Identification and Analysis of Differentially Expressed Genes in Sugarcane Roots Under Different Potassium Application Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.3. Field Experiments
2.3.1. Overview of the Experimental Site
2.3.2. Field Experimental Methods
2.3.3. Sugarcane Planting and Fertilization Management
2.3.4. Determination of Sugarcane Growth Indicators
2.3.5. Indoor Hydroponic Experiments
2.4. Transcriptome Sequencing Analysis of Sugarcane Roots
2.4.1. Collection and Processing of Root Transcriptome Samples in Field Experiments
2.4.2. RNA Extraction and cDNA Library Construction and Sequencing
2.4.3. Alignment of Sequencing Data with the Reference Genome Sequence
2.4.4. Gene Functional Annotation and Differentially Expressed Genes
2.4.5. Mining of Differentially Expressed Genes
2.4.6. Statistical Analyses
3. Results
3.1. The Growth of Sugarcane Seedlings and Tillers Under Varying Application Rates of Potassium Fertilizer
3.2. Yield and Sucrose Content of Sugarcane in the Field Under Different K Application Levels
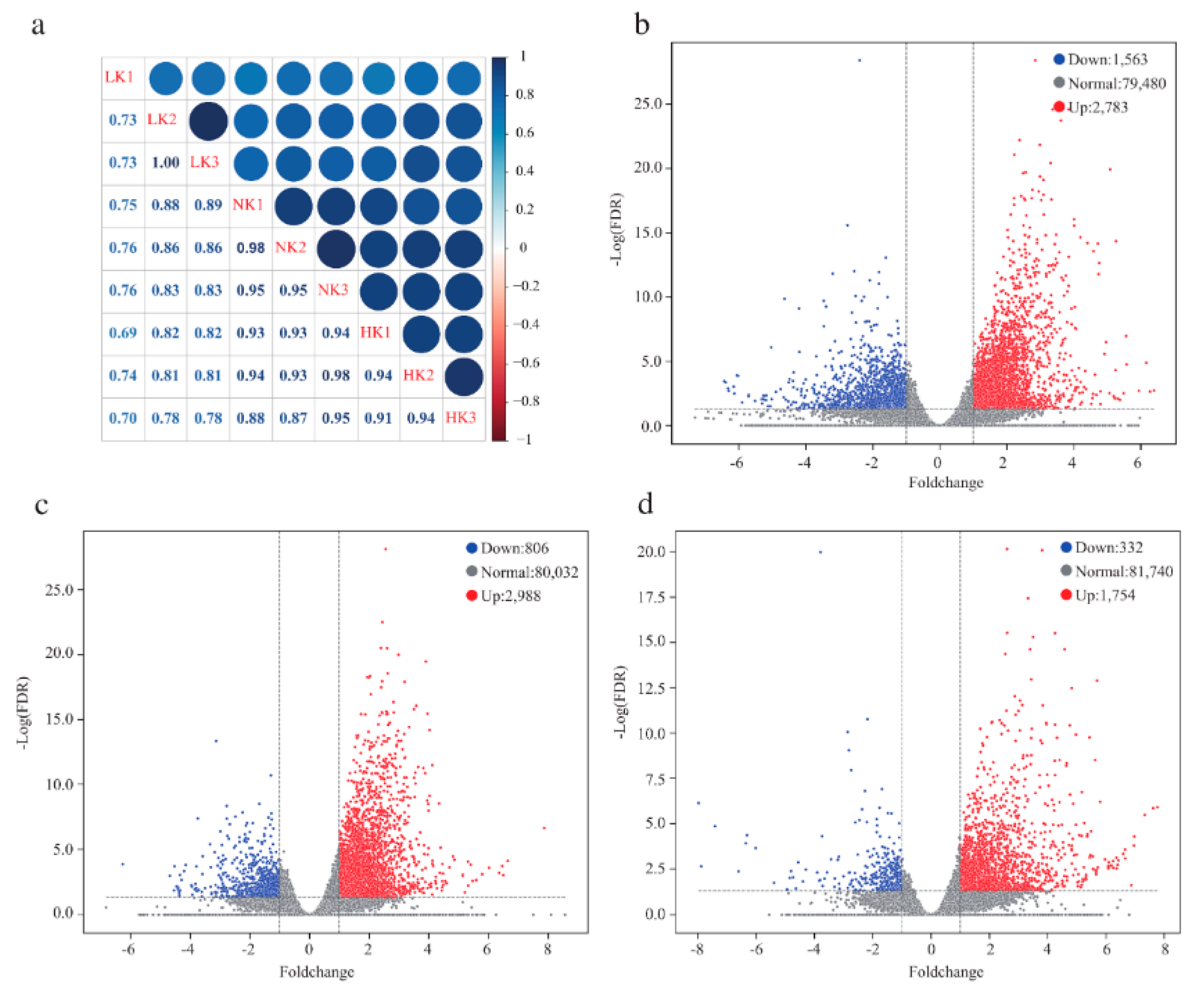
3.3. Analysis of the Sequencing Data
3.4. The Transcriptomic Responses of Sugarcane Root Systems Under Different K Treatments
3.5. GO Enrichment Analysis of Differentially Expressed Genes
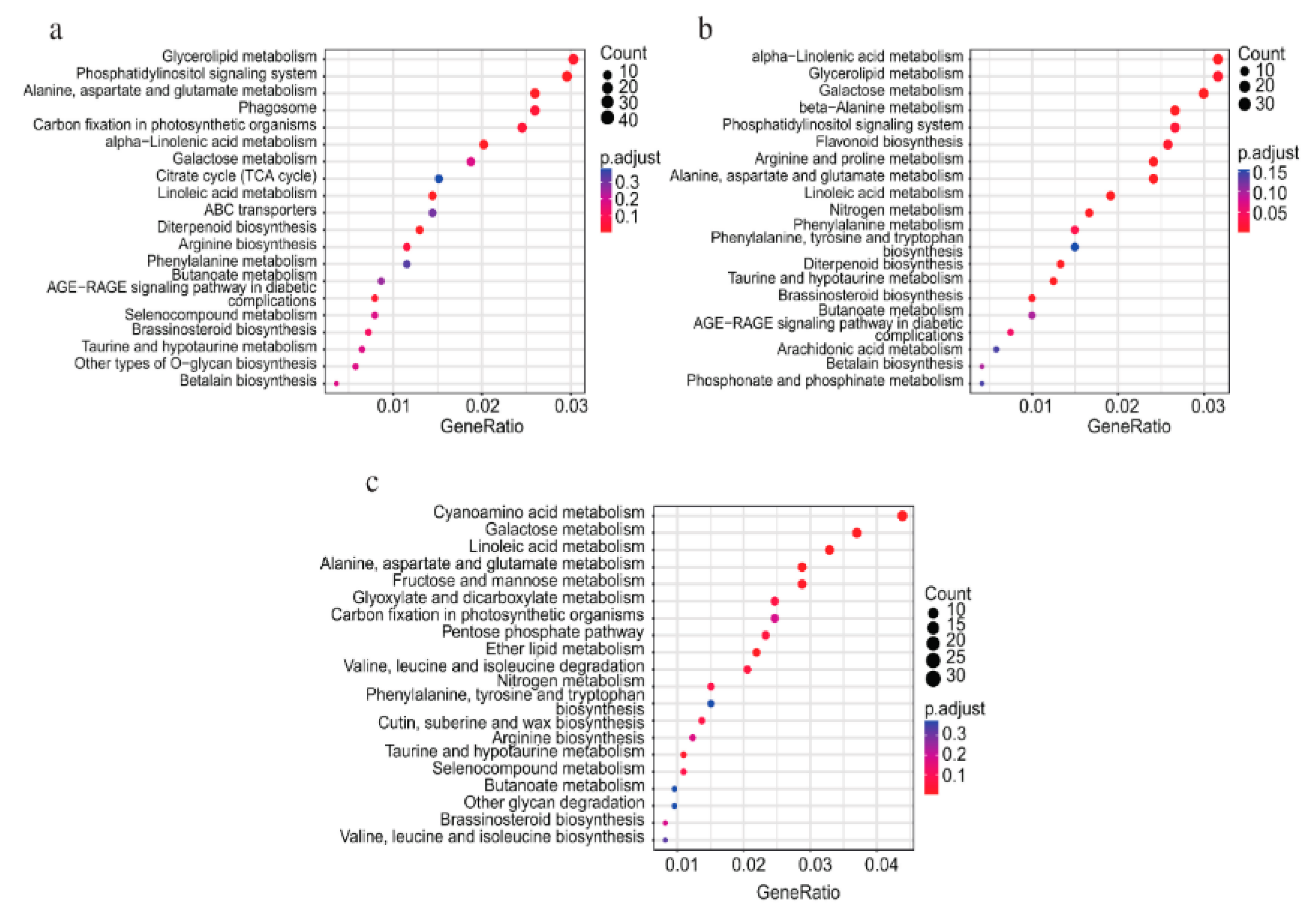
3.6. KEGG Enrichment Analysis of Differentially Expressed Genes
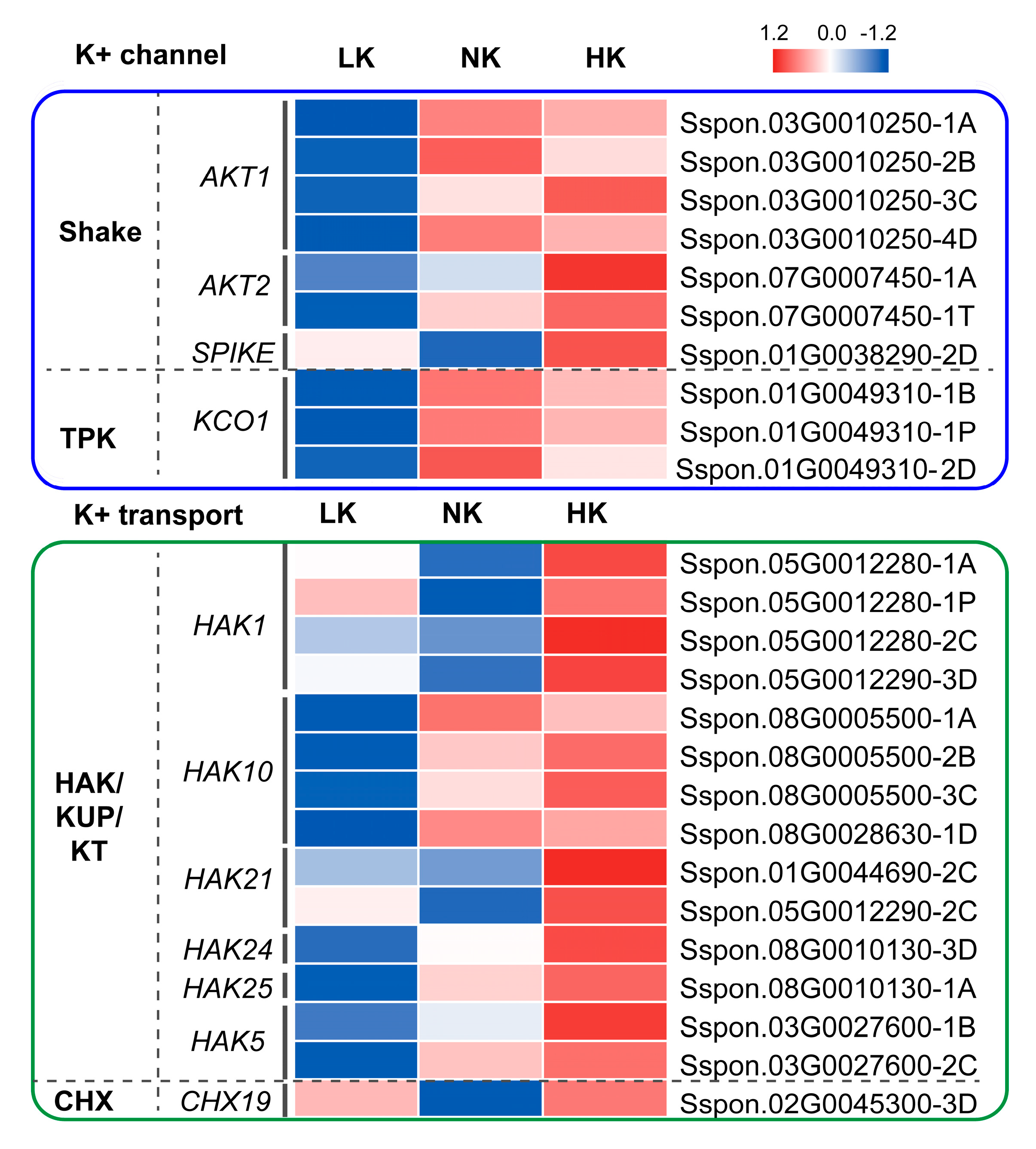
3.7. The Potassium Transport Elements in Sugarcane Roots Under Different External Potassium Concentrations
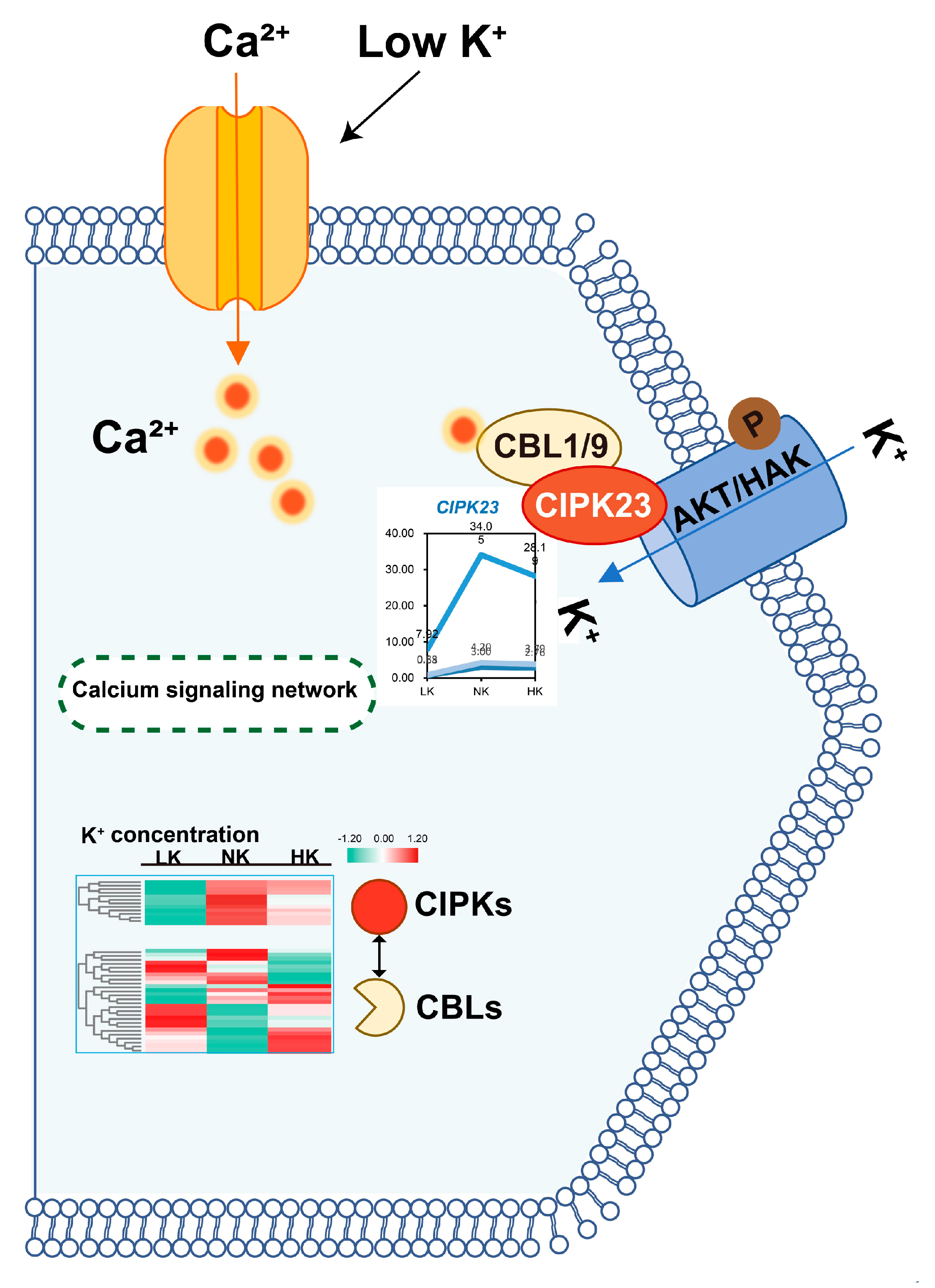
3.8. Ca2+ Signaling and Activation of Potassium Channels
3.9. The Co-Expression Network of Key Genes Involved in Potassium Uptake by Sugarcane Roots Participates in the Regulation of Economic Traits of Sugarcane
3.10. Analysis of the Functional Characterization and Co-Expression Network of Agronomic Trait-Associated Gene Modules
4. Discussion
4.1. Potassium Supply Levels Exert Dual Regulatory Effects on Sugarcane Growth and Quality
4.2. Transcriptomic Signatures and Physiological Effects of Biphasic Potassium Regulation in Sugarcane
4.3. Transport Proteins Mediate Potassium Uptake to Regulate Growth and Development Processes in Sugarcane
4.4. Calcium-Mediated Signaling Coordinates Plant Potassium Homeostasis Through the CBL/CIPK-AKT1/HAK5 Regulatory Module
4.5. Optimal Potassium Nutrition Induces Transcriptional Reprogramming in Root Systems, Thereby Enhancing High-Yield and High-Sucrose Phenotypic Expression in Sugarcane
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Dinesh Babu, K.S.; Janakiraman, V.; Palaniswamy, H.; Kasirajan, L.; Gomathi, R.; Ramkumar, T.R. A short review on sugarcane: Its domestication, molecular manipulations and future perspectives. Genet. Resour. Crop Evol. 2022, 69, 2623–2643. [Google Scholar] [CrossRef] [PubMed]
- Boschiero, B.N.; Mariano, E.; Torres-Dorante, L.O.; Sattolo, T.M.S.; Otto, R.; Garcia, P.L.; Dias, C.T.S.; Trivelin, P.C.O. Nitrogen fertilizer effects on sugarcane growth, nutritional status, and productivity in tropical acid soils. Nutr. Cycl. Agroecosyst. 2020, 117, 367–382. [Google Scholar] [CrossRef]
- Katal, K.; Alipour, A.; Zahedi, H.; Sharghi, Y.; Alavifazel, M. Effects of different irrigation levels and phosphate fertilizer doses with or without nano iron application on sugar beet growth and physiology. Russ. J. Plant Physiol. 2024, 71, 190. [Google Scholar] [CrossRef]
- Zeng, X.P.; Zhu, K.; Lu, J.M.; Jiang, Y.; Yang, L.T.; Xing, Y.X.; Li, Y.R. Long-term effects of different nitrogen levels on growth, yield, and quality in sugarcane. Agronomy 2020, 10, 353. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Campos, M.D.; Martello, J.M.; Alves, C.J.; Nascimento, C.A.C.; Pereira, J.C.D.R.; Cantarella, H. Organomineral fertilizer as source of P and K for sugarcane. Sci. Rep. 2020, 10, 5398. [Google Scholar] [CrossRef]
- Cui, J.; Tcherkez, G. Potassium dependency of enzymes in plant primary metabolism. Plant Physiol. Biochem. 2021, 166, 522–530. [Google Scholar] [CrossRef]
- Deng, S. Modern Sugarcane Production and Technology; Guangdong Science and Technology Press: Guangzhou, China, 1991. [Google Scholar]
- Cavalcante, V.; Prado, R.; Júnior de Almeida, H.; Cruz, F.; Santos, D.M.M. Gaseous exchanges, growth and foliar anatomy of sugarcane plants grown in potassium (K) deprived nutrient solution. Aust. J. Crop Sci. 2015, 9, 577–584. [Google Scholar]
- Xie, J.L.; Li, C.N.; Li, Y.; Liang, Q.; Liu, X.Y.; Luo, T.; Lin, L.; Liang, T.; He, W.Z.; Tang, H.Y. Effects of potassium fertilizer application amount on sugarcane yield, sugar accumulation and stress resistance. Soil Fert. Sci. China 2019, 2, 133–138. [Google Scholar]
- Liu, Z.F.; Lin, D.; Peng, C.Y. Effect of applying potassium on yield and quality of sugarcane. Sugar Crops China 2009, 4, 34–35+43. [Google Scholar]
- Shukla, S.K.; Yadav, R.L.; Singh, P.N.; Singh, I. Potassium nutrition for improving stubble bud sprouting, dry matter partitioning, nutrient uptake and winter initiated sugarcane ratoon yield. Eur. J. Agron. 2009, 30, 27–33. [Google Scholar] [CrossRef]
- Tan, H.W.; Zhou, L.Q.; Xie, R.L.; Huang, M.F. Potassium uptake and its utilization in sugarcane under the different fertilization levels. J. South. Agric. 2011, 42, 295–298. [Google Scholar]
- Huang, Z.R.; Zhou, W.L.; Ao, J.H.; Cheng, D.W.; Yang, Y.H.; Jiang, Y.; Li, Q.W. Sugarcane yield and soil potassium balance in potassium application of four consecutive years. Chin. J. Trop. Crops 2020, 41, 1347–1353. [Google Scholar]
- Salokhe, S. Development of an efficient protocol for production of healthy sugarcane seed cane through meristem culture. J. Agric. Food Res. 2021, 4, 100126. [Google Scholar] [CrossRef]
- Zhao, Y.; Ai, J.; Wang, Y.T.; Zhang, Z.F.; Yang, H.Q.; Li, J.Q.; Guo, Z.J.; Liu, H.J.; Qin, W.; Deng, J.; et al. Research on the production potential of main sugarcane varieties under different planting modes in hilly and mountainous areas. Sci. Agric. Sin. 2024, 57, 3743–3757. [Google Scholar]
- Sharma, R.; Sindhu, S.S.; Glick, B.R. Potassium solubilizing microorganisms as potential biofertilizer: A sustainable climate-resilient approach to improve soil fertility and crop production in agriculture. J. Plant Growth Regul. 2024, 43, 2503–2535. [Google Scholar] [CrossRef]
- Xu, X.X.; Du, X.; Wang, F.; Sha, J.C.; Chen, Q.; Tian, G.; Zhu, Z.L.; Ge, S.F.; Jiang, Y.M. Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front. Plant Sci. 2020, 11, 904. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Y.H.; Zhang, X.M.; Hu, W.J.; Lin, L.J.; Deng, Q.X.; Xia, H.; Liang, D.; Lv, X.L. Effects of potassium-containing fertilizers on sugar and organic acid metabolism in grape fruits. Int. J. Mol. Sci. 2024, 25, 2828. [Google Scholar] [CrossRef]
- Soumare, A.; Sarr, D.; Diedhiou, A. Potassium sources, microorganisms, and plant nutrition challenges and future research directions: A review. Pedosphere 2022, 33, 105–115. [Google Scholar] [CrossRef]
- Li, X.T.; Tong, X.N.; Yu, Q.H.; Wang, N.; Wang, X.G.; Cao, M.J. Potassium efficiency of different low K tolerant soybean varieties. Soybean Sci. 2014, 33, 385–388. [Google Scholar]
- Lu, T.; Chen, H.T.; Shen, Z.G.; Chen, X. Advance of potassium channels and transporters in plant. Acta Agric. Boreali-Sin. 2019, 34, 372–379. [Google Scholar]
- Wang, W.; Chen, Q.; Xu, S.; Liu, W.C.; Zhu, X.; Song, C.P. Trehalose-6-phosphate phosphatase E modulates ABA-controlled root growth and stomatal movement in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 1518–1534. [Google Scholar] [CrossRef]
- Hao, D.L.; Li, X.H.; Kong, W.Y.; Chen, R.R.; Liu, J.X.; Guo, H.L.; Zhou, J.Y. Phosphorylation regulation of nitrogen, phosphorus, and potassium uptake systems in plants. Crop J. 2023, 11, 1034–1047. [Google Scholar] [CrossRef]
- Luo, H.B.; Huang, C.M.; Cao, H.Q.; Wei, Y.W.; Xu, L.; Wu, K.C.; Deng, Z.N.; Wu, X.J.; Ye, L.P.; Yi, X.P. Molecular cloning and functional analysis of Shak10 gene promoter from sugarcane (Saccharum officinarum L.). Trop. Plant Biol. 2024, 17, 204–213. [Google Scholar]
- Li, K.L.; Xue, H.; Tang, R.J.; Luan, S. TORC pathway intersects with a calcium sensor kinase network to regulate potassium sensing in Arabidopsis. Proc. Natl. Acad. Sci. USA 2023, 120, e2316011120. [Google Scholar] [CrossRef]
- Li, W.F.; Fang, Y.H.; Chen, X.K.; Xia, H.M.; Li, F.Q.; Wang, Y. Rapid determination method of cane sugar content. Sugar Crops China 2009, 2, 14–15. [Google Scholar]
- Wang, Y.; Chen, Y.F.; Wu, W.H. Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol. 2021, 63, 34–52. [Google Scholar] [CrossRef]
- Deeken, R.; Geiger, D.; Fromm, J.; Koroleva, O.; Ache, P.; Langenfeld-Heyser, R.; Sauer, N.; May, S.T.; Hedrich, R. Loss of the AKT2/3 potassium channel affects sugar loading into the phloem of Arabidopsis. Planta 2002, 216, 334–344. [Google Scholar] [CrossRef]
- Gopalasundaram, P.; Bhaskaran, A.; Rakkiyappan, P. Integrated nutrient management in sugarcane. Sugar Tech 2012, 14, 3–20. [Google Scholar] [CrossRef]
- Su, G. The Potassium Nutrition of Sugarcane; Guangdong Agricultural Sciences: Guangzhou, China, 1981. [Google Scholar]
- Li, Q.W.; Lu, Y.L.; Zhou, W.L.; Chen, D.W.; Ao, J.H.; Huang, Y.; Huang, Z.R.; Jiang, Y. Effects of low potassium stress on growth and photosynthetic characteristics of different sugarcane lines. Sugarcane Cane Sugar 2011, 6, 1–5. [Google Scholar]
- El-Tilib, M.; Elnasikh, M.; Elamin, E.A. Phosphorus and potassium fertilization effects on growth attributes and yield of two sugarcane varieties grown on three soil series. J. Plant Nutr. 2004, 27, 663–699. [Google Scholar] [CrossRef]
- Jin, Y.F.; Chen, D.W.; Li, Q.W.; Huang, Y.; Huang, Z.R. Effect of different levels of potassium on sugarcane germination and seedling growth. Guangdong Agric. Sci. 2013, 40, 72–74. [Google Scholar]
- Zheng, C.; Li, Q.W.; Huang, Z.R.; Ao, J.H. Potassium uptake difference of different sugarcane varieties. Chin. J. Trop. Crops 2011, 32, 2221–2225. [Google Scholar]
- Zhang, H.W.; Xiao, W.; Yu, W.W.; Yao, L.; Li, L.G.; Wei, J.H.; Li, R.F. Foxtail millet SiHAK1 excites extreme high-affinity K+ uptake to maintain K+ homeostasis under low K+ or salt stress. Plant Cell Rep. 2018, 37, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.M.; Wang, Y.J.; Zhang, N.N.; Wu, Z.L.; Zeng, Q.Y.; Wu, J.Y.; Wu, X.B.; Wang, L.; Zhang, J.S.; Qi, Y.W. Genome-wide systematic characterization of the HAK/KUP/KT gene family and its expression profile during plant growth and in response to low-K+ stress in Saccharum. BMC Plant Biol. 2020, 20, 20. [Google Scholar] [CrossRef]
- Feng, H.M.; Tang, Q.; Cai, J.; Xu, B.C.; Xu, G.H.; Yu, L. Rice OsHAK16 functions in potassium uptake and translocation in shoot, maintaining potassium homeostasis and salt tolerance. Planta 2019, 250, 549–561. [Google Scholar] [CrossRef]
- Chen, G.; Hu, Q.D.; Luo, L.; Yang, T.Y.; Zhang, S.; Hu, Y.B.; Yu, L.; Xu, G.H. Rice potassium transporter OsHAK1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges. Plant Cell Environ. 2015, 38, 2747–2765. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Ruan, B.; Guo, L.B.; Zeng, D.L.; Gao, Z.Y.; Zhu, L.; Hu, J.; Ren, D.Y.; Yu, L.; et al. OsHAK1 controls the vegetative growth and panicle fertility of rice by its effect on potassium-mediated sugar metabolism. Plant Sci. 2018, 274, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Chang, C.L.; Wang, P.H.; Tsai, M.C.; Hsu, P.H.; Chang, I.F. A type III ACC synthase, ACS7, is involved in root gravitropism in Arabidopsis thaliana. J. Exp. Bot. 2013, 64, 4343–4360. [Google Scholar] [CrossRef]
- Zhou, W.K.; Lozano-Torres, J.L.; Blilou, I.; Zhang, X.Y.; Zhai, Q.Z.; Smant, G.; Li, C.Y.; Scheres, B. A jasmonate signaling network activates root stem cells and promotes regeneration. Cell 2019, 177, 942–956.e14. [Google Scholar] [CrossRef]
- Gratz, R.; Manishankar, P.; Ivanov, R.; Köster, P.; Mohr, I.; Trofimov, K.; Steinhorst, L.; Meiser, J.; Mai, H.J.; Drerup, M.; et al. CIPK11-dependent phosphorylation modulates FIT activity to promote Arabidopsis iron acquisition in response to calcium signaling. Dev. Cell 2019, 48, 726–740.e10. [Google Scholar] [CrossRef]
- Lin, Q.F.; Gong, J.X.; Zhang, Z.L.; Meng, Z.Z.; Wang, J.Y.; Wang, S.; Sun, J.; Gu, X.; Jin, Y.T.; Wu, T.; et al. The Arabidopsis thaliana trehalose-6-phosphate phosphatase gene AtTPPI regulates primary root growth and lateral root elongation. Front. Plant Sci. 2022, 13, 1088278. [Google Scholar] [CrossRef]
- Luan, S.; Lan, W.; Lee, S.C. Potassium nutrition, sodium toxicity, and calcium signaling: Connections through the CBL–CIPK network. Curr. Opin. Plant Biol. 2009, 12, 339–346. [Google Scholar] [CrossRef]
- Yu, Q.Y.; An, L.J.; Li, W.L. The CBL–CIPK network mediates different signaling pathways in plants. Plant Cell Rep. 2014, 33, 203–214. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, Q.Y.; Zhao, P.; Eickelkamp, A.; Ma, C.M.; He, G.F.; Li, F.J.; Wallrad, L.; Becker, T.; Li, Z.H.; et al. The potassium channel GhAKT2BD is regulated by CBL–CIPK calcium signalling complexes and facilitates K+ allocation in cotton. FEBS Lett. 2022, 596, 1904–1920. [Google Scholar] [CrossRef] [PubMed]
- Li, K.L.; Tang, R.J.; Wang, C.; Luan, S. Potassium nutrient status drives posttranslational regulation of a low-K response network in Arabidopsis. Nat. Commun. 2023, 14, 360. [Google Scholar] [CrossRef]
- Scherzer, S.; Böhm, J.; Krol, E.; Shabala, L.; Kreuzer, I.; Larisch, C.; Bemm, F.; Al-Rasheed, K.A.S.; Shabala, S.; Rennenberg, H.; et al. Calcium sensor kinase activates potassium uptake systems in gland cells of Venus flytraps. Proc. Natl. Acad. Sci. USA 2015, 112, 7309–7314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.C.; Yin, W.L.; Xia, X.L. Shaker-like potassium channels in Populus, regulated by the CBL–CIPK signal transduction pathway, increase tolerance to low-K+ stress. Plant Cell Rep. 2010, 29, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Ragel, P.; Ródenas, R.; García-Martín, E.; Andrés, Z.; Villalta, I.; Nieves-Cordones, M.; Rivero, R.M.; Martínez, V.; Pardo, J.M.; Quintero, F.J.; et al. The CBL-interacting protein kinase CIPK23 regulates HAK5-mediated high-affinity K+ uptake in Arabidopsis roots. Plant Physiol. 2015, 169, 2863–2873. [Google Scholar]
- Xu, J.; Li, H.D.; Chen, L.Q.; Wang, Y.; Liu, L.L.; He, L.; Wu, W.H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 2006, 125, 1347–1360. [Google Scholar] [CrossRef]
- Ling, Q.P.; Zeng, Q.Y.; Hu, F.; Chen, J.W.; Wu, J.Y.; Li, Q.W.; Qi, Y.W. Molecular cloning and expression analysis of CIPK23 gene from sugarcane under low potassium, drought and salt stress. Mol. Plant Breed. 2015, 13, 1329–1335. [Google Scholar]
- Sánchez-Barrena, M.J.; Chaves-Sanjuan, A.; Raddatz, N.; Mendoza, I.; Cortés, Á.; Gago, F.; González-Rubio, J.M.; Benavente, J.L.; Quintero, F.J.; Pardo, J.M.; et al. Recognition and activation of the plant AKT1 potassium channel by the kinase CIPK231. Plant Physiol. 2020, 182, 2143–2153. [Google Scholar] [CrossRef] [PubMed]





| Macroelements | Microelements | ||
|---|---|---|---|
| Nutritional Salts | Density/g·L−1 | Nutritional Salts | Density/g·L−1 |
| Ca(NO3)2·4H2O | 236.00 | H3BO3 | 2.86 |
| KNO3 | 101.00 | MnCl2·4H2O | 1.81 |
| MgSO4·7H2O | 98.00 | ZnSO4·7H2O | 0.22 |
| KH2PO4 | 27.00 | CuSO4·5H2O | 0.08 |
| KCl | 90.00 | Na2MoO4·2H2O | 0.03 |
| NaNO3 | 42.50 | FeSO4·7H2O | 5.56 |
| NaH2PO4 | 43.50 | EDTA-Na2 | 7.49 |
| Na2SO4 | 28.40 | ||
| Sample | Raw Reads (Gb) | Clean Reads | Clean Bases (Gb) | GC Content (%) | Q20 (%) | Q30 (%) |
|---|---|---|---|---|---|---|
| LK1 | 8.83 | 58,878,858 | 8.82 | 54.05 | 96.78 | 90.06 |
| LK2 | 6.52 | 43,448,294 | 6.50 | 51.50 | 96.76 | 89.79 |
| LK3 | 6.48 | 43,206,584 | 6.47 | 51.81 | 96.85 | 90.11 |
| NK1 | 7.94 | 52,907,244 | 7.92 | 52.27 | 97.01 | 90.57 |
| NK2 | 8.38 | 55,896,526 | 8.37 | 51.93 | 97.11 | 90.91 |
| NK3 | 6.46 | 43,037,984 | 6.43 | 52.09 | 98.29 | 94.43 |
| HK1 | 6.85 | 45,662,804 | 6.83 | 53.11 | 97.94 | 93.17 |
| HK2 | 6.36 | 42,398,626 | 6.34 | 52.15 | 98.04 | 93.45 |
| HK3 | 6.46 | 43,037,796 | 6.44 | 52.93 | 97.66 | 92.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Zhang, Z.; Li, Y.; Zhao, Y.; Liu, J.; Deng, J. Identification and Analysis of Differentially Expressed Genes in Sugarcane Roots Under Different Potassium Application Levels. Agronomy 2025, 15, 2060. https://doi.org/10.3390/agronomy15092060
Li R, Zhang Z, Li Y, Zhao Y, Liu J, Deng J. Identification and Analysis of Differentially Expressed Genes in Sugarcane Roots Under Different Potassium Application Levels. Agronomy. 2025; 15(9):2060. https://doi.org/10.3390/agronomy15092060
Chicago/Turabian StyleLi, Rudan, Zhongfu Zhang, Yanye Li, Yong Zhao, Jiayong Liu, and Jun Deng. 2025. "Identification and Analysis of Differentially Expressed Genes in Sugarcane Roots Under Different Potassium Application Levels" Agronomy 15, no. 9: 2060. https://doi.org/10.3390/agronomy15092060
APA StyleLi, R., Zhang, Z., Li, Y., Zhao, Y., Liu, J., & Deng, J. (2025). Identification and Analysis of Differentially Expressed Genes in Sugarcane Roots Under Different Potassium Application Levels. Agronomy, 15(9), 2060. https://doi.org/10.3390/agronomy15092060







