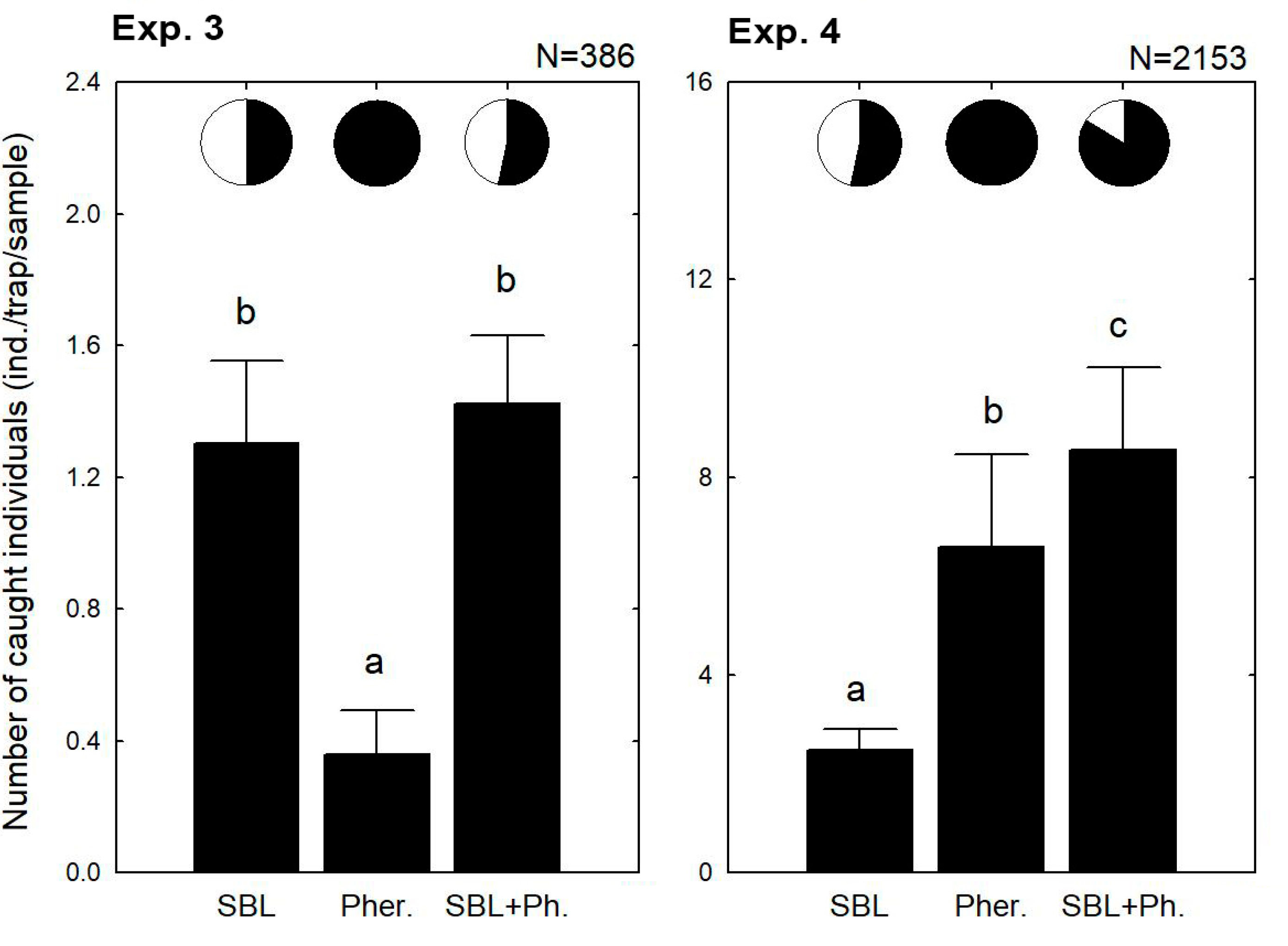
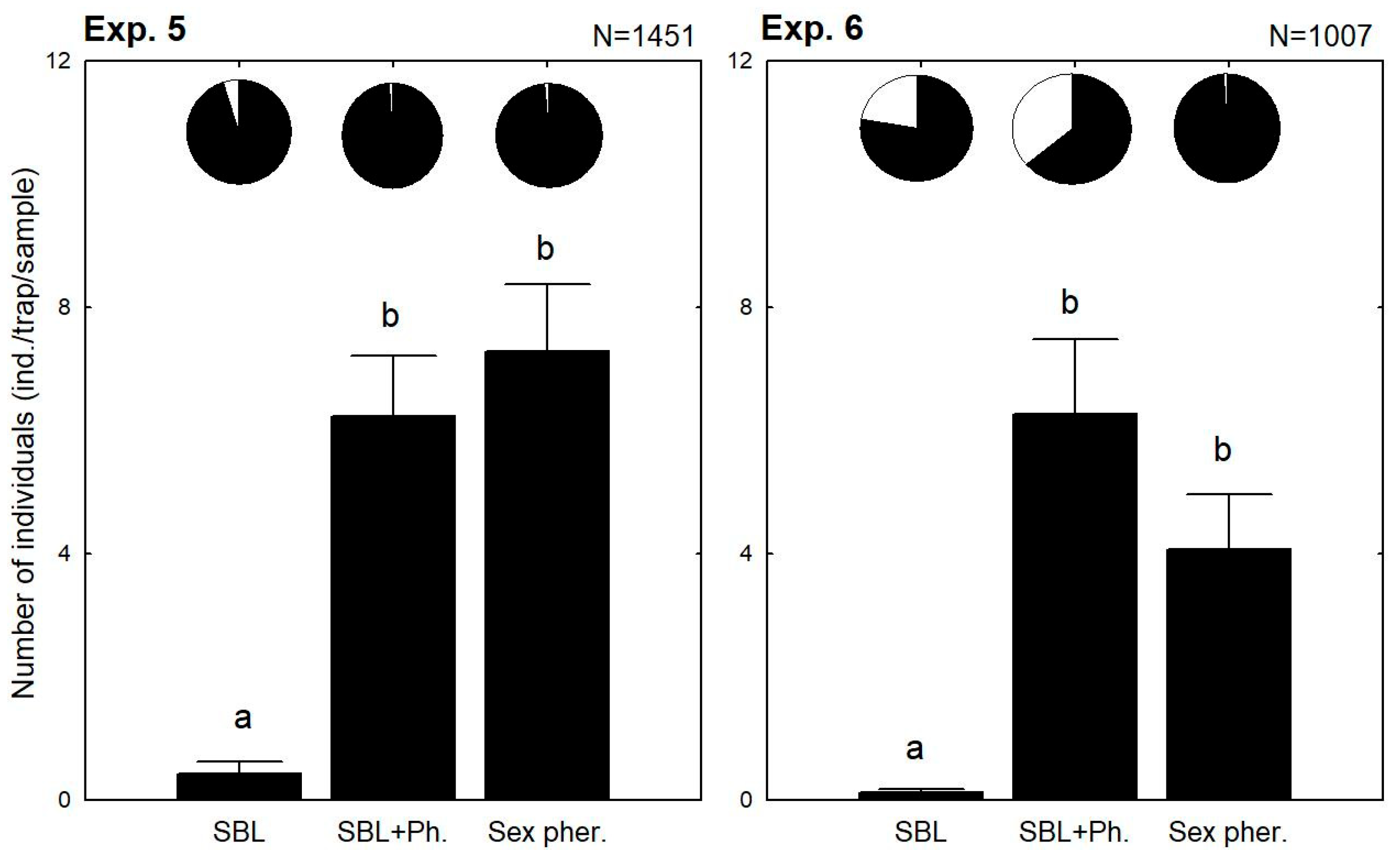
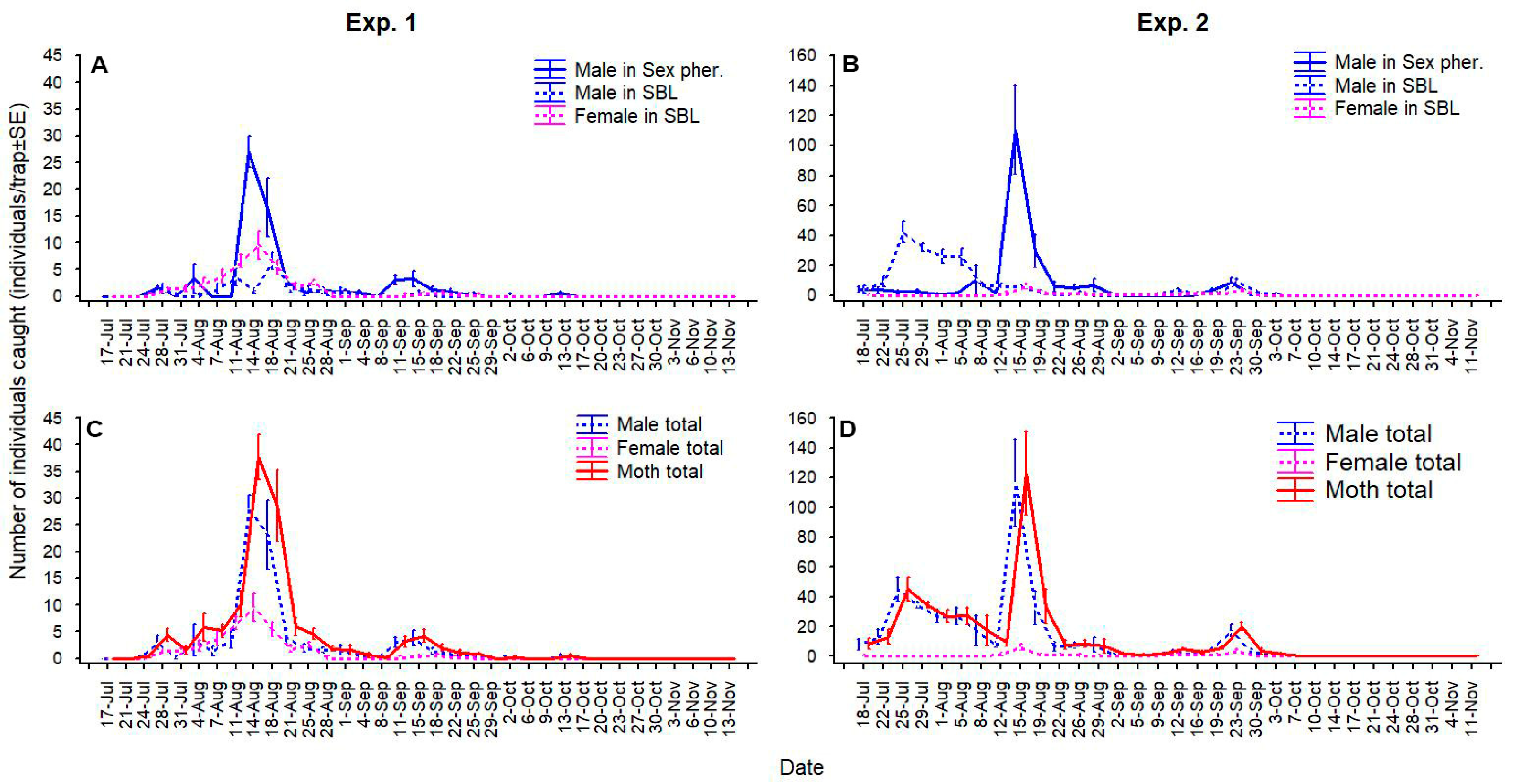
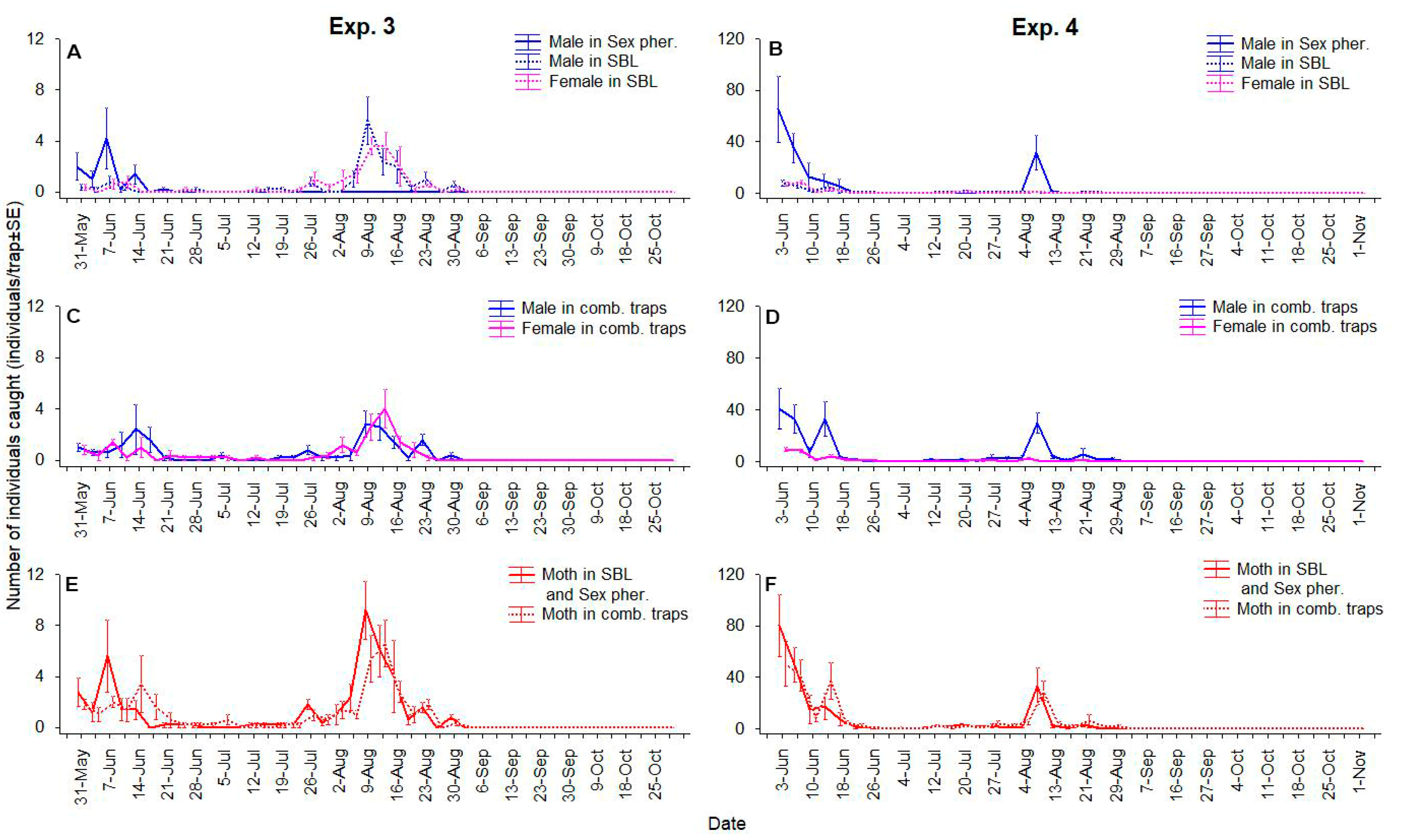
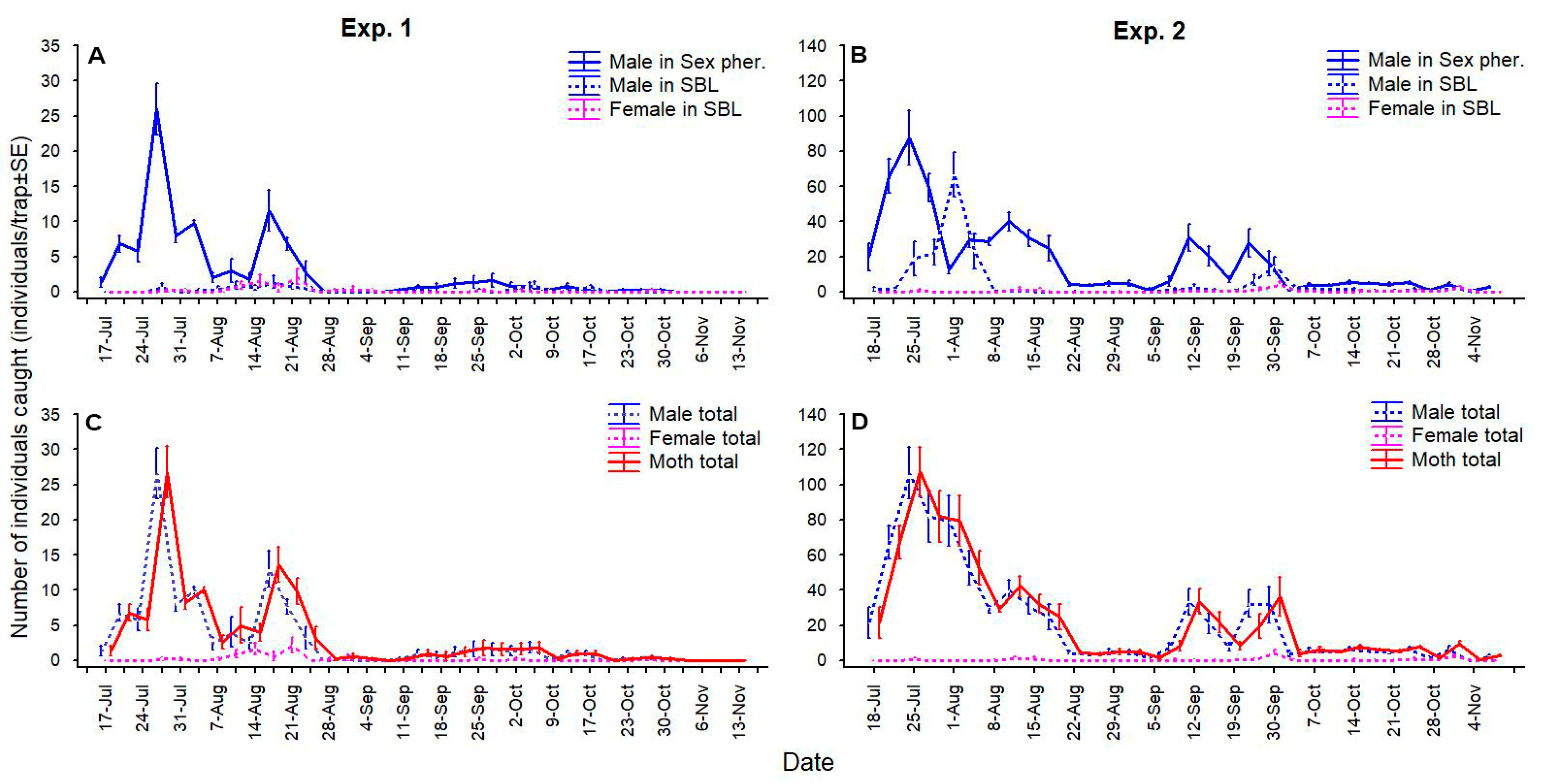
3. Results
3.1. Parallel Use of Traps with Sex Pheromone and SBL
3.2. Combined Use of the Lures in the Same Dispenser
3.3. Combined Use of the Lures in the Same Trap Together
3.4. Effect of the Phenology
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roelofs, W.L.; Carde, R.T. Responses of Lepidoptera to synthetic sex pheromone chemicals and their analogues. Annu. Rev. Entomol. 1977, 22, 377–405. [Google Scholar] [CrossRef]
- Yela, J.L.; Holyoak, M. Effects of moonlight and meteorological factors on light and bait trap catches of noctuid moths (Lepidoptera: Noctuidae). Environ. Entomol. 1997, 26, 1283–1290. [Google Scholar] [CrossRef]
- Jonason, D.; Franzén, M.; Ranius, T. Surveying moths using light traps: Effects of weather and time of year. PLoS ONE 2014, 9, e92453. [Google Scholar] [CrossRef]
- Williams, C.B. Comparing the Efficiency of Insect Traps. Bull. Entomol. Res. 1951, 42, 513–517. [Google Scholar] [CrossRef]
- Hardwick, D.F. A brief review of the principles of light trap design with a description of an efficient trap for collecting noctuid moths. J. Lepid. Soc. 1968, 22, 65–75. [Google Scholar]
- Nowinszky, L. The Handbook of Light Trapping; Savaria University Press: Szombathely, Hungary, 2003; p. 267. (In Hungarian) [Google Scholar]
- McGeachie, W.J. The effects of moonlight illuminance, temperature and wind speed on light-trap catches of moths. Bull. Entomol. Res. 1989, 79, 185–192. [Google Scholar] [CrossRef]
- Truxa, C.; Fiedler, K. Attraction to light-from how far do moths (Lepidoptera) return to weak artificial sources of light? Eur. J. Entomol. 2012, 109, 77–84. [Google Scholar] [CrossRef]
- Infusino, M.; Brehm, G.; Di Marco, C.; Scalercio, S. Assessing the efficiency of UV LEDs as light sources for sampling the diversity of macro-moths (Lepidoptera). Eur. J. Entomol. 2017, 114, 25–33. [Google Scholar] [CrossRef]
- Regier, J.C.; Mitter, C.; Mitter, K.; Cummings, M.P.; Bazinet, A.L.; Hallwachs, W.; Janzen, D.H.; Zwick, A. Further progress on the phylogeny of Noctuoidea (Insecta: Lepidoptera) using an expanded gene sample. Syst. Entomol. 2016, 42, 82–93. [Google Scholar] [CrossRef]
- Keegan, K.L.; Rota, J.; Zahiri, R.; Zilli, A.; Wahlberg, N.; Schmidt, B.C.; Lafontaine, J.D.; Goldstein, P.Z.; Wagner, D.L. Toward a Stable Global Noctuidae (Lepidoptera) Taxonomy. Insect Syst. Divers. 2021, 5, 1–24. [Google Scholar] [CrossRef]
- Leraut, P. Moths of Europe Vol. 6–Noctuids 2; N.A.P Editions: Verrières-le-Buisson, France, 2019; p. 575. [Google Scholar]
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. Available online: http://www.pherobase.com (accessed on 1 December 2024).
- Landolt, P.J. New chemical attractants for trapping Laconobia subjuncta, Mamestra configurata, and Xestia c-nigrum (Lepidoptera: Noctuidae). J. Econ. Entomol. 2000, 93, 101–106. [Google Scholar] [CrossRef]
- Landolt, P.J.; Pantoja, A.; Hagerty, A.; Crabo, L.; Green, D. Moths trapped in Alaska with feeding attractant lures and the seasonal flight patterns of potential agricultural pests. Can. Entomol. 2007, 139, 278–291. [Google Scholar] [CrossRef]
- Tóth, M.; Szarukán, I.; Dorogi, B.; Gulyás, A.; Nagy, P.; Rozgonyi, Z. Male and female noctuid moths attracted to synthetic lures in Europe. J. Chem. Ecol. 2010, 36, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Tóth, M.; Szarukán, I.; Nagy, A.; Gém, F.; Nyitrai, R.; Kecskés, Z.; Krakkó, L.; Jósvai, J.K.; Bélai, I. Semisynthetic “bisex” lures for catching females and males of pest insects. Növényvédelem 2015, 51, 197–205. (In Hungarian) [Google Scholar]
- Nagy, A.; Szarukán, I.; Gém, F.; Nyitrai, R.; Füsti-Molnár, B.; Némerth, A.; Kozák, L.; Molnár, A.; Katona, K.; Szanyi, S.; et al. Preliminary data on the effect of semi-synthetic baits for Noctuidae (Lepidoptera) on the non-target Lepidoptera species. Acta Agrar. Debreceniensis 2015, 65, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Szanyi, S.; Nagy, A.; Molnár, A.; Katona, K.; Tóth, M.; Varga, Z. Night-active Macroheterocera species in traps with synthetic attractants in the Velyka Dobron game reserve (Transcarpathia, Ukraine). Acta Zool. Acad. Sci. Hung. 2017, 63, 97–114. [Google Scholar] [CrossRef]
- Wakamura, S.; Struble, D.I.; Matsuura, H.; Sato, M.; Kegasawa, K. Sex pheromone of the black cutworm moth, Agrotis ipsilon Hüfnagel (Lepidoptera: Noctuidae): Attractant synergist and improved formulation. Appl. Entomol. Zool. 1986, 21, 299–304. [Google Scholar] [CrossRef]
- Causse, R.; Buès, R.; Barthes, J.; Toubon, J.F. Mise en evidence expérimentale de nouveaux constituants des phéromones sexuelles de Scotia ipsilon Hüfn. et Mamestra suasa Schiff (Lépidoptères, Noctuidae). In Médiateurs Chimiques: Comportement et Systématique des Lépidoptères. Application en Agronomie; INRA: Paris, France, 1988; pp. 75–82. [Google Scholar]
- Arn, H.; Esbjerg, P.; Buès, R.; Toth, M.; Szocs, G.; Guerin, P.; Rauscher, S. Field attraction of Agrotis segetum males in four European countries to mixtures containing three homologous acetates. J. Chem. Ecol. 1983, 9, 267–276. [Google Scholar] [CrossRef]
- Underhill, E.W.; Steck, W.F.; Chisholm, M.D. Sex Pheromone of the Clover Cutworm Moth, Scotogramma trifolii: Isolation, Identification and Field Studies. Environ. Entomol. 1976, 5, 307–310. [Google Scholar] [CrossRef]
- Descoins, C.; Priesner, E.; Gallois, M.; Arn, H.; Martin, G. Sur la sécrétion phéromonale des femelles vierges de Mamestra brasskae L. et de Mamestra oleracea L. (Lépidoptères, Noctuidac, Hadeninae). Comptes Rendus De ľAcadémie Des Sci. De Paris 1978, 286, 77–80. [Google Scholar]
- Landolt, P.J.; Alfaro, J.F. Trapping Laconobia subjuncta, Xestia c-nigrum, and Mamestra configurata (Lepidoptera: Noctuidae) with acetic acid and 3-methyl-1-butanol in controlled release dispensers. Environ. Entomol. 2001, 30, 656–662. [Google Scholar] [CrossRef]
- Landolt, P.J.; Higbee, B.S. Both sexes of the true armyworm (Lepidoptera: Noctuidae) trapped with the feeding attractant composed of acetic acid and 3-methyl-1-butanol. Fla. Entomol. 2002, 85, 182–185. [Google Scholar] [CrossRef]
- Meagher, R.L. Collection of soybean looper and other noctuids in phenylacetaldehyde-baited field traps. Fla. Entomol. 2001, 84, 154–155. [Google Scholar] [CrossRef]
- Szanyi, S.; Molnár, A.; Szanyi, K.; Tóth, M.; Jósvai, J.K.; Varga, Z.; Nagy, A. Semiochemical-baited traps as a new method supplementing light traps for faunistic and ecological studies of Macroheterocera (Lepidoptera). Sci. Rep. 2024, 14, 1–15. [Google Scholar] [CrossRef]
- Esbjerg, P.; Sigsgaard, L. Phenology and pest status of Agrotis segetum in a changing climate. Crop. Prot. 2014, 62, 64–71. [Google Scholar] [CrossRef]
- Guo, J.; Fu, X.; Wu, X.; Zhao, X.; Wu, K. Annual Migration of Agrotis segetum (Lepidoptera: Noctuidae): Observed on a Small Isolated Island in Northern China. PLoS ONE 2015, 10, e0131639. [Google Scholar] [CrossRef]
- Wang, P.; Jin, M.; Wu, C.; Peng, Y.; He, Y.; Wang, H.; Xiao, Y. Population genomics of Agrotis segetum provide insights into the local adaptive evolution of agricultural pests. BMC Biol. 2024, 22, 42. [Google Scholar] [CrossRef]
- Ferkau, C.; Fischer, K. Costs of reproduction in male Bicyclus anynana and Pieris napi butterflies: Effects of mating history and food limitation. Ethology 2006, 112, 1117–1127. [Google Scholar] [CrossRef]
- Service, P.M. The effect of mating status on lifespan, egg laying, and starvation resistance in Drosophila melanogaster in relation to selection on longevity. J. Insect Physiol. 1989, 35, 447–452. [Google Scholar] [CrossRef]
- Gilg, M.R.; Kruse, K.C. Reproduction decreases life span in the giant waterbug (Belostoma flumineum). Am. Midl. Nat. 2003, 149, 306–319. [Google Scholar] [CrossRef]
- Kemp, D.J.; Rutowski, R.L. A survival cost to mating in a polyandrous butterfly, Colias eurytheme. Oikos 2004, 105, 65–70. [Google Scholar] [CrossRef]
- Arnqvist, G.; Rowe, L. Sexual Conflict; Princeton University Press: Princeton, NJ, USA, 2005; p. 352. [Google Scholar]
- Taylor, M.L.; Price, T.A.R.; Wedell, N. Polyandry in Nature: A Global Analysis. Trends Ecol. Evol. 2014, 29, 376–383. [Google Scholar] [CrossRef]
- Torres-Vila, L.M. Polyandry-fecundity relationship in insects: Methodological and conceptual problems. J. Evol. Biol. 2012, 26, 325–334. [Google Scholar] [CrossRef]
- Arnqvist, G.; Nilsson, T. The Evolution of Polyandry: Multiple Mating and Female Fitness in Insects. Anim. Behav. 2000, 60, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Kvarnemo, C.; Simmons, L.W. Polyandry as a mediator of sexual selection before and after mating. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120042. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vila, L.M.; Rodríguez-Molina, M.C.; Jennions, M.D. Polyandry and fecundity in the lepidoptera: Can methodological and conceptual approaches bias outcomes? Behav. Ecol. Sociobiol. 2004, 55, 315–324. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Rodríguez-Molina, M.C.; Gragera, J.; Bielza-Lino, P. Polyandry in Lepidoptera: A heritable trait in Spodoptera exigua Hübner. Heredity 2001, 86, 177–183. [Google Scholar] [CrossRef]
- Santostefano, F.; Galarza, J.A.; Mappes, J. Testing the direct and genetic benefit hypotheses of polyandry in the wood tiger moth. Behav. Ecol. Sociobiol. 2018, 72, 109. [Google Scholar] [CrossRef]
- Valtonen, A.; Ayres, M.P.; Roininen, H.; Pöyry, J.; Leinonen, R. Environmental controls on the phenology of moths: Predicting plasticity and constraint under climate change. Oecologia 2011, 165, 237–248. [Google Scholar] [CrossRef]
- Teder, T. Phenological responses to climate warming in temperate moths and butterflies: Species traits predict future changes in voltinism. Oikos 2020, 129, 1051–1060. [Google Scholar] [CrossRef]
- Hill, G.M.; Kawahara, A.Y.; Daniels, J.C.; Bateman, C.C.; Scheffers, B.R. Climate change effects on animal ecology: Butterflies and moths as a case study. Biol. Rev./Biol. Rev. Camb. Philos. Soc. 2021, 96, 2113–2126. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Ma, C.-S.; Lann, C.L.; Van Baaren, J. Effects of Climate Change on Insect Phenology. In Effects of Climate Change on Insects: Physiological, Evolutionary, and Ecological Responses; González-Tokman, D., Dáttilo, W., Eds.; Oxford University Press: Oxford, UK, 2024; pp. 89–110. [Google Scholar] [CrossRef]
- Mironidis, G.K.; Stamopoulos, D.C.; Savopoulou-Soultani, M. Overwintering Survival and Spring Emergence of Helicoverpa armigera (Lepidoptera: Noctuidae) in Northern Greece. Environ. Entomol. 2010, 39, 1068–1084. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hao, H.F. Effects of climate change and crop planting structure on the abundance of cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Ecol. Evol. 2020, 10, 1324–1338. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szanyi, S.; Pálóczi, S.; Jósvai, J.K.; Varga, Z.; Tóth, M.; Nagy, A. Monitoring of Harmful Noctuid Pests with Synthetic Sex Pheromones and Semisynthetic Bisexual Lures (SBL): Benefits and Limitations of Separate, Parallel and Combined Use of the Methods. Agronomy 2025, 15, 2086. https://doi.org/10.3390/agronomy15092086
Szanyi S, Pálóczi S, Jósvai JK, Varga Z, Tóth M, Nagy A. Monitoring of Harmful Noctuid Pests with Synthetic Sex Pheromones and Semisynthetic Bisexual Lures (SBL): Benefits and Limitations of Separate, Parallel and Combined Use of the Methods. Agronomy. 2025; 15(9):2086. https://doi.org/10.3390/agronomy15092086
Chicago/Turabian StyleSzanyi, Szabolcs, Szilvia Pálóczi, Júlia Katalin Jósvai, Zoltán Varga, Miklós Tóth, and Antal Nagy. 2025. "Monitoring of Harmful Noctuid Pests with Synthetic Sex Pheromones and Semisynthetic Bisexual Lures (SBL): Benefits and Limitations of Separate, Parallel and Combined Use of the Methods" Agronomy 15, no. 9: 2086. https://doi.org/10.3390/agronomy15092086
APA StyleSzanyi, S., Pálóczi, S., Jósvai, J. K., Varga, Z., Tóth, M., & Nagy, A. (2025). Monitoring of Harmful Noctuid Pests with Synthetic Sex Pheromones and Semisynthetic Bisexual Lures (SBL): Benefits and Limitations of Separate, Parallel and Combined Use of the Methods. Agronomy, 15(9), 2086. https://doi.org/10.3390/agronomy15092086





