Genetic Diversity Analysis of Phenotypic Traits in Jujube Germplasm Resources
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Botanical Trait Investigation and Sampling Methods
2.2.2. Fruit Quality Sampling and Determination
2.3. Data Processing
3. Results
3.1. Analysis of Botanical Trait Surveys
3.1.1. Statistical Comparison of Descriptive Traits
3.1.2. Statistical Comparison of Quantitative Traits
3.2. Analysis of Fruit Quality Trait Survey
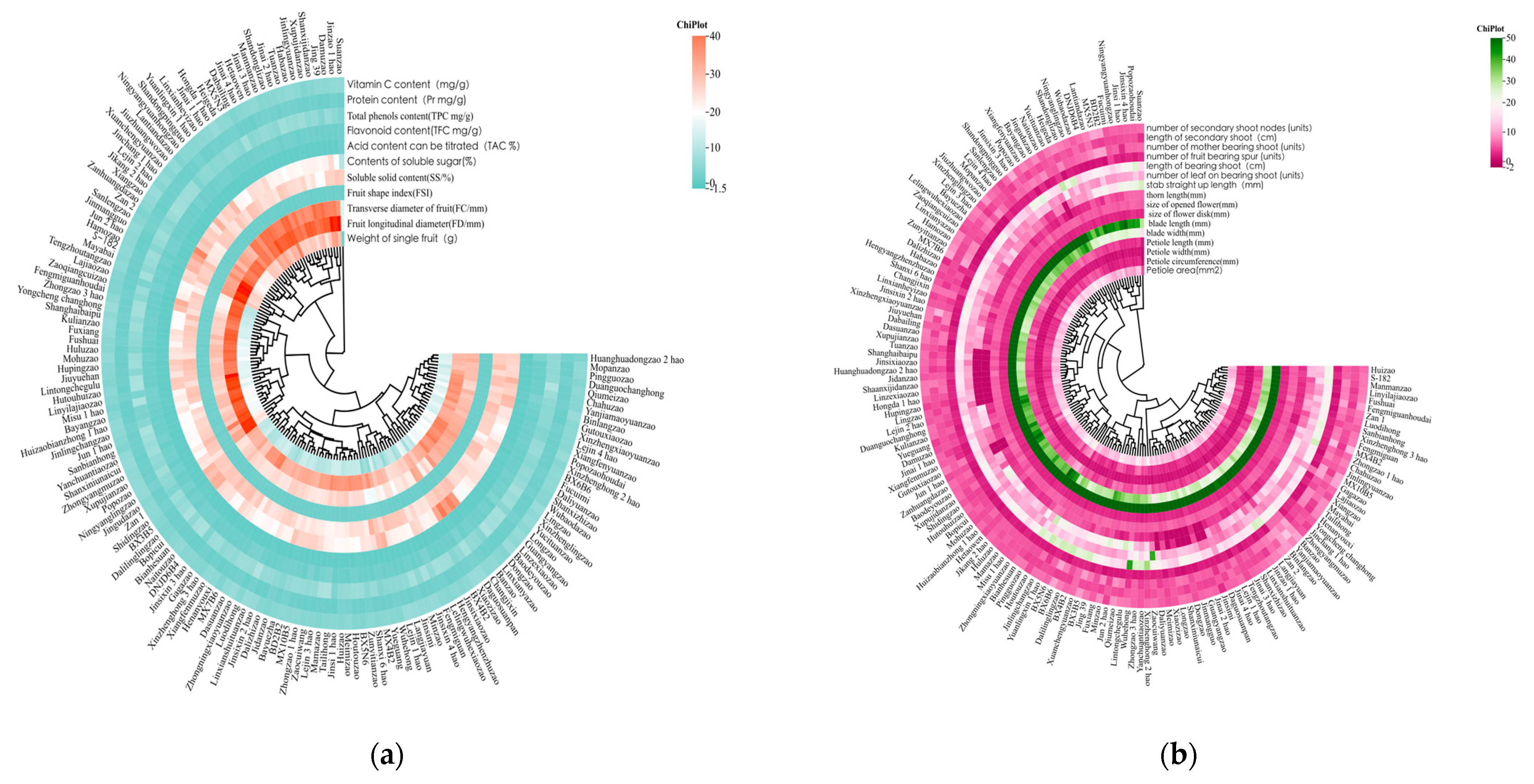
3.3. Cluster Analysis
3.3.1. Cluster Analysis of Morphological Traits
3.3.2. Cluster Analysis of Fruit Quality Traits
3.4. Comprehensive Evaluation
3.4.1. Principal Component Analysis
3.4.2. Factor Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Min | Minimum |
| Max | Maximum |
| Mean | Arithmetic mean |
| SD | Standard deviation |
| CV | Coefficient of variation |
References
- Li, D.K.; Wang, Y.K.; Xue, X.F.; Ren, H.Y.; Zhao, A.L.; Wu, H. Advances of Research and Utilization of Jujube (Zizphus) Germplasm in China. J. Fruit Resour. 2021, 1350, 63–72. [Google Scholar]
- Luo, Y.; Chen, W.; Pan, Y.; Ge, L.; Wu, C.; Wang, J.; Liu, M.; Yan, F. Comparison and Genetic Variation Analysis of Important Fruit Traits in Jujube F1 Hybrids by Different Male Parents. Agronomy 2024, 14, 459. [Google Scholar] [CrossRef]
- Hu, X.; Yin, K.H.; Nie, G.W.; Li, K.; Zhang, Y.; Zhang, X.; Tian, Y. Analysis and comprehensive evaluation of fruit quality of 39 sweet cherry accessions in Shanxi region. J. Fruit Resour. 2025, 42, 752–764. [Google Scholar]
- Li, Y.; Zhang, S.H.; Guo, Y.; Zhang, X.F.; Wang, G.P. Catkin Phenotypic Diversity and Cluster Analysis of 211 Chinese Chestnut Germplasms. Sci. Agric. Sin. 2020, 53, 4667–4682. [Google Scholar]
- Ma, C.H.; Li, D.L.; Wang, R. The Diversity Analysis of Blade Color of Genus Pyrus in China. J. Plant Genet. Resour. 2014, 15, 1232–1238. [Google Scholar]
- Sin, Y.X.; Dang, Z.J.; Hu, W.S. Diversity Analysis of Loquat (Eriobotrya) Defoliation Color. Acta Hortic. Sin. 2017, 4, 755–767. [Google Scholar]
- Su, W.L.; Zhao, A.L.; Wang, Y.K.; Fu, J.T.; Ren, H.Y.; Xue, X.F.; Shi, M.J.; Liu, L.; Li, Y.; Li, D.K. Diversity Analysis of Fruit Texture Traits in Jujube. J. Plant Genet. Resour. 2024, 25, 1830–1840. [Google Scholar]
- Xuan, J.; Ma, Q.; Ge, L.; Yan, F.; Yu, J.; Wang, J.; Wu, C.; Liu, M. Variation analysis and comparison of leaf and fruit traits of triploid hybrid progeny in jujube. Front. Plant Sci. 2025, 16, 1553316. [Google Scholar] [CrossRef]
- Nikmatullah, A.; Nairfana, I.; Dewi, S.M.; Sarjan, M. Morphological diversity of Indian jujube (Ziziphus mauritiana) in Sumbawa Island, West Nusa Tenggara, Indonesia. Biodiversitas J. Biol. Divers. 2023, 24, 4597–4608. [Google Scholar] [CrossRef]
- Xu, M.Q.; Lu, C.H.; Zhu, G.R.; Shao, Y.; Li, Y.; Wu, J.; Xie, J.; Wang, X.; Wang, L. Phenotypic diversity analysis of 133 accession local peach germplasm in Southern Xinjiang. J. Fruit Resour. 2024, 41, 2369–2376. [Google Scholar]
- Wu, H.; Su, W.L.; Shi, M.J.; Xue, X.; Ren, H.-Y.; Wang, Y.; Zhao, A.; Li, D. Diversity Analysis and Comprehensive Evaluation of Jujube Fruit Traits. J. Plant Genet. Resour. 2022, 23, 1613–1625. [Google Scholar]
- Khadivi, A.; Beigi, F. Morphological and chemical characterizations of jujube (Ziziphus jujuba Mill.) to select superior accessions. Food Sci. Nutr. 2022, 10, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.X.; Wang, X.L.; Mao, Y.M.; Wang, Y.; Ren, Y.X.; Ren, L.L.; Shen, L.Y. Analysis of Genetic Diversity of Wild Jujube Germplasm Resources Based on Quantitative Characters. Acta Hortic. Sin. 2023, 50, 2568–2576. [Google Scholar]
- Yang, R.; Li, J.; Huang, H.; Wu, X.; Wu, R.; Bai, Y. Analysis of Phenotypic Trait Variation in Germplasm Resources of Lycium ruthenicum Murr. Agronomy 2024, 14, 1930. [Google Scholar] [CrossRef]
- Li, D.K.; Wang, Y.K. Germplasm Resources of Chinese Jujube; China Forestry Publishing House: Beijing, China, 2016. [Google Scholar]
- Sun, B.W.; Wang, M.Q.; Qin, H.Y.; Wu, M.Y.; Ma, M.J.; Liu, G.L.; Yuan, P.Q.; Lu, W.P. Comprehensive evaluation of fruit quality of 88 accessions of Actinidia arguta germplasm resources based on principal component analysis and correlation analysis. J. Fruit Resour. 2025, 4, 765–774. [Google Scholar]
- Martinez-Nicolas, J.J.; Melgarejo, P.; Legua, P.; Garcia-Sanchez, F.; Hernández, F. Genetic diversity of pomegranate germplasm collection from Spain determined by fruit, seed, leaf and flower characteristiccs. PeerJ 2016, 4, e2214. [Google Scholar] [CrossRef]
- Yang, L.; Jia, P.P.; Jin, J.; Abudoukayoumu, A.Y.M.; Zhang, Y.F.; Wang, G.Y.; Hao, Q.; Niu, J.X. Analysis on phenotypic trait diversity of 118 Ziziphus jujuba cultivars. J. Plant Genet. Resour. Environ. 2023, 1, 50–60. [Google Scholar]
- Shen, Y.N.; Hu, F.Y.; Liu, L.N.; Wang, H.J.; Zhang, H.W.; Li, H.; Zhang, H.K. Genetic diversity of phenotypic traits of Zizyphus jujuba ‘Pingding’ germplasm resources in Chaoyang, Liaoning Province. China Fruits 2023, 59, 46–51. [Google Scholar]
- Chen, W.; Kong, D.C.; Cui, Y.-H.; Cao, M.; Pang, X.M.; Li, Y.Y. Phenotypic genetic diversity of a core collection of Ziziphus jujuba and correlation analysis of dehiscent characters. J. Beijing For. Univ. 2017, 39, 78–84. [Google Scholar]
- Khadivi, A.; Mirheidari, F.; Moradi, Y.; Paryan, S. Identification of superior jujube (Ziziphus jujuba Mill.) genotypes based on morphological and fruit characterizations. Food Sci. Nutr. 2021, 9, 3165–3176. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Chauhan, B.S. Genetic Diversity, Conservation, and Utilization of Plant Genetic Resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, T.; Zhang, J.; Wang, J.J. Genetic Diversity Analysis of Chinese Cherry Landraces (Prunus pseudocerasus) Based on Phenotypic Traits. Acta Hortic. Sin. 2016, 43, 2119–2132. [Google Scholar]
- Han, C.H.; Yang, L.; Li, L.L.; A, L.M.; Wu, Y.F.; Liu, Y.B.; Geng, W.J. Genetic Diversity Analysis of Phenotypic Traits in Offspring of Ziziphus jujuba Mill. Mol. Plant Breed. 2025, 1–10. Available online: https://link.cnki.net/urlid/46.1068.S.20250211.1200.002 (accessed on 30 July 2025).
- Zheng, C.L.; Yu, W.C.; Liu, N.F.; Hu, L.X.; Xu, Q. Phenotypic Diversity Analysis of the Different Germplasms of 85 Materials of the Grain Amaranth. Acta Agrestia Sin. 2023, 5, 1435–1444. [Google Scholar]
- Liu, Q.L.; Sheng, W.; Luo, Q.H. Comprehensive Evaluation of Fruit Quality of Different Varieties of Xinjiang Elaeagnus moorcroftii. Chin. Agric. Sci. Bull. 2024, 40, 58–62. [Google Scholar]
- Nie, J.Y.; Zhang, H.J.; Ma, Z.Y.; Yang, Z.F.; Li, J. The Application of Cluster Analysis in the Fruit Research in China and Its Problems. J. Fruit Sci. 2000, 17, 128–130. [Google Scholar]
- Zhang, H.F.; Feng, L.L.; Duan, J.Z.; Liu, G.Z.; Liu, H.J.; Qi, X.L.; Yan, Z.L.; Zhuo, W.F.; Chen, H.Y.; Qi, H.Z.; et al. Genetic diversity analysis and comprehensive evaluation of 118 wheat cultivars based on 14 traits. Jiangsu Agric. Sci. 2022, 50, 99–108. [Google Scholar]
- Duan, B.H.; Feng, Y.F.; Lin, M.J.; Wu, C.Y. Principal Component Analysis and Comprehensive Evaluation of Quality Traits of the 16 Introduced Fresh Jujube Cultivars. Xinjiang Agric. Sci. 2017, 54, 2198–2210. [Google Scholar]
- Xue, X.F.; Zhao, A.L.; Wang, Y.K.; Sui, C.L.; Ren, H.Y.; Li, D.K.; Liang, Q. Fruit quality analysis and comprehensive evaluation of different jujube varieties. China Fruits 2016, 03, 11–15. [Google Scholar] [CrossRef]
- Cao, G.; Yao, Y.; He, X.; Lu, M.S.; Feng, Y.F. Analysis on the Variation of Fruit Characters in the Progeny of ‘Fucuimi’ Jujube. North. Fruits 2022, 7–11. [Google Scholar] [CrossRef]
- Liu, S.Y.; Mao, Y.M.; Wang, X.L.; Qiu, X.J.; Li, Z.H.; Shen, L.Y. Investigation on Germplasm Resources of Zizyphus jujube. North. Hortic. 2023, 16, 34–42. [Google Scholar]
- Fan, Y.C.; Chai, S.S.; Zhang, M.M.; Zhao, X.H.; Shen, X. Phenotypic Genetic Diversity of Elaeagnus angustifolia Resources from Ningxia. North. Hortic. 2018, 23, 37–43. [Google Scholar]



| Descriptive Traits | Trait Characteristics | Frequency (%) | H′ | Descriptive Traits | Trait Characteristics | Frequency (%) | H′ |
|---|---|---|---|---|---|---|---|
| type of bark dry cracking | strip | 99.3 | 5.01 | depth of stalk cavity | shallow | 14.1 | 4.97 |
| lumpy | 0.7 | intermediate | 66.7 | ||||
| colour of extension shoot | yellow brown | 4.7 | 4.96 | deep | 18.1 | ||
| fusco rufous | 40.7 | width of stalk cavity | narrow | 8.7 | 4.99 | ||
| taupe | 10 | intermediate | 78.7 | ||||
| purple brown | 36 | broad | 12.7 | ||||
| light grey | 5.3 | lubricity of fruit skin | smooth | 93.3 | 4.96 | ||
| celadon | 3.3 | rough | 2.7 | ||||
| wax layer on the surface of extension shoot | dense | 34 | 4.95 | raised | 4 | ||
| sparse | 57.3 | size of fruit dot | small | 10.7 | 4.97 | ||
| none | 8.7 | intermediate | 50.7 | ||||
| curvature degree of secondary shoot | ≤15° | 24.7 | 4.95 | large | 38.7 | ||
| 15°~30° | 60.7 | density of Fruit dot | sparse | 51.3 | 4.92 | ||
| ≥30° | 14 | intermediate | 33.3 | ||||
| 0° | 0.7 | dense | 15.3 | ||||
| leaf lustre | dull | 4.7 | 4.99 | fruit size | small | 4.7 | 4.96 |
| glossier | 85.3 | medium-small | 17.3 | ||||
| glossy | 10 | medium | 41.3 | ||||
| leaf colour | light green | 9.3 | 4.97 | medium-large | 28.7 | ||
| green | 57.3 | large | 8 | ||||
| dark green | 33.3 | extra-large | 0 | ||||
| leaf state | curving | 18 | 4.97 | fruit colour | light red | 1.3 | 4.97 |
| flat | 68.7 | red | 83.3 | ||||
| back curving | 13.3 | mauve | 4.7 | ||||
| leaf shape | ellipse | 27.3 | 4.94 | reddish brown | 10 | ||
| ovoid | 44 | orange | 0.7 | ||||
| egg-oviform | 28.7 | colour of fruit flesh | white | 0.7 | 4.97 | ||
| shape of leaf margin | round shape | 35.3 | 4.84 | light green | 92.7 | ||
| heart shape | 2 | green | 6.7 | ||||
| cut shape | 1.3 | thickness of fruit skin | thin | 6 | 4.98 | ||
| round-cuneiform | 9.3 | intermediate | 52 | ||||
| inclination shape | 52 | thick | 42 | ||||
| Shape of leaf apex | sharp tine | 10 | 4.97 | fruit flavour | sour | 0.7 | 5.05 |
| blunt tine | 55.3 | sweet-sour | 26.7 | ||||
| rapid tine | 32 | sour-sweet | 16.7 | ||||
| acute-recessed | 32 | sweet | 52 | ||||
| shape of leaf margin | minute sawtooth | 31.3 | 4.97 | extremely sweet | 4 | ||
| bicrenate | 68.7 | texture of fruit flesh | loose | 8.7 | 4.96 | ||
| stigma state | keep | 4.7 | 6.92 | crisp | 50.7 | ||
| remnant | 93.3 | intermediate | 30.7 | ||||
| desquamate | 2 | compact | 10 | ||||
| sepal attitude | keep | 0.7 | 4.99 | coarseness of fruit flesh | delicate | 18.7 | 4.96 |
| remnant | 22 | intermediate | 62 | ||||
| desquamate | 77.3 | coarse | 19.3 | ||||
| fruit uniformity | same size | 14 | 4.98 | juice of fruit flesh | lack | 36 | 4.96 |
| relatively same size | 72 | medium | 50.7 | ||||
| different size | 14 | rich | 13.3 | ||||
| fruit cracking | yes | 6.7 | 4.97 | state of stone shell | contain | 97.3 | 5.01 |
| rich | 4.7 | remnant | 1.3 | ||||
| slight | 39.3 | none | 1.3 | ||||
| no | 49.3 | stone shape | globose | 1.3 | 4.96 | ||
| fruit shape | globose | 2.7 | 4.88 | ellipse | 44 | ||
| oblate | 27.3 | spindly | 28 | ||||
| oblong globose | 21.3 | inverted spindly | 25.3 | ||||
| ovoid | 13.3 | kernel size | small | 4 | 4.96 | ||
| obovate | 10.7 | medium | 44 | ||||
| coniform | 4.7 | medium-large | 44.7 | ||||
| cylinder | 16 | large | 5.3 | ||||
| millstone | 0.7 | colour of flower disc | milky | 14 | 4.96 | ||
| flat cylinder | 2.7 | whitish yellow | 29.3 | ||||
| teapot shape | 0.6 | yellowish green | 56.7 | ||||
| shape of fruit shoulder | flat | 74.7 | 4.96 | shape of fruit top | concave | 40 | 4.91 |
| convex | 25.3 | flat | 47.3 | ||||
| tine | 6.7 | ||||||
| convex | 6 |
| Serial Number | Quantitative Trait | Min | Max | Range | Mean | S | CV |
|---|---|---|---|---|---|---|---|
| 1 | number of secondary shoot nodes (units) | 3 | 12 | 9 | 6.58 | 1.22 | 18.54 |
| 2 | length of secondary shoot (cm) | 3.13 | 12.71 | 9.58 | 5.17 | 0.95 | 18.37 |
| 3 | number of mother-bearing shoots(units) | 3 | 12 | 9 | 6.19 | 0.97 | 15.67 |
| 4 | number of fruit-bearing spurs (units) | 1.1 | 6.67 | 5.57 | 2.11 | 0.43 | 20.37 |
| 5 | length of bearing shoot (cm) | 9.37 | 42.1 | 32.73 | 18.64 | 2.61 | 14.00 |
| 6 | number of leaves on bearing shoot (units) | 6.67 | 41.1 | 34.73 | 10.66 | 1.72 | 16.13 |
| 7 | stab straight up length (mm) | 2.97 | 27.95 | 24.98 | 10.62 | 1.61 | 15.16 |
| 8 | thorn length (mm) | 1.73 | 7.03 | 5.3 | 3.29 | 0.5 | 15.2 |
| 9 | size of opened flower (mm) | 4.19 | 7.39 | 3.2 | 5.71 | 0.29 | 5.07 |
| 10 | size of flower disc (mm) | 2.19 | 3.66 | 1.47 | 2.72 | 0.2 | 7.35 |
| 11 | blade length (mm) | 30.34 | 91.75 | 61.41 | 62.35 | 5.9 | 9.46 |
| 12 | blade width (mm) | 15.59 | 52.58 | 36.99 | 31.56 | 3.23 | 10.23 |
| 13 | blade area (mm2) | 666.9 | 2879.59 | 2212.1 | 1385.71 | 245.5 | 17.72 |
| 14 | blade circumference (mm) | 81.93 | 262.36 | 180.43 | 147.77 | 16.71 | 11.31 |
| 15 | petiole length (mm) | 1.77 | 11.06 | 9.29 | 5.02 | 0.95 | 18.92 |
| 16 | petiole width (mm) | 0.78 | 2.45 | 1.67 | 1.4 | 0.3 | 21.43 |
| 17 | petiole circumference (mm) | 4.7 | 22.19 | 17.49 | 13.5 | 2.33 | 17.26 |
| 18 | petiole area (mm2) | 0.69 | 9.24 | 8.55 | 3.96 | 0.72 | 18.18 |
| Fruit Quality Traits | Min | Max | Mean | S | CV | Diversity Index |
|---|---|---|---|---|---|---|
| single-fruit weight (g) | 2.05 | 39.29 | 17.09 | 2.2 | 12.87 | 4.92 |
| longitudinal diameter of fruit (mm) | 16.23 | 56.12 | 38.42 | 1.95 | 5.08 | 4.99 |
| transverse diameter of the fruit (mm) | 15.57 | 42.84 | 29.72 | 1.56 | 5.25 | 4.99 |
| fruit shape index | 0.92 | 2.09 | 1.32 | 0.07 | 5.3 | 4.99 |
| soluble solids content (%) | 19.77 | 31.93 | 26.37 | 1.12 | 4.25 | 5.01 |
| soluble sugar content (%) | 9.84 | 39.93 | 24.66 | 2.52 | 10.37 | 4.99 |
| titratable acid (%) | 0.2 | 1.51 | 0.35 | 0.04 | 11.07 | 4.96 |
| total flavonoids (mg/g) | 0.95 | 3.25 | 1.5 | 0.2 | 13.48 | 4.97 |
| total phenol (mg/g) | 2.67 | 9.42 | 5.62 | 0.55 | 10.13 | 4.96 |
| protein content(mg/g) | 0.95 | 2.64 | 1.51 | 0.12 | 8.29 | 4.94 |
| vitamin C content (mg/g) | 2.86 | 6.99 | 4.59 | 0.26 | 5.71 | 4.99 |
| Fruit Quality | Principal Component | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| soluble sugar | 0.98 | 0.14 | −0.05 | −0.04 | −0.01 | 0.001 |
| titratable acid | −0.11 | −0.12 | 0.71 | −0.43 | −0.01 | 0.17 |
| soluble solids | −0.04 | 0.07 | 0.02 | 0.09 | −0.002 | 0.94 |
| total flavonoids | −0.05 | 0.11 | 0.28 | 0.24 | 0.67 | −0.33 |
| total phenol | −0.1 | −0.11 | −0.02 | 0.9 | −0.02 | 0.1 |
| protein | −0.01 | −0.12 | −0.21 | −0.17 | 0.82 | 0.17 |
| vitamin C | −0.02 | 0.11 | 0.81 | 0.14 | −0.02 | −0.09 |
| single-fruit weight | 0.95 | −0.18 | −0.02 | −0.05 | −0.001 | −0.07 |
| longitudinal diameter of fruit | 0.84 | 0.52 | −0.01 | −0.06 | −0.03 | 0.03 |
| transverse diameter of the fruit | 0.9 | −0.41 | −0.09 | 0.07 | 0.01 | −0.04 |
| fruit shape index | −0.08 | 0.98 | 0.04 | −0.08 | −0.04 | 0.06 |
| eigenvalue | 3.46 | 1.62 | 1.29 | 1.15 | 1.07 | 0.97 |
| contribution rate | 31.41 | 14.76 | 11.76 | 10.42 | 9.74 | 8.79 |
| cumulative contribution | 31.41 | 46.17 | 57.93 | 68.35 | 78.08 | 86.88 |
| Variety | Score | Ranking | Variety | Score | Ranking |
|---|---|---|---|---|---|
| sanlengzao | 0.75 | 1 | jiuzhuangwozao | 0.50 | 76 |
| linyilajiaozao | 0.71 | 2 | xinzhenglingzao | 0.50 | 77 |
| zan 2 | 0.70 | 3 | tengzhoutangzao | 0.50 | 78 |
| jinmangguo | 0.69 | 4 | xiangzao | 0.50 | 79 |
| jing 39 | 0.68 | 5 | tailihong | 0.49 | 80 |
| jinzao 1 | 0.68 | 6 | yucituanzao | 0.49 | 81 |
| damuzao | 0.66 | 7 | s-182 | 0.49 | 82 |
| hamazao | 0.66 | 8 | xiangfenyuanzao | 0.49 | 83 |
| ningyanglingzao | 0.66 | 9 | dalilinglingzao | 0.49 | 84 |
| longzao | 0.65 | 10 | jinkang 2 | 0.49 | 85 |
| jinai 1 | 0.64 | 11 | xiangfenmuzao | 0.49 | 86 |
| jinai 4 | 0.64 | 12 | yongchengchanghong | 0.49 | 87 |
| jun 2 | 0.63 | 13 | xiaozizao | 0.49 | 88 |
| xuanchengyuanzao | 0.63 | 14 | chahuzao | 0.48 | 89 |
| dabailing | 0.63 | 15 | hutouhuizao | 0.48 | 90 |
| mx5n3 | 0.62 | 16 | mx4b2 | 0.48 | 91 |
| jingudazao | 0.62 | 17 | shanxiniunaicui | 0.48 | 92 |
| linxianheyizao | 0.62 | 18 | henanyouxi 1 | 0.47 | 93 |
| shandongpingguo | 0.61 | 19 | bianhesuan | 0.47 | 94 |
| zan 1 | 0.61 | 20 | xinzhenghong 3 | 0.47 | 95 |
| yuanlingxin 1 hao | 0.61 | 21 | houtouzao | 0.47 | 96 |
| lajiaozao | 0.61 | 22 | wuhehong | 0.47 | 97 |
| jinai 3 | 0.60 | 23 | jidanzao | 0.47 | 98 |
| bayangzao | 0.60 | 24 | linzexiaozao | 0.47 | 99 |
| ningyangyuanhongzao | 0.60 | 25 | luodihong | 0.47 | 100 |
| yanjiamaoyuanzao | 0.60 | 26 | bx3b5 | 0.47 | 101 |
| daguosuanpan | 0.59 | 27 | bayuezha | 0.47 | 102 |
| hongdayihao | 0.59 | 28 | zunyitianzao | 0.47 | 103 |
| jinai 2 | 0.59 | 29 | zhongzao 3 | 0.47 | 104 |
| tuanzao | 0.59 | 30 | lingzao | 0.46 | 105 |
| shandonglizao | 0.59 | 31 | gagazao | 0.46 | 106 |
| lantiandazao | 0.59 | 32 | dongzao | 0.46 | 107 |
| manmanzao | 0.59 | 33 | xinzhenghong 2 | 0.46 | 108 |
| misu 1 | 0.58 | 34 | bd2b2 | 0.46 | 109 |
| jinlingyuanzao | 0.58 | 35 | bopicui | 0.46 | 110 |
| heigeda | 0.58 | 36 | qiumeizao | 0.46 | 111 |
| xupujianzao | 0.58 | 37 | pingguozao | 0.46 | 112 |
| huizaobianzhong 1 | 0.58 | 38 | changjixin | 0.45 | 113 |
| hetaowen | 0.57 | 39 | jinsixin 3 | 0.45 | 114 |
| huanghuadongzao 2 | 0.57 | 40 | daliyuanzao | 0.44 | 115 |
| lejin 2 | 0.56 | 41 | huizao | 0.44 | 116 |
| xupujidanzao | 0.56 | 42 | bx5n6 | 0.44 | 117 |
| wubaodazao | 0.56 | 43 | bx6b6 | 0.43 | 118 |
| zhongyangmuzao | 0.56 | 44 | mopanzao | 0.43 | 119 |
| hupingzao | 0.56 | 45 | meimizao | 0.43 | 120 |
| popozao | 0.56 | 46 | lejin 3 | 0.43 | 121 |
| zanhuangdazao | 0.56 | 47 | fucuimi | 0.43 | 122 |
| shanghaibaipu | 0.56 | 48 | jinsimi | 0.43 | 123 |
| sanbianhong | 0.55 | 49 | fengmiguan | 0.43 | 124 |
| yanchuantiaozao | 0.55 | 50 | shanxiliuhao | 0.43 | 125 |
| binlangzao | 0.55 | 51 | guangyangzao | 0.43 | 126 |
| shanxijidanzao | 0.55 | 52 | duanguochanghong | 0.43 | 127 |
| fuxiang | 0.55 | 53 | mx10b5 | 0.43 | 128 |
| fushuai | 0.54 | 54 | Zhongningxiaoyuan zao | 0.42 | 129 |
| habazao | 0.54 | 55 | naitouzao | 0.42 | 130 |
| banzao | 0.53 | 56 | jinsixin 1 | 0.42 | 131 |
| zaoqiangcuizao | 0.53 | 57 | descendants of popozao | 0.42 | 132 |
| jinchang 1 | 0.53 | 58 | zhongzao 1 | 0.42 | 133 |
| shidingzao | 0.53 | 59 | lejin 1 | 0.42 | 134 |
| jiuyuehan | 0.53 | 60 | dnjd6b4 | 0.41 | 135 |
| mayabai | 0.53 | 61 | jinsixin 2 | 0.41 | 136 |
| gutouxiaozao | 0.53 | 62 | dalizhizao | 0.41 | 137 |
| kulinzao | 0.53 | 63 | langjiayuan | 0.40 | 138 |
| mohuzao | 0.52 | 64 | wenzao | 0.40 | 139 |
| linxianyazao | 0.52 | 65 | mx7b6 | 0.40 | 140 |
| huluzao | 0.52 | 66 | linxianshuituanzao | 0.40 | 141 |
| jun 1 | 0.52 | 67 | dasuanzao | 0.39 | 142 |
| zaocuiwang | 0.51 | 68 | lelingwuhexiaozao | 0.38 | 143 |
| descendants of fengmiguan | 0.51 | 69 | mamazao | 0.38 | 144 |
| shanxizhizao | 0.51 | 70 | bx4b2 | 0.37 | 145 |
| lejin 4 | 0.51 | 71 | jinsixiaozao | 0.35 | 146 |
| lintongchegulu | 0.51 | 72 | hengyangzhenzhuzao | 0.33 | 147 |
| yueguang | 0.51 | 73 | baodeyouzao | 0.33 | 148 |
| jinlingchangzao | 0.50 | 74 | jinsixin 4 | 0.32 | 149 |
| Xinzhengxiaoyuan zao | 0.50 | 75 | suanzao | 0.28 | 150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Xie, J.; Tong, T.; Zhou, X.; Yuan, Z.; Zhang, Y.; Li, X.; Wu, C. Genetic Diversity Analysis of Phenotypic Traits in Jujube Germplasm Resources. Agronomy 2025, 15, 2063. https://doi.org/10.3390/agronomy15092063
Bai Y, Xie J, Tong T, Zhou X, Yuan Z, Zhang Y, Li X, Wu C. Genetic Diversity Analysis of Phenotypic Traits in Jujube Germplasm Resources. Agronomy. 2025; 15(9):2063. https://doi.org/10.3390/agronomy15092063
Chicago/Turabian StyleBai, Yiqun, Jingmei Xie, Taohong Tong, Xiaofeng Zhou, Ze Yuan, Yingxia Zhang, Xiangyu Li, and Cuiyun Wu. 2025. "Genetic Diversity Analysis of Phenotypic Traits in Jujube Germplasm Resources" Agronomy 15, no. 9: 2063. https://doi.org/10.3390/agronomy15092063
APA StyleBai, Y., Xie, J., Tong, T., Zhou, X., Yuan, Z., Zhang, Y., Li, X., & Wu, C. (2025). Genetic Diversity Analysis of Phenotypic Traits in Jujube Germplasm Resources. Agronomy, 15(9), 2063. https://doi.org/10.3390/agronomy15092063






