Abstract
Constrained by site conditions and water resources, pear tree cultivation faces increasing drought stress. Paecilomyces variotii extract (PVE), a novel biostimulant extracted from wild sea buckthorn root-isolated strains and containing chitin, humic/fulvic acids, and beneficial microbes, has gained attention due to its high activity and efficacy in alleviating plant stresses (e.g., drought). In this study, Pyrus pyrifolia ‘Qiu Yue’ was used as the experimental material, and pot experiments were conducted to examine the drought-mitigating effects of different PVE concentrations. Drought stress was achieved by maintaining soil water content at 35–45% of water holding capacity for 45 days under natural evaporation conditions in rain shelters. The growth status of pear trees, soil enzyme activity, and metabolite levels were analyzed. The results showed that the application of 5 ng/mL PVE promoted pear tree growth, enhanced leaf antioxidant enzyme activity, and improved photosynthetic capacity and soil enzyme activity. Under normal water conditions, the shoot growth length, plant height, stem diameter, and root system activity of the 5 ng/mL PVE group were 31.91%, 12.05%, 3.54%, and 10.94% higher than those of the control group, respectively. Under drought stress, these values increased by 25.12%, 8.87%, 12.21%, and 16.98%, respectively. The addition of 5 ng/mL PVE facilitates trehalose release and upregulates starch-sucrose, glycerophospholipid, and galactose metabolic pathways, thereby potentiating drought stress tolerance in pear trees. However, at 20 ng/mL, reductions were observed in pear tree growth indicators, leaf antioxidant enzyme activity, soil enzyme activity, and trehalose content in root exudates compared to the 5 ng/mL PVE treatment. Overall, 5 ng/mL PVE effectively promotes pear tree growth and enhances drought resistance, making it suitable for broader use in pear cultivation practices.
1. Introduction
Pear trees, an economically valuable fruit crop, are widely cultivated in arid sandy soils and mountainous/hilly regions. These environments present distinct limitations: sandy soils, characterized by low organic matter and poor water retention, lead to rapid runoff during rainy seasons and deep desiccation in dry periods; hilly areas, susceptible to erosion due to slopes, have shallow, nutrient-deficient topsoil [1]. The resulting dual water and nutrient stress presents a major obstacle to pear production. Water shortages hinder root growth and photosynthesis, and a lack of nutrients—especially nitrogen, potassium, and trace elements—limits fruit quality development [2]. Additionally, these stressors have a compounding effect: drought decreases nutrient absorption efficiency, and nutrient shortages weaken physiological responses to drought [3]. Therefore, developing scientific strategies to support pear tree growth and improve stress tolerance has become a vital focus for growers [4].
Biostimulants—active agents derived from natural substances and applied to plants, seeds, soil, or growth media—are defined as functional substances containing specific compounds and/or microorganisms that enhance nutrient uptake, crop stress tolerance, and crop quality, with their mechanisms differing from those of fertilizers irrespective of nutritional content [5,6]. Main categories include humic acids, seaweed extracts, chitin/chitosan derivatives, amino acids and peptides, beneficial microorganisms, and mineral-based compounds [7].
Paecilomyces variotii extract (PVE), a novel biostimulant (sold as “ZhiNengCong” or ZNC) developed by Shandong Pengbo Biotechnology Co., Ltd., has attracted significant research interest due to its high bioactivity [8], eco-friendly properties, and multifunctional features [9,10,11]. This composite substance is produced through ethanol extraction of Paecilomyces variotii strains isolated from wild sea buckthorn roots. It contains chitin, humic and fulvic acids, plant and animal protein hydrolysates, seaweed extracts, and beneficial rhizosphere microorganisms [12]. Among these components, humic/fulvic acids are known to exhibit hormone-like activities (e.g., auxin-like effects promoting root growth), while chitin derivatives act as microbe-associated molecular patterns (MAMPs) triggering plant immune responses and potentially modulating root microbiome composition to enhance symbiotic interactions [13]. Protein hydrolysates may serve as bioavailable nitrogen sources and signaling molecules, and seaweed extracts often contain bioactive compounds (e.g., betaines, polysaccharides) that contribute to osmoprotection and stress tolerance [14]. Functionally, PVE primarily influences plant physiology through two mechanisms: stimulating plant immune responses by upregulating disease-related genes (enhancing resistance to biotic and abiotic stress [15]) and enhancing nutrient uptake to promote growth and development [10]. For example, PVE significantly increases expression of nitrogen/phosphorus transport proteins (e.g., AMT1;1, PHT1;5) and auxin synthesis genes (e.g., YUC3, YUC5) in Arabidopsis, boosting nutrient absorption and root growth. Studies on various crops such as rice, potato, cucumber, and ginger have shown that PVE at concentrations of 0.05–10 ppb can significantly promote crop growth and enhance stress resistance [8,11,12,16,17], with its efficacy being dozens of times higher than that of traditional brassinolide. Under stresses like salinity, low temperature, and drought, PVE may enhance stress resistance by inducing the synthesis of osmotic regulatory substances (e.g., proline, soluble sugars, etc.), regulating the abscisic acid (ABA) signaling pathway, or the reactive oxygen species (ROS) scavenging system. However, its specific impact on key physiological responses of pear trees under drought stress and its concentration-dependent effects have not yet been studied.
This study aims to investigate the concentration-dependent effects of PVE on the growth parameters, physiological responses, rhizosphere soil enzyme activities, and root metabolite profiles of pear trees under well-watered and drought conditions, thereby laying a scientific foundation for the optimal application of PVE in pear tree cultivation. The following hypotheses were formulated: (i) a higher concentration of 20 ng/mL PVE can enhance the drought resistance of pear trees, based on previous findings that higher concentrations of PVE increase disease resistance; (ii) a lower concentration of 5 ng/mL PVE can promote increases in new shoot elongation, plant height, root activity, photosynthetic efficiency, and root metabolite production.
2. Materials and Methods
2.1. Experimental Materials
Two-year-old Pyrus pyrifolia ‘Qiu Yue’ seedlings grafted onto Prunus mume rootstock were used as test material. Uniform, vigorous specimens were selected and pruned to a height of 60 cm with a root length of 17 cm before planting. The PVE stock solution (5 mg/mL) was supplied by Shandong Pengbo Biotechnology Co., Ltd. in Tai’an, Shandong Province, China, and PVE working solutions (5 ng/mL and 20 ng/mL) were prepared by serial dilution of the stock solution with deionized water immediately prior to each application. The plastic pots used in the experiment had a top diameter of 35.5 cm, a bottom diameter of 26 cm, a height of 31 cm, and a pot volume of 24 L. In the pot experiments, topsoil (0–20 cm depth) was collected from a pear orchard in Xigudui Village, Ningyang County, Shandong Province, China (35°51′26″ N, 117°11′59″ E). After air-drying and removal of visible plant residues and stones, the soil was sieved through a 5 mm mesh. The soil was not sterilized to preserve its natural microbial community. Soil properties were characterized as follows: pH 5.88, organic matter 25.83 g/kg, total nitrogen 0.86 g/kg, available phosphorus 24.91 mg/kg, available potassium 121 mg/kg, and alkali-hydrolyzable nitrogen 31.85 mg/kg.
2.2. Experimental Design
The pot experiment was set up with six treatments in a randomized-block design, with three biological replicates (individual plants) per treatment. It included three PVE solution concentrations (0, 5, and 20 ng/mL) and two water regimes (regular irrigation and drought stress). PVE was applied as a root drench (2000 mL per pot per application) at three key timepoints prior to differential irrigation treatments: dormancy stage (during transplantation, 6 November 2021), bud burst stage (15 March 2022), and early stage of new shoot growth (18 April 2022). On 6 November 2021, pear tree seedlings were transplanted into individual plastic pots. Each pot was filled with 16 kg of air-dried and sieved soil, amended with 0.03 kg/pot of humic acid compound fertilizer (N-P2O5-K2O: 17-17-17) as a basal application. Three drainage holes (approx. 1 cm diameter) were drilled in the bottom of each pot. Potted plants were placed in an open-sided transparent polyethylene rain shelter (with a light transmittance of approximately 85%) to exclude natural precipitation. The ambient temperature and relative humidity inside the shelter were comparable to those in open-field conditions. Consistent soil moisture management for all treatments was maintained until 30 April 2022. During winter (November–February), 2 L of water was applied approximately every 2–3 weeks, while during spring (March–April), irrigation with 2 L of water occurred every 4–7 days to ensure adequate moisture. Water stress regimes commenced on 3 May 2022, coinciding with the shoot elongation period, after pear seedlings had stabilized in pots for 6 months. Soil moisture was monitored daily using the Time Domain Reflectometer and adjusted through supplemental irrigation to maintain treatment-specific levels: 70–80% of water holding capacity (WHC) for well-watered treatments and 35–45% WHC for drought-stressed treatments. This regime was maintained until the experiment concluded on 16 June 2022 (45 days of differential moisture treatment). Details of the treatments are shown in Table 1.

Table 1.
Experimental design of this study.
After the experiment, pear seedlings were carefully removed, with the above-ground parts cut off at the crown. Roots were sequentially rinsed with tap water, followed by three to four rinses with deionized water. Surface moisture was blotted with quantitative filter paper before root activity was determined after weighing. For each biological replicate (n = 3 plants per treatment), healthy leaves from the middle section of current-year shoots were randomly collected and immediately flash-frozen in liquid nitrogen, then stored at −80 °C for analysis of antioxidant enzymes. Rhizosphere soil was dislodged from roots by mechanical agitation. One subsample was lyophilized for non-targeted metabolomics; another was air-dried, ground, and sieved through 18-mesh and 100-mesh screens for physico-chemical analysis.
2.3. Measurement of Physiological Indicators of Pear Growth
2.3.1. Investigation on the Growth of Pear Trees
Plant height was determined by measuring the vertical distance from the soil surface to the apical meristem with a calibrated ruler. The basal stem diameter was recorded 2 cm above the soil with a vernier caliper. Total new shoot elongation was quantified using a precision dendrometer tape from the basal node to the terminal bud. Measurements were made at the end of the experimental period (16 June 2022).
For each plant, root activity was determined using the triphenyl tetrazolium chloride (TTC) reduction method. Specifically, fresh root tips were excised, accurately weighed (0.5 g), and placed in a 50 mL centrifuge tube. A 0.4% TTC solution and 0.1 mol/L phosphate buffer were added to the centrifuge tube, which was then incubated in a 37 °C water bath for 2 h in the dark. Subsequently, 1 mol/L sulfuric acid solution was added to terminate the reaction. The roots were rinsed three times with deionized water, cut into pieces, and ground with absolute ethanol to extract the reduced product, triphenyl formazan (TTF). The grinding solution was centrifuged at 4000 r/min for 10 min, and the supernatant was taken, diluted to volume with absolute ethanol, and its absorbance (OD value) was measured at a wavelength of 485 nm. Root activity was expressed as micrograms of TTF produced per gram of fresh root per hour (μg TTF/g/h).
2.3.2. Measurement of Physiological Indicators of Pear Trees
For each biological replicate (n = 3), fresh leaf tissue (0.2 g) was finely chopped and homogenized with 1.8 mL of ice-cold physiological saline. The resulting homogenate was transferred into 2 mL centrifuge tubes, then centrifuged at 12,000 r/min and 4 °C for 15 min. The supernatant was collected for further analysis of soluble protein (BCA method), superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) activities and glutathione (GSH) content using the WB6501 kit (New Sem Bio-Technology Co., Ltd., San Jose, CA, USA) and A001-1, A084-3, A007-1, A006-2 kits (Nanjing Jiancheng Bio-Engineering Research Institute, Nanjing, China). Each biochemical assay was performed in triplicate (technical replicates) per biological sample.
2.3.3. Measurement of Chlorophyll SPAD Values and Photosynthetic Rates
Drought tolerance assessments induced by PVE were conducted 45 days after implementing moisture treatment. Chlorophyll content was measured using a SPAD-502Plus chlorophyll meter (Konica Minolta, Tokyo, Japan) on the third fully expanded leaf from the apex of current-year shoots. Three readings per leaf were averaged, and measurements were taken on three leaves per plant (n = 3 biological replicates, 9 readings per treatment). Gas exchange parameters, including net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transpiration rate (Tr), were measured between 9:00 and 11:00 a.m. with a portable photosynthesis system (LC Pro-SD, ADC BioScientific Ltd., Hoddesdon, UK) equipped with a broad-leaf cuvette (cuvette area 6.25 cm2). Measurements were performed under ambient CO2 concentration (approx. 400 µmol mol−1) and natural sunlight conditions. Photosynthetically active radiation (PAR) during measurements ranged from 1000 to 1200 µmol/m2/s, and leaf temperature was maintained at 25 ± 2 °C by the cuvette Peltier system. Measurements were taken on the leaves selected for SPAD readings (three leaves per plant).
2.4. Measurement of Soil Enzyme Activity and Root Exudates
2.4.1. Measurement of Soil Enzyme Activity
The methods used to measure the activity of soil urease, sucrase, and phosphatase were the sodium phenolate-sodium hypochlorite colorimetric method, the 3,5-dinitrosalicylic acid colorimetric method, and the sodium phosphate colorimetric method, respectively [18]. Assays were performed in triplicate (technical replicates) for each soil sample (n = 3 biological replicates).
2.4.2. Measurement of Non-Targeted Metabolism in Root Systems
For each biological replicate (n = 3), 50 mg of lyophilized rhizosphere soil was weighed into a 1.5 mL centrifuge tube, followed by the addition of 400 μL extraction solvent (acetonitrile and methanol, 1:1 v/v). The mixture was vortexed for 30 s and subjected to ultrasonic-assisted extraction at 5 °C (40 kHz) for 30 min. After equilibration at −20 °C for 30 min, samples were centrifuged at 13,000× g for 15 min at 4 °C. The supernatant was transferred, dried under a nitrogen stream, and reconstituted in 120 μL of resuspension solution (acetonitrile and water, 1:1 v/v). Following a secondary ultrasonic-assisted extraction (5 °C, 40 kHz, 5 min), the sample was centrifuged again at 13,000× g at 4 °C for 5 min. The final supernatant was transferred to an autosampler vial with an insert for LC-MS analysis. Quality control (QC) samples were prepared by combining equal volumes of all sample extracts. During analytical runs, QC samples were injected after every 10 experimental samples to monitor system reproducibility.
The instrument platform used for this LC-MS analysis was AB SCIEX’s ultra-high-performance liquid chromatography tandem time-of-flight mass spectrometry UPLC-TripleTOF system.
2.5. Statistical Analysis
Data processing and visualization were performed using Microsoft Excel 2020 and Origin 2021 (OriginLab Corporation, Northampton, MA, USA). Statistical analyses were conducted with SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). All data are shown as mean ± standard deviation (Mean ± SD) of three biological replicates (n = 3). Data were tested for normality (Shapiro-Wilk) and variance homogeneity (Levene’s). Non-compliant datasets underwent Box-Cox transformation (with reciprocal or logarithmic methods as needed). Two-way or one-way ANOVA was applied based on the experimental design. Differences between treatments were evaluated using Duncan’s multiple range test (p < 0.05).
Metabolomics data were processed using Progenesis QI software (version 3.0; Waters Corporation, Milford, CA, USA) with sum normalization. Variables (metabolite peaks) exhibiting a relative standard deviation (RSD) > 30% across QC injections were excluded from subsequent statistical analysis to ensure data quality. This threshold is commonly used in metabolomics to filter out poorly measured features while retaining the majority of robust metabolites. The excluded features were typically low-abundance ions potentially affected by instrument noise or inconsistent extraction. Metabolite identification was achieved by matching MS and MS/MS spectra against established public databases (HMDB, Metlin). Principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were performed using the ropls package (v1.6.2) in R, with model stability assessed through sevenfold cross-validation. Student t-tests and fold-change analyses were conducted, and KEGG database annotation was used to identify pathways containing differentially expressed metabolites. Pathway enrichment analysis was implemented using the Python package scipy.stats (version 1.7.0; SciPy.org, Pasadena, CA, USA), with biologically relevant pathways determined by Fisher’s exact test.
3. Results
3.1. Pear Tree Growth Conditions
Under well-watered conditions, different concentrations of PVE treatment had varying effects on the vegetative growth of pear trees. PVE5-W showed a significant increase of 31.93% in new shoot growth length compared to the control group (p < 0.05, Table 2), while PVE20-W also exhibited similar growth promotion (35.56% increase vs. W-W). The plant height showed PVE5-W > W-W ≈ PVE20-W, with the stem diameter in the PVE5-W group increasing by 19.08% compared to the PVE20-W group, demonstrating a dose-response effect. Under drought stress, the PVE5-D treatment exhibited the best growth-promoting effect, with significantly higher new shoot growth compared to the W-D and PVE20-D groups (p < 0.05). However, there were no statistically significant differences in plant height among the treatments (p > 0.05). Stem diameter showed a gradient change of PVE5-D ≥ W-D > PVE20-D, with PVE5-D increasing significantly by 29.90% compared to PVE20-D. Notably, the high-concentration PVE20 treatment displayed growth inhibition under drought conditions, indicating enhanced concentration sensitivity of plants to growth regulators under stress conditions.

Table 2.
Growth characteristics of pear trees under different treatments.
Water status significantly influences root metabolic activity, with drought stress causing a decline in root activity in the W-D group (Table 2). Under well-watered conditions, root activity in the PVE5-W treatment was 10.94% higher than in the control group, with the treatments ordered as follows: PVE5-W ≥ PVE20-W > W-W. During drought conditions, the root activity of the PVE5-D group was significantly higher than that of the PVE20-D and W-D groups (p < 0.05). However, the root activity under PVE20-D treatment only reached parity with the W-D group, demonstrating a concentration threshold effect of PVE on plant growth regulation.
3.2. Pear Leaf Antioxidant Enzyme Activity
Under normal moisture conditions, the SOD activity in the PVE5-W and PVE20-W treatments increased by 16.08% and 14.64%, respectively, compared to the control group (Table 3). Under drought conditions, PVE treatment reversed the decline in SOD activity caused by stress, with PVE20-D and PVE5-D showing increases of 16.53% and 12.82%, respectively, compared to the W-D group, and no significant advantage was observed with higher concentrations (p > 0.05).

Table 3.
Physiological characteristics of pear trees under different treatments.
POD was unaffected by PVE treatment under adequate moisture conditions. However, under drought conditions, PVE5-D and PVE20-D treatments increased their activity by 23.46% and 30.17%, respectively, compared to the W-D group. CAT activity reached a peak value of 193.38 U/mgprot during PVE5-W treatment, representing a 21.96% increase compared to the W-W group. Under drought conditions, the CAT activity in the PVE20-D treatment reached 12.59%, significantly higher than that of the PVE5-D group (6.86%). Increased POD and CAT activities in PVE20-D suggest potential oxidative stress, though ROS markers (e.g., H2O2, MDA) were not quantified.
The changes in GSH content also showed a concentration-dependent relationship with PVE. Under both moisture conditions, the GSH content in the PVE5 treatment group was significantly higher than in the other treatment groups (PVE5 ≥ PVE20 > control, p < 0.05), confirming that low concentrations of PVE are more beneficial for maintaining the thiol-disulfide redox cycle.
3.3. Pear Leaf Chlorophyll SPAD Values and Photosynthetic Rates
Drought conditions significantly decreased leaf chlorophyll (Chlorophyll) SPAD values (p < 0.05, Figure 1), with this effect being more evident in the PVE treatment groups. Under well-watered conditions, the SPAD values of the PVE5-W and PVE20-W groups increased by 13.54% and 18.55%, respectively, compared to the control (W-W). There were no statistically significant differences in SPAD values among treatment groups under drought stress (p > 0.05).
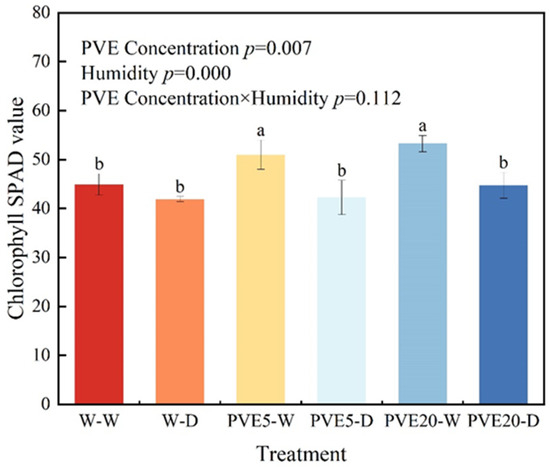
Figure 1.
Chlorophyll SPAD values of pear trees under different treatments. Different lowercase letters above the bars indicate significant differences between treatments at p < 0.05. The results of a two-way ANOVA for PVE concentration, water regime, and their interaction are shown. W-W: deionized water application under well-watered conditions; W-D: deionized water application under drought stress; PVE5-W: 5 ng/mL PVE application under well-watered conditions; PVE5-D: 5 ng/mL PVE application under drought stress; PVE20-W: 20 ng/mL PVE application under well-watered conditions; PVE20-D: 20 ng/mL PVE application under drought stress. The same below.
Drought stress greatly reduced the photosynthetic performance of pear trees, as shown by decreases in net photosynthetic rate (Pn), stomatal conductance (Gs), and transpiration rate (Tr). Meanwhile, intercellular CO2 concentration (Ci) increased (Table 4). The application of PVE partly eased the negative effects of drought.

Table 4.
Photosynthetic characteristics of pear trees under different treatments.
Under normal water supply, the Pn value treated with PVE5-W was the highest at 8.75 µmol/m2/s, showing a 23.07% increase compared to the control group (W-W) (p < 0.05). Drought significantly decreased Gs and Tr. Under normal watering, Gs in the PVE treatments (PVE5-W, PVE20-W) increased by 22.22% and 50.00%, respectively, compared to W-W, while Tr increased by 13.46% and 14.07%, respectively. Under drought conditions, Gs in PVE treatments (PVE5-D, PVE20-D) remained higher than in W-D, but PVE treatments had no significant effect on Tr (Table 4). Under normal conditions, Ci values for the PVE treatments (PVE5-W, PVE20-W) were 6.08% and 7.99% lower than those of W-W (p < 0.05). Drought stress generally caused an increase in Ci, but PVE still showed a specific regulatory effect.
3.4. Soil Enzyme Activity
Under well-watered conditions, soil urease activity showed significant differences across treatments (p < 0.05), with the W-W treatment exhibiting the lowest activity (Figure 2a). The PVE5-W and PVE20-W treatments experienced increases of 17.39% and 35.78%, respectively, compared to the W-W treatment. For sucrase activity, the PVE5-W treatment had the highest levels, increasing by 10.88% and 24.81% relative to W-W and PVE20-W (Figure 2b). The phosphatase activity of PVE5-W treatment was 17.07% and 77.78% higher than W-W and PVE20-W treatments, respectively (Figure 2c).
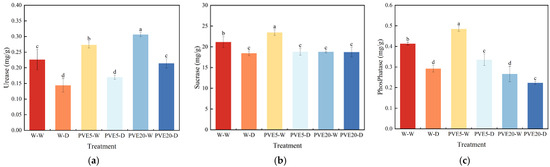
Figure 2.
Activities of urease (a), sucrase (b), and phosphatase (c) in soil under different treatments. Different lowercase letters above bars within each panel indicate statistically significant differences between treatments at p < 0.05. For abbreviations, see Figure 1.
Under drought conditions, soil urease activity showed a gradient change, with the W-D treatment displaying the lowest activity and the PVE20-D treatment the highest. Sucrase activity did not differ significantly among the PVE treatments (p > 0.05). PVE5-D treatment had 17.24% and 54.55% higher phosphatase activity than those of the W-D and PVE20-D treatments, respectively.
3.5. Rhizosphere Soil Untargeted Metabolomics
Based on non-targeted metabolomics analysis, this study detected a total of 2800 metabolic peaks in positive ion mode. After comparing first- and second-order mass spectrometry data and matching them with databases, 388 metabolic compounds were ultimately identified. Among these, 365 metabolites were annotated in public databases such as HMDB and Lipidmaps, and 238 metabolites were successfully mapped to the KEGG metabolic pathway database. In negative-ion mode, fewer mass spectrometry peaks (2232 peaks) were detected compared to positive-ion mode. After comparing first- and second-order mass spectrometry data, 142 metabolites were finally identified, with 136 and 96 of them annotated in the HMDB/Lipidmaps and KEGG databases, respectively (Table 5).

Table 5.
Statistical table for total ion numbers and identification of non-targeted metabolism.
Metabolite clustering analysis showed that the metabolic profile of the 20 ng/mL PVE treatment group (PVE20-W) did not show significant changes compared to the control treatment (W-W) (Figure 3). Notably, the levels of trehalose were significantly increased in the 5 ng/mL PVE treatment groups (PVE5-D and PVE5-W). As a non-reducing disaccharide produced by plant-promoting bacteria, trehalose plays a key protective role in plants’ responses to abiotic stresses such as drought and high salinity. Its molecules can form protective membrane structures on the cell surface, effectively maintaining protein conformation stability, which activates the plant defense system and reduces damage caused by drought stress.
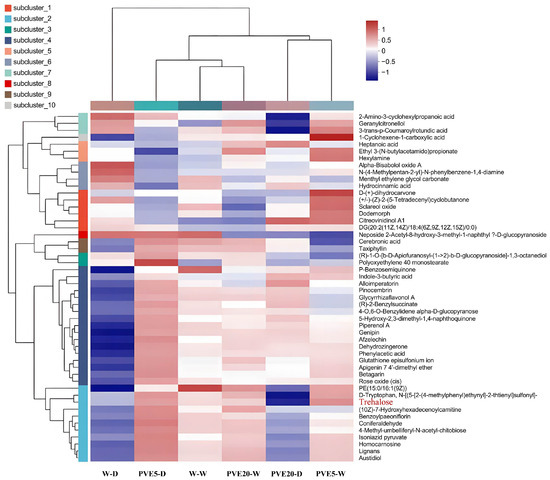
Figure 3.
Hierarchical clustering analysis of root metabolite profiles under different treatments. The heatmap displays relative metabolite abundance (color scale: −1 = low, 1 = high) with metabolites grouped into 10 distinct subclusters (subcluster_1 to subcluster_10) based on accumulation patterns. The Euclidean algorithm was used, and the top 50 metabolites with differential expression levels were selected for clustering to more intuitively display the variation trends of differential metabolites within the same group. On the left side of the figure is a dendrogram showing the clustering of metabolites in the samples. The closer two metabolite branches are to each other, the more similar their expression levels are. At the top of the figure is a dendrogram representing the clustering of samples. The closer two sample branches are, the more similar the expression patterns of all metabolites in these two samples are—specifically, the more consistent the variation trends of metabolite expression levels are. For abbreviations of treatments, see Figure 1.
Under drought stress conditions, the 5 ng/mL PVE-treated group (PVE5-D) showed significant trehalose accumulation (Figure 4a). This suggests that PVE at an appropriate concentration may promote trehalose biosynthesis and release by regulating the metabolic activity of plant-promoting bacteria, thereby improving the plant’s drought resistance. KEGG pathway enrichment analysis further indicated that treatment with 5 ng/mL PVE greatly activated the starch and sucrose metabolism, glycerophospholipid metabolism, and galactose metabolism pathways (Figure 4b). As a key metabolite in the starch and sucrose metabolism pathway, trehalose accumulation supplies an essential osmotic regulatory material to help plants cope with drought stress. This finding offers new experimental evidence for understanding the molecular mechanism by which PVE enhances plant drought resistance.
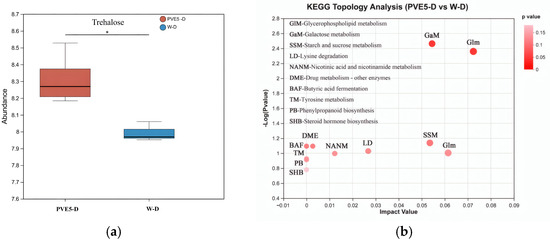
Figure 4.
Trehalose accumulation and KEGG pathway enrichment in response to PVE5 treatment under drought stress. (a) Trehalose content in rhizosphere soil under drought conditions: PVE5-D (5 ng/mL PVE) vs. W-D (deionized water). (b) KEGG topology analysis of enriched metabolic pathways in PVE5-D vs. W-D comparison. * Note: significant difference between PVE5-D and W-D treatments in trehalose production.
4. Discussion
The complex mechanisms of biostimulants, involving interactions among multiple bioactive components, often pose challenges to understanding how they work. However, it has been shown that plants generally respond similarly to different biostimulants by improving nutrient absorption and enhancing stress tolerance [19]. In this study, PVE significantly improved growth and drought resistance in pear trees, with 5 ng/mL identified as the optimal concentration due to its dual role in promoting growth and maintaining redox homeostasis. This dose-dependent effect can be attributed to the synergistic action of PVE’s bioactive components, including chitin, humic acids, and beneficial microorganisms, which collectively regulate plant physiological processes [10,13].
As a new biostimulant, PVE can greatly boost the growth of pear trees, as seen in increased plant height, stem thickness, shoot extension, and root system health (Table 2). The growth-promoting effect of low-dose PVE may stem from its ability to activate root growth signaling pathways: humic acids in PVE can mimic auxin-like effects to promote root cell elongation [13], while beneficial rhizosphere microorganisms stimulate the release of growth-promoting substances (e.g., trehalose) to enhance nutrient uptake efficiency [15].
Physiologically, PVE treatments significantly boosted the antioxidant metabolic activity of pear tree leaves, especially under drought stress, by regulating key antioxidant enzymes (CAT, SOD, and POD), thereby effectively reducing oxidative damage (Table 3). Particularly, 5 ng/mL PVE provided the optimal effect in activating the antioxidant enzyme system and maintaining redox homeostasis. Redox homeostasis is critical for plant stress adaptation, as it balances ROS production and scavenging. Under drought, plants generate excessive ROS (e.g., H2O2, O2−) that damage lipids and proteins [20]. The 5 ng/mL PVE treatment enhanced SOD (to dismutate O2− to H2O2), POD, and CAT (to decompose H2O2), ensuring ROS levels stay within a tolerable range (redox capacity limit). This enzymatic synergy prevents oxidative burst while avoiding excessive energy consumption, which is consistent with previous findings that balanced antioxidant activity correlates with improved stress tolerance [21]. These enzymes are critically linked to plant stress resistance, helping reduce drought damage through cellular structural protection, especially important under water stress.
Additionally, 5 ng/mL PVE boosted chlorophyll production, improved photosynthetic efficiency, and increased soil enzyme activity, collectively increasing drought tolerance (Figure 1 and Figure 2, Table 4). Notably, significant trehalose buildup was observed in the 5 ng/mL PVE group. This non-reducing disaccharide, produced by plant-growth-promoting bacteria, forms protective layers during drought [22], maintaining protein structure and activating defense responses [23]. KEGG pathway analysis confirmed that 5 ng/mL PVE promoted starch and sucrose metabolism (Figure 4b), where trehalose acts as an osmoprotectant to stabilize cell membranes and maintain water potential, further explaining its role in drought resistance [21].
However, too high PVE levels negatively affected growth, physiological functions, soil enzymes, and root metabolism [24]. Under drought stress, the 20 ng/mL treatment showed reductions of 53.28%, 9.05%, 23.02%, and 11.29% in new shoot growth, plant height, stem diameter, and root activity, respectively, compared to the 5 ng/mL group. Antioxidant enzymes, soil enzymes, and trehalose levels were also significantly lower. The inhibitory effect of high-dose PVE may result from excessive activation of plant immune responses: chitin in PVE, as a microbe-associated molecular pattern (MAMP), can overstimulate defense signaling when in high concentration, diverting energy from growth to stress responses [10,13]. Additionally, excessive humic acids may disrupt nutrient uptake balance by interfering with ion transporters in roots, leading to metabolic disorders [13]. These results suggest that excessive PVE concentrations not only fail to boost drought tolerance but may hinder growth, cause water loss, and inhibit development [19,25].
A notable observation is the decoupling of chlorophyll SPAD values and Pn in PVE-treated groups: under well-watered conditions, PVE5-W shows slightly lower SPAD values but slightly higher Pn than PVE20-W (Table 4, Figure 1). This may be due to PVE5-W enhancing photosynthetic efficiency per unit chlorophyll rather than simply increasing chlorophyll content—possibly by upregulating Rubisco activity or optimizing Gs [26], as indicated by higher Gs in PVE5-W compared to PVE20-W (Table 4). In contrast, high PVE concentration (20 ng/mL) may increase chlorophyll synthesis but impair photosynthetic machinery (e.g., thylakoid membrane stability), leading to uncoupling of chlorophyll content and Pn.
Another biological confounder was the inverse relationship between high urease activity and low growth in PVE20-D (Figure 2a, Table 2). Urease activity reflects microbial nitrogen mineralization capacity, but elevated urease in PVE20-D may indicate microbial dysbiosis: excessive PVE could alter rhizosphere microbial composition, favoring urease-producing microbes that mineralize nitrogen too rapidly, leading to transient ammonium toxicity or imbalanced C/N ratio, which inhibits plant growth [15,27].
As environmentally friendly agricultural formulations, biostimulants offer significant benefits for improving crop stress tolerance, lowering production costs, and reducing environmental pollution [28]. They can provide substantial economic and social advantages, making them ideal solutions for tackling climate change and environmental degradation through ecological, green, and organic farming systems [21]. However, PVE-represented biostimulants face several practical challenges: (1) despite the booming global market, the product standardization system has not yet been perfected [27], leading to inconsistent product quality [29]; (2) optimal application methods (seed treatment, foliar spraying, fertigation) vary by biostimulant type. For PVE, drip-irrigated root drenching shows potential for targeted delivery; however, future studies should compare foliar and soil application to enhance efficacy and minimize costs; (3) excessive application can cause significant inhibitory effects [7,8,30]. Given its high bioactivity and low application rates, PVE is particularly susceptible to improper dosing risks. Thus, establishing standardized protocols and clarifying their mechanisms of action [23] are critical for future research. Additionally, future work should incorporate cost-benefit analyses for large-scale orchard use and long-term monitoring of PVE’s effects on soil health to facilitate agricultural scaling.
5. Conclusions
The study demonstrated significant regulatory effects of Paecilomyces variotii extract (PVE) on pear tree growth, development, and drought resistance. At optimal conditions (well-watered conditions), PVE increased plant height (by 12.05%), stem diameter (by 3.54%), and new shoot growth (by 31.91%), while boosting leaf net photosynthetic rate (by 23.07%). Applying 5 ng/mL PVE to roots improved drought resistance by greatly raising rhizosphere trehalose levels. However, higher concentration inhibited leaf antioxidant enzyme activity, decreased soil enzyme activity, and disrupted root trehalose metabolism, indicating that strict concentration control is vital for field use. This study yields significant implications for sustainable horticulture: (i) as a natural bioactive compound, PVE offers an eco-alternative to synthetic bioregulators, advancing green inputs for crop management; (ii) the trehalose-mediated drought resistance mechanism elucidates a novel stress-adaptation pathway, providing a theoretical framework for next-generation bioprotectants; (iii) identification of the optimal concentration window (5 ng/mL) enables precise resource-efficient orchard operations, minimizing input waste while maximizing efficacy. This study has certain limitations that warrant consideration: first, the experimental material used was two-year-old seedlings, so the differences in physiological responses between seedlings and mature trees, as well as across varieties, remain unclear; second, the short-term drought simulation may not fully reflect the regulatory effects of PVE or the dynamics of plant-soil microbial interactions under long-term field conditions. In practical applications, the precise control of PVE concentration (affected by soil texture and organic matter), environmental stability (temperature/pH sensitivity), and compatibility with other agricultural materials still need to be broken through. Future efforts should integrate field validation and technological innovation to advance PVE’s industrial application. Further research should explore the signaling pathways controlling trehalose biosynthesis and drought-responsive genes, verify optimal concentration ranges for key commercial crops, and monitor PVE migration in soil-plant systems to assess long-term ecological safety.
Author Contributions
Conceptualization, H.W.; methodology, Y.W. and G.H.; software, Z.Y. and Y.Z. (Yawei Zhang); validation, H.P. and Q.Y.; formal analysis, Y.W. and W.Y.; investigation, Y.W.; resources, Y.Z. (Yuping Zhuge); data curation, Y.W.; writing—original draft preparation, Z.G.; writing—review and editing, H.W. and Y.Z. (Yuping Zhuge); visualization, Z.G. and Z.Y.; supervision, Y.L.; project administration, H.W.; funding acquisition, Y.Z. (Yuping Zhuge). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Modern Agriculture Industrial Technology Systems Project of Shandong Province (Grant number: SDAIT-29-01) and the Major Science and Technology Innovation Projects in Shandong Province (Grant numbers: 2020CXGC010803 and 2021CXGC010801).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| PVE | Paecilomyces variotii extract |
| EBIC | European Biostimulants Industry Committee |
| ZNC | ZhiNengCong |
| W-W | Treatment of deionized water application under well-watered conditions |
| W-D | Treatment of deionized water application under drought stress |
| PVE5-W | Treatment of 5 ng/mL PVE application under well-watered conditions |
| PVE5-D | Treatment of 5 ng/mL PVE application under drought stress |
| PVE20-W | Treatment of 20 ng/mL PVE application under well-watered conditions |
| PVE20-D | Treatment of 20 ng/mL PVE application under drought stress |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| CAT | Catalase |
| GSH | Glutathione |
| Pn | Net photosynthetic rate |
| Gs | Stomatal conductance |
| Ci | Intercellular CO2 concentration |
| Tr | Transpiration rate |
References
- Carvalho, M.L.; de Moraes, M.T.; Cerri, C.E.P.; Cherubin, M.R. Biochar amendment enhances water retention in a tropical sandy soil. Agriculture 2020, 10, 62. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.; Wang, Z.; Wang, J.; Li, Y.; Sun, C. Distribution characteristics of soil water and nutrients in pear orchard and their relationship with yields in loess hilly region. J. Appl. Ecol. 2021, 32, 3159–3166. [Google Scholar] [CrossRef]
- Karlova, R.; Boer, D.; Hayes, S.; Testerink, C. Root plasticity under abiotic stress. Plant Physiol. 2021, 187, 1057–1070. [Google Scholar] [CrossRef]
- Dumanoğlu, H.; Şahin, Ö.; Sarıkamış, G. Drought stress response of Pears and quince as Pear rootstocks induced by D-mannitol assessed with proliferation and growth, antioxidant enzymes and mineral elements of in vitro microshoots. S. Afr. J. Bot. 2025, 178, 382–389. [Google Scholar] [CrossRef]
- Plant Biostimulants. Available online: https://biostimulants.eu/plant-biostimulants/ (accessed on 7 July 2025).
- What Are Biostimulants? Available online: http://www.biostimulantcoalition.org/about/ (accessed on 11 July 2025).
- Hakkoum, Z.; Minaoui, F.; Chabili, A.; Douma, M.; Mouhri, K.; Loudiki, M. Biofertilizing effect of soil cyanobacterium Anabaena cylindrica–based formulations on wheat growth, physiology, and soil fertility. Agriculture 2025, 15, 189. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Y.; Chen, B.; Zhang, M.; Liu, Z.; Wang, Q.; Ma, J. Paecilomyces variotii extracts and controlled-release urea synergistically increased nitrogen use efficiency and rice yield. ACS Omega 2020, 5, 13303–13311. [Google Scholar] [CrossRef]
- Jiang, Y.; Yue, Y.; Wang, Z.; Lu, C.; Yin, Z.; Li, Y.; Ding, X. Plant biostimulant as an environmentally friendly alternative to modern agriculture. J. Agric. Food Chem. 2024, 72, 5107–5121. [Google Scholar] [CrossRef]
- Lu, C.; Liu, H.; Jiang, D.; Wang, L.; Jiang, Y.; Tang, S.; Hou, X.; Han, X.; Liu, Z.; Zhang, M.; et al. Paecilomyces variotii extracts (ZNC) enhance plant immunity and promote plant growth. Plant Soil 2019, 441, 383–397. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, A.; Wang, Q.; Song, Y.; Zhang, M.; Ding, X.; Li, Y.; Geng, Q.; Zhu, C. Ultrahigh-activity immune inducer from Endophytic Fungi induces tobacco resistance to virus by SA pathway and RNA silencing. BMC Plant Biol. 2020, 20, 169. [Google Scholar] [CrossRef]
- Chen, Q.; Qu, Z.; Zhang, Z.; Ma, G.; Zhu, M.; Dan, J.; Wang, J.; Zhang, S.; Ding, X.; Zhang, M.; et al. Coated diammonium phosphate combined with Paecilomyces variotii extracts improves root architecture, enhances spring low temperature tolerance, and increases wheat yield. Soil Tillage Res. 2023, 227, 105613. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L. Physiological responses to humic substances as plant growth promoter. Chem. Biol. Technol. Agric. 2014, 1, 3. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, X.F.; Chaparro, J.M.; Badri, D.V.; Manter, D.K.; Vivanco, J.M.; Guo, J. Root and bacterial secretions regulate the interaction between plants and PGPR leading to distinct plant growth promotion effects. Plant Soil 2016, 401, 259–272. [Google Scholar] [CrossRef]
- Cao, J.; Liu, B.; Xu, X.; Zhang, X.; Zhu, C.; Li, Y.; Ding, X. Plant endophytic fungus extract ZNC improved potato immunity, yield, and quality. Front. Plant Sci. 2021, 12, 707256. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Yin, Z.; Li, Y.; Lu, C.; Wang, Q.; Ding, X. A novel guanine elicitor stimulates immunity in Arabidopsis and rice by ethylene and jasmonic acid signaling pathways. Front. Plant Sci. 2022, 13, 841228. [Google Scholar] [CrossRef] [PubMed]
- Lu, R. Analytical Methods of Soil Agrochemistry, 1st ed.; China Agricultural Science and Technology Press: Beijing, China, 2000; pp. 249–254. [Google Scholar]
- Chabili, A.; Hakkoum, Z.; Minaoui, F.; Douma, M.; Meddich, A.; Loudiki, M. Germination screen of eco-extracts from soil cyanobacteria and microalgae for their biostimulant effects on wheat seeds emergence and vigor. Algal Res. 2025, 89, 104087. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, P.; Yu, X.; Xu, J.; Liu, G. Physiological and molecular mechanisms of rice tolerance to salt and drought stress: Advances and future directions. Int. J. Mol. Sci. 2024, 25, 9404. [Google Scholar] [CrossRef]
- Mohanan, A.; Kodigudla, A.; Raman, D.R.; Bakka, K.; Challabathula, D. Trehalose accumulation enhances drought tolerance by modulating photosynthesis and ROS-antioxidant balance in drought sensitive and tolerant rice cultivars. Physiol. Mol. Biol. Plants 2023, 29, 2035–2049. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Yuan, R.; Yang, X.; Xi, H.; Zhuo, M.; Wei, M. Salinity-responsive key endophytic bacteria in the propagules of Kandelia obovata enhance salt tolerance in rice. J. Integr. Agric. 2025, 24, 1738–1753. [Google Scholar] [CrossRef]
- He, C.; Wu, T.; Li, J.; Zhang, X.; Zheng, Z.; Gao, Y.; Zhang, C.; Zhong, T.; Zhang, Y.; Du, F. Bio-stimulant based nanodelivery system for pesticides with high adhesion and growth stimulation. Chem. Eng. J. 2024, 491, 151904. [Google Scholar] [CrossRef]
- Moreno-Gavíra, A.; Diánez, F.; Sánchez-Montesinos, B.; Santos, M. Paecilomyces variotii as a plant-growth promoter in horticulture. Agronomy 2020, 10, 597. [Google Scholar] [CrossRef]
- Tiebel, K.; Karge, A. Influence of drought stress on the seed germination and seedling survival of Pinus sylvestris and Larix decidua. For. Ecol. Manag. 2025, 591, 122852. [Google Scholar] [CrossRef]
- Lin, L.; Yuan, K.; Qi, K.; Xie, Z.; Huang, X.; Zhang, S. Synergistic interaction between PbbZIP88 and PbSRK2E enhances drought resistance in Pear through regulation of PbATL18 expression and stomatal closure. Plant Cell Environ. 2025, 48, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Sible, C.N.; Seebauer, J.R.; Below, F.E. Plant biostimulants: A categorical review, their implications for row crop production, and relation to soil health indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- Lau, S.E.; Lim, L.W.T.; Hamdan, M.F.; Chan, C.; Saidi, N.B.; Ong-Abdullah, J.; Tan, B.C. Enhancing plant resilience to abiotic stress: The power of biostimulants. Phyton-Int. J. Exp. Bot. 2025, 94, 1–31. [Google Scholar] [CrossRef]
- Khoulati, A.; Ouahhoud, S.; Taibi, M.; Ezrari, S.; Mamri, S.; Merah, O.; Hakkou, A.; Addi, M.; Maleb, A.; Saalaoui, E. Harnessing biostimulants for sustainable agriculture: Innovations, challenges, and future prospects. Discov. Agric. 2025, 3, 56. [Google Scholar] [CrossRef]
- Falgreen, S.; Laursen, M.B.; Bødker, J.S.; Kjeldsen, M.K.; Schmitz, A.; Nyegaard, M.; Johnsen, H.E.; Dybkær, K.; Bøgsted, M. Exposure time independent summary statistics for assessment of drug dependent cell line growth inhibition. BMC Bioinform. 2014, 15, 168. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).