Expression and Functional Analysis of the ABORTED MICROSPORES (AMS) Gene in Marigold (Tagetes erecta L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. RNA Preparation
2.3. Expression Analysis of TeAMS
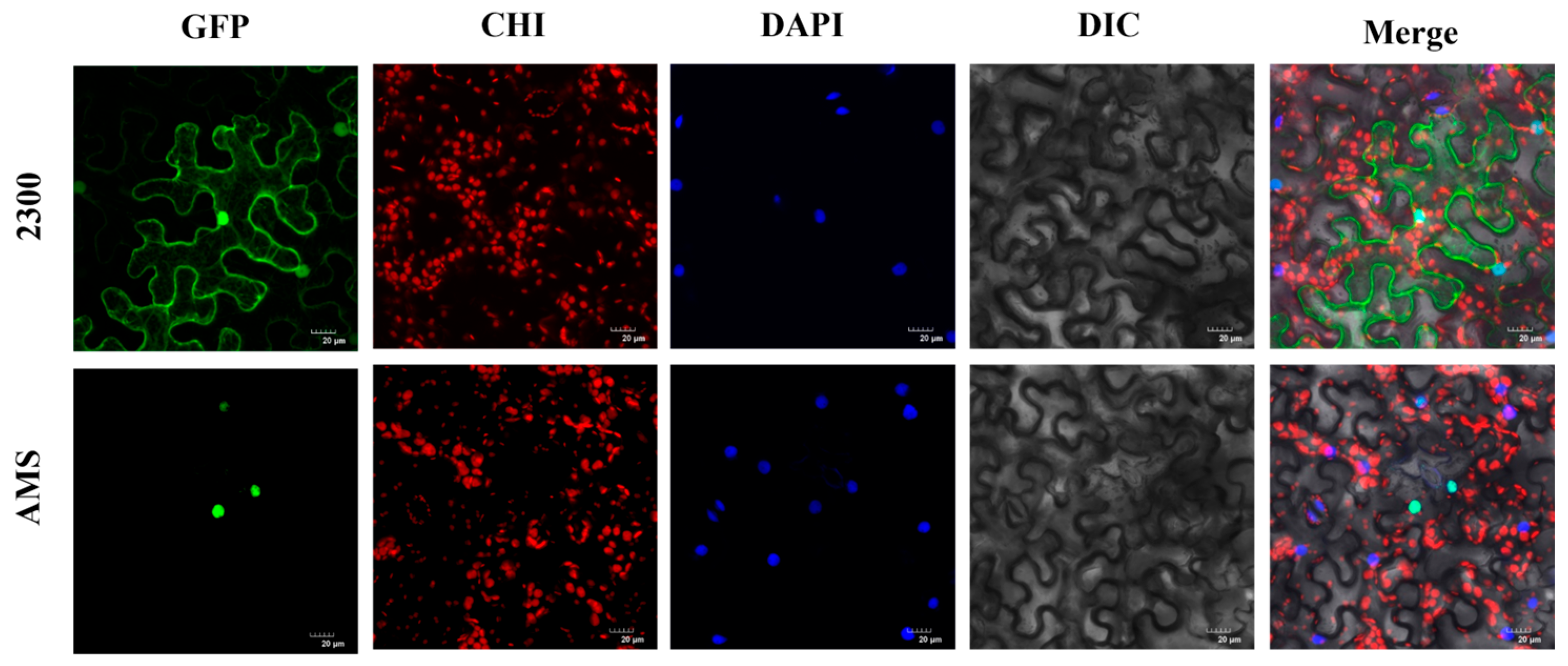
2.4. Subcellular Localization of TeAMS
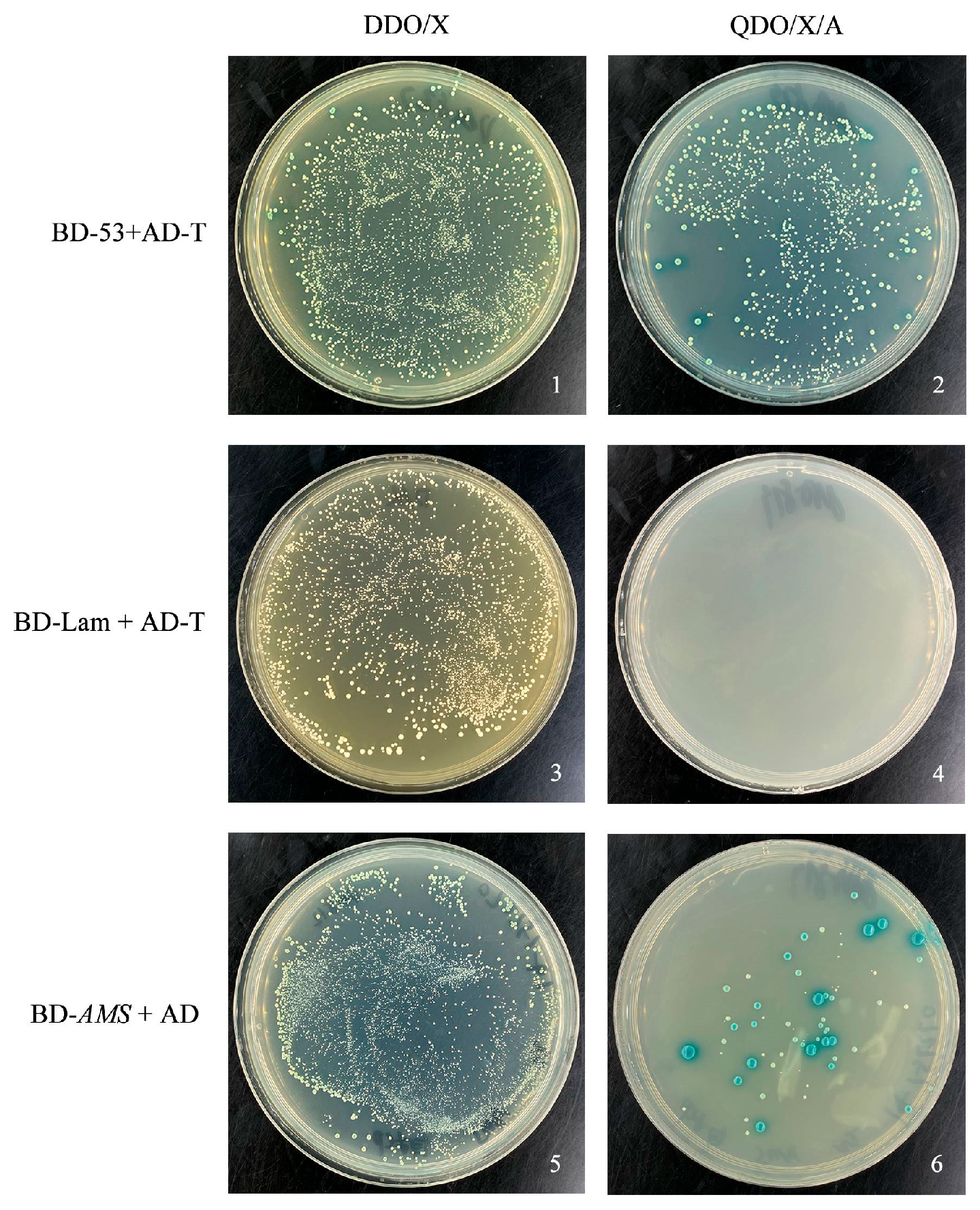
2.5. Verification of Transcription Self-Activation of TeAMS
2.6. Construction of TeAMS-sGFP-1300 Overexpression Vector
2.7. Transformation of Tobacco Using the Leaf Disc Method
2.8. Expression Analysis and Phenotypic Characterization of TeAMS-Transgenic Tobacco
2.9. Pollen Viability and Morphology of Transgenic Tobacco
2.10. Construction of the pTRV2-TeAMS VIGS Vector and Transformation of N. benthamiana
2.11. Molecular Detection of TRV2
2.12. Determination of TeAMS VIGS Expression Efficiency
2.13. Plant Phenotype, Pollen Viability, and Pollen Morphology of the Silenced N. benthamiana Plants
3. Results
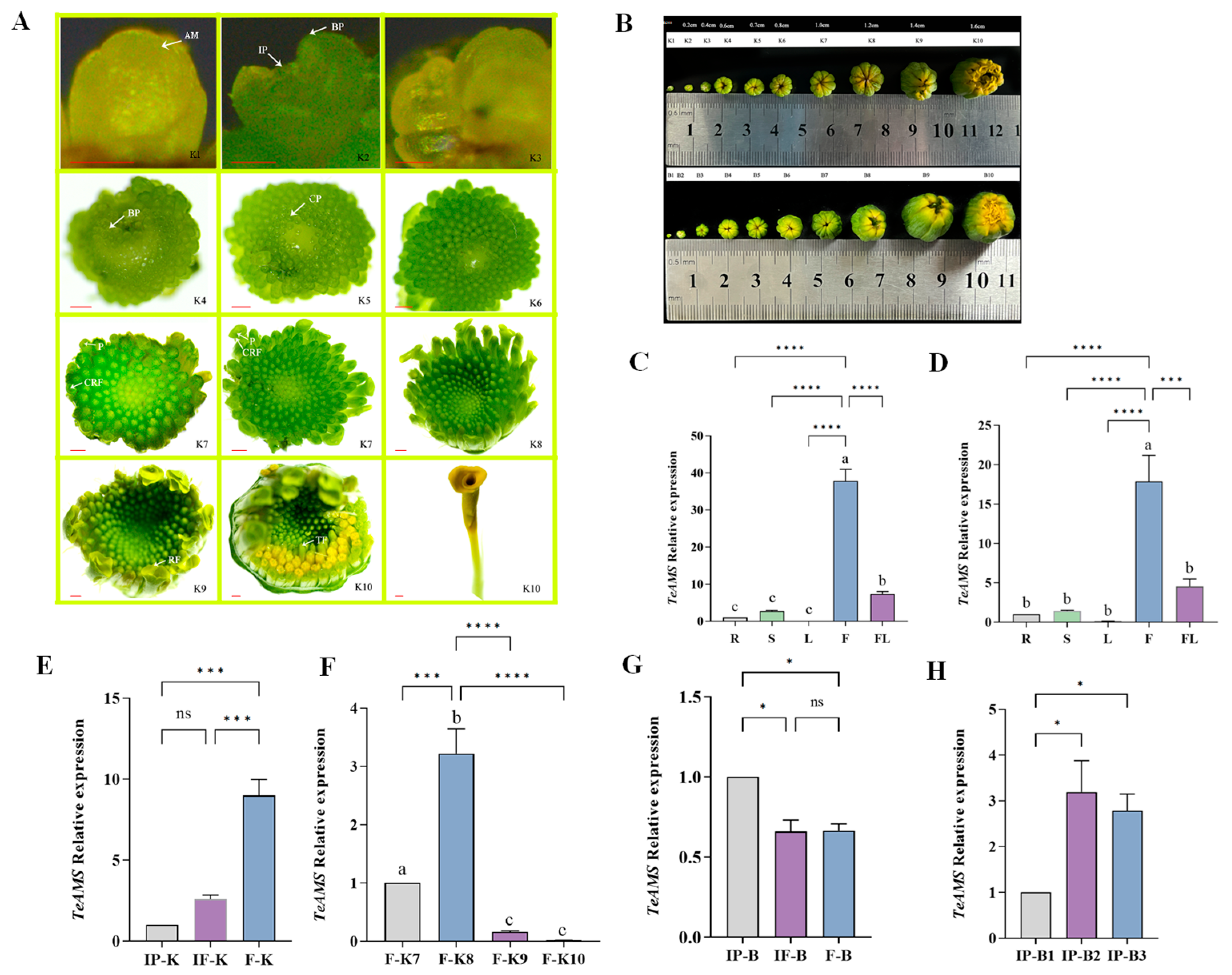
3.1. Morphological Differentiation Process and Expression Analysis of Marigold Inflorescence and Florets
3.2. TeAMS Is Localized in the Nucleus
3.3. TeAMS Possesses Self-Activation Property
3.4. Construction of the sGFP-1300-TeAMS Overexpression Vector and Transformation of Tobacco
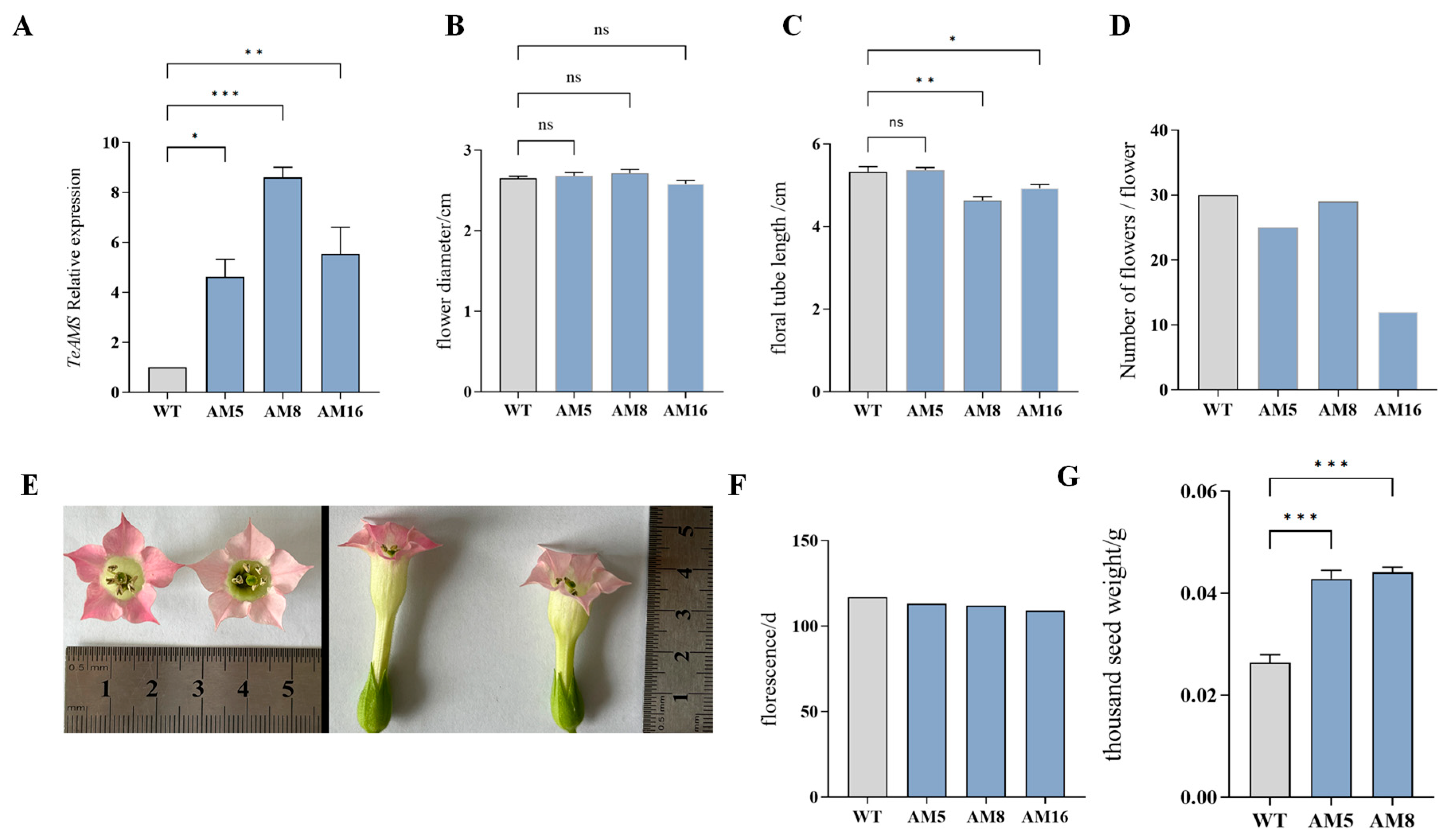
3.5. Determination of Relative Expression and Phenotypic Observation of TeAMS in Transgenic Tobacco
3.6. Pollen Viability of Transgenic Tobacco
3.7. Pollen Morphology of Transgenic Tobacco
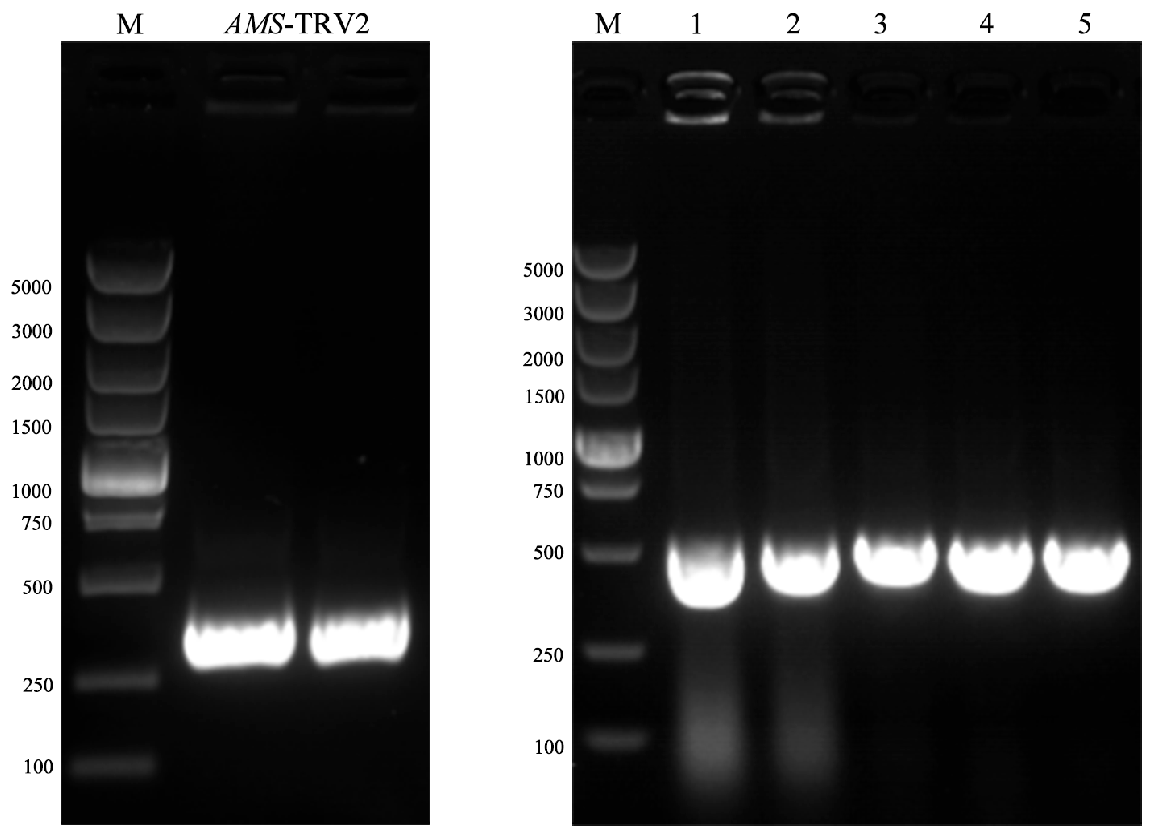
3.8. Construction of the pTRV2-TeAMS VIGS Vector
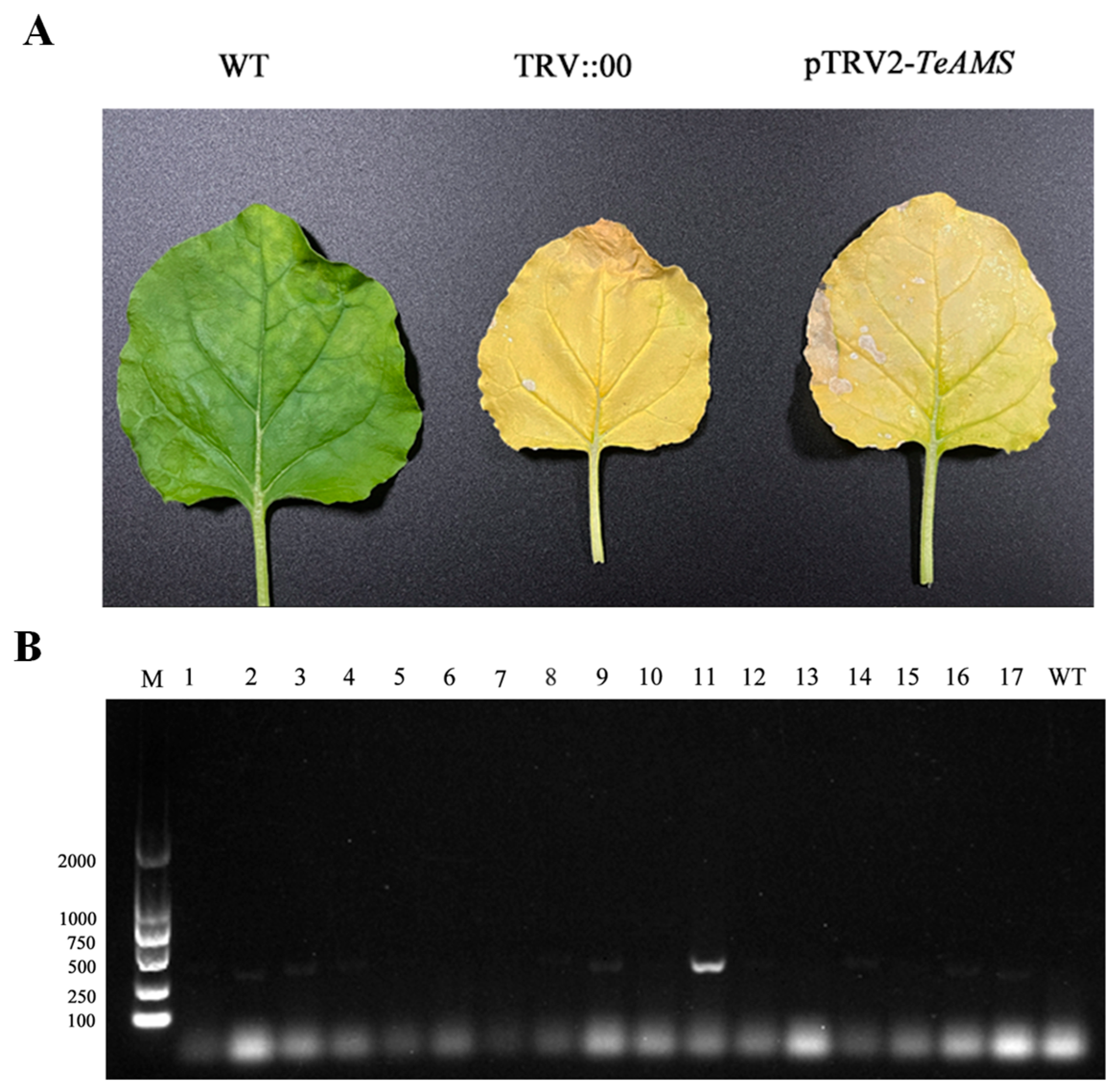
3.9. Successful Viral Infection Mediated by TRV2 Vectors
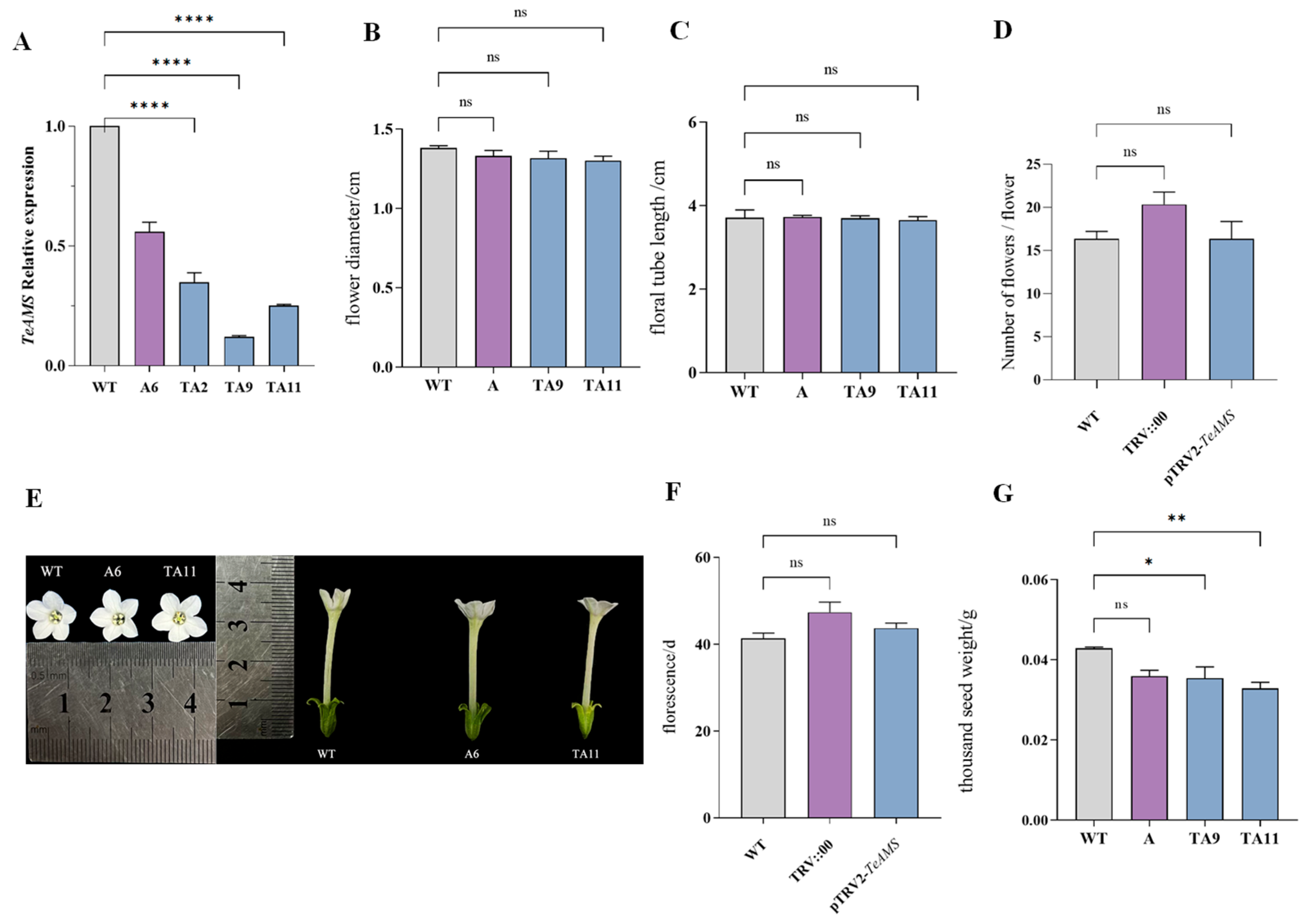
3.10. Determination of Relative Expression and Phenotypic Observation of TeAMS in Silenced Plants
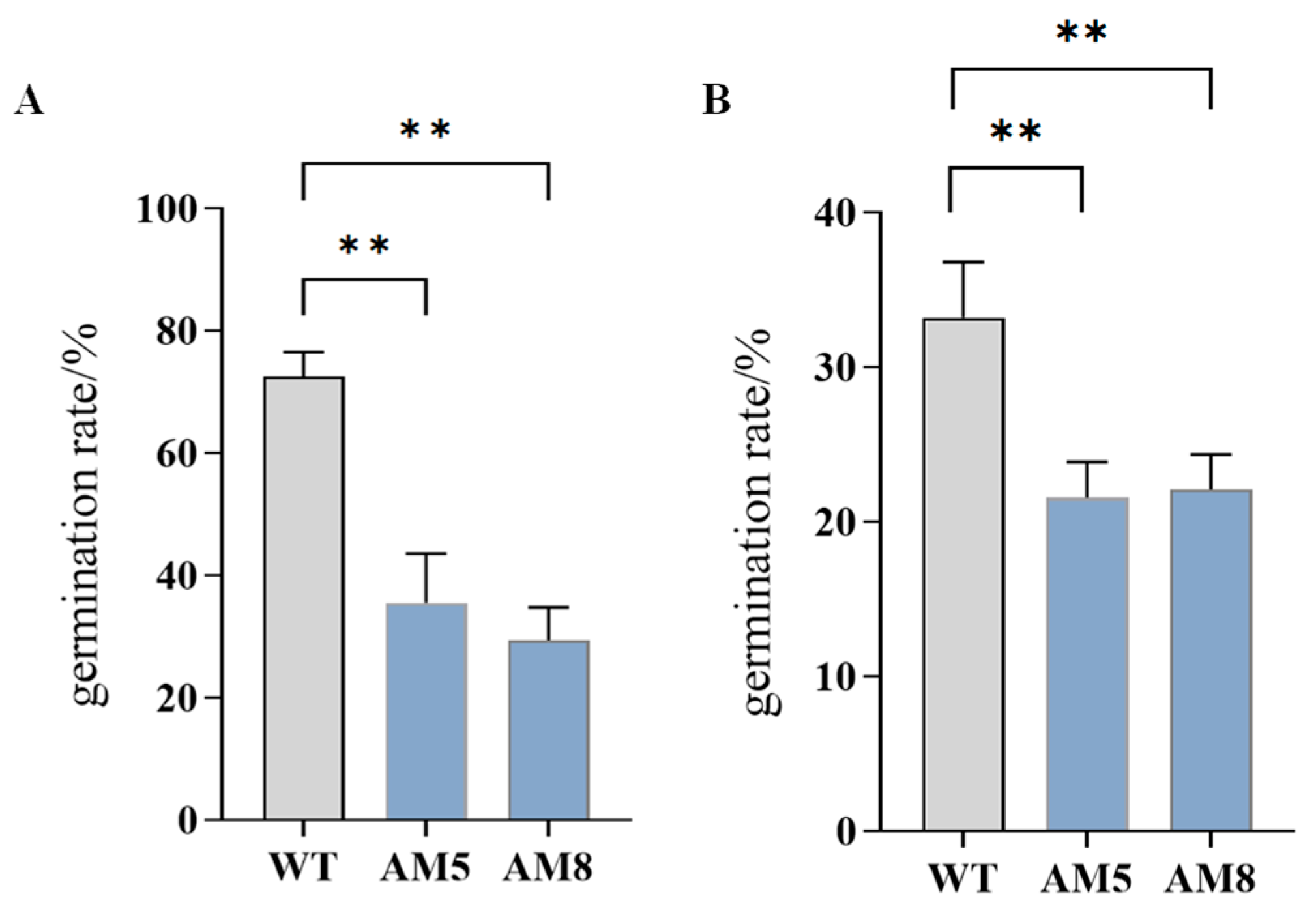
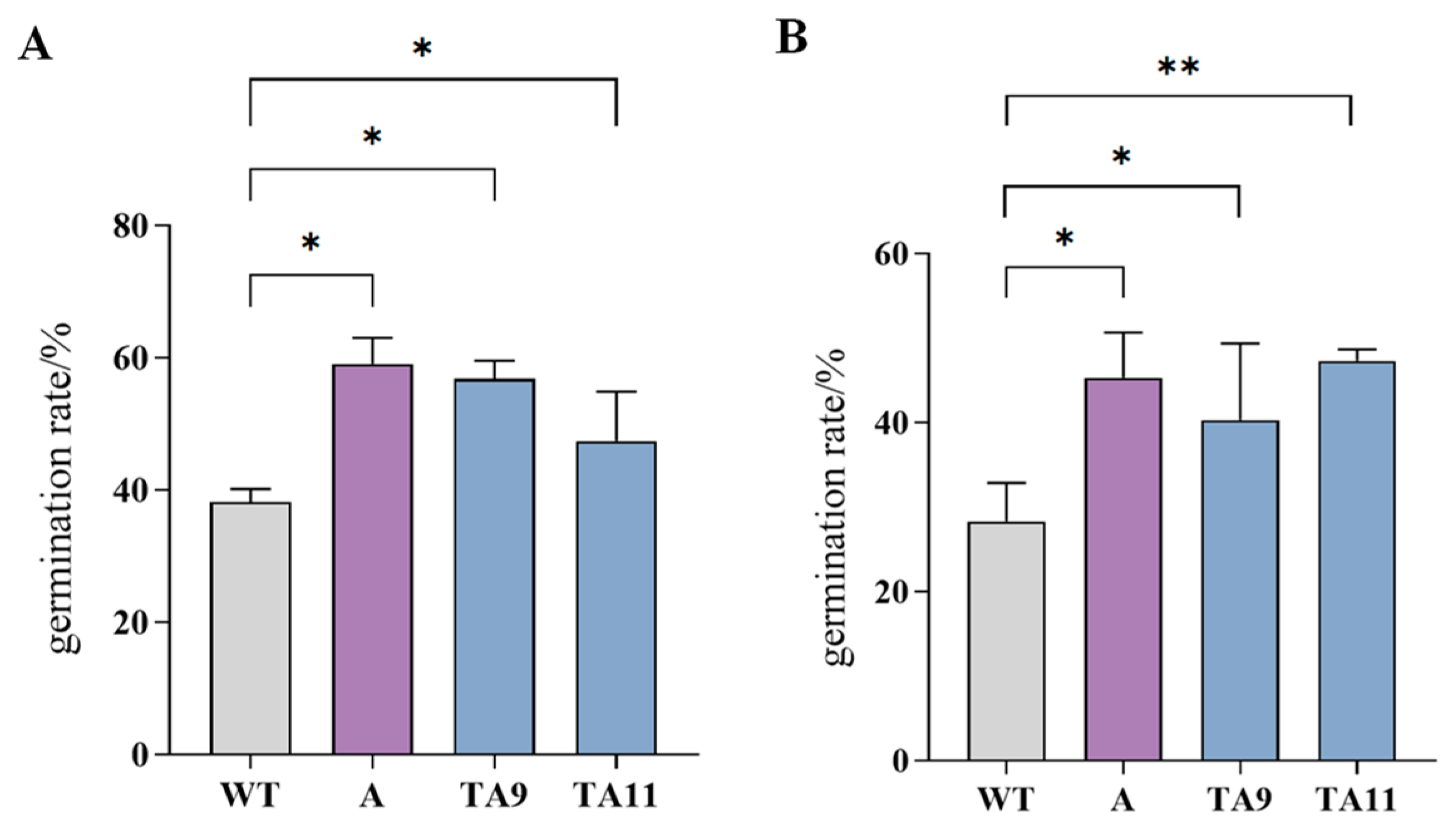
3.11. Pollen Viability of pTRV-TeAMS N. benthamiana Silenced Plants
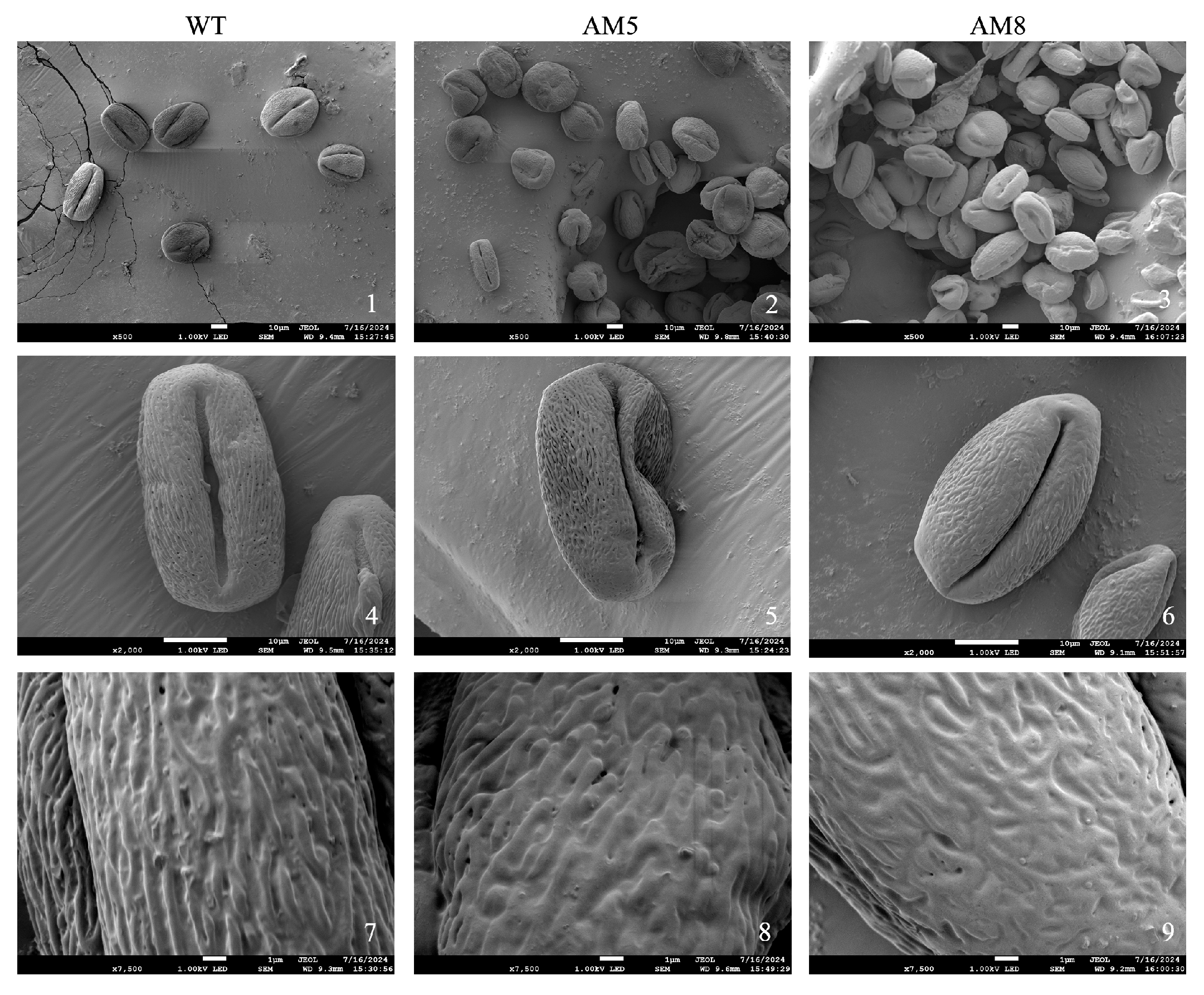
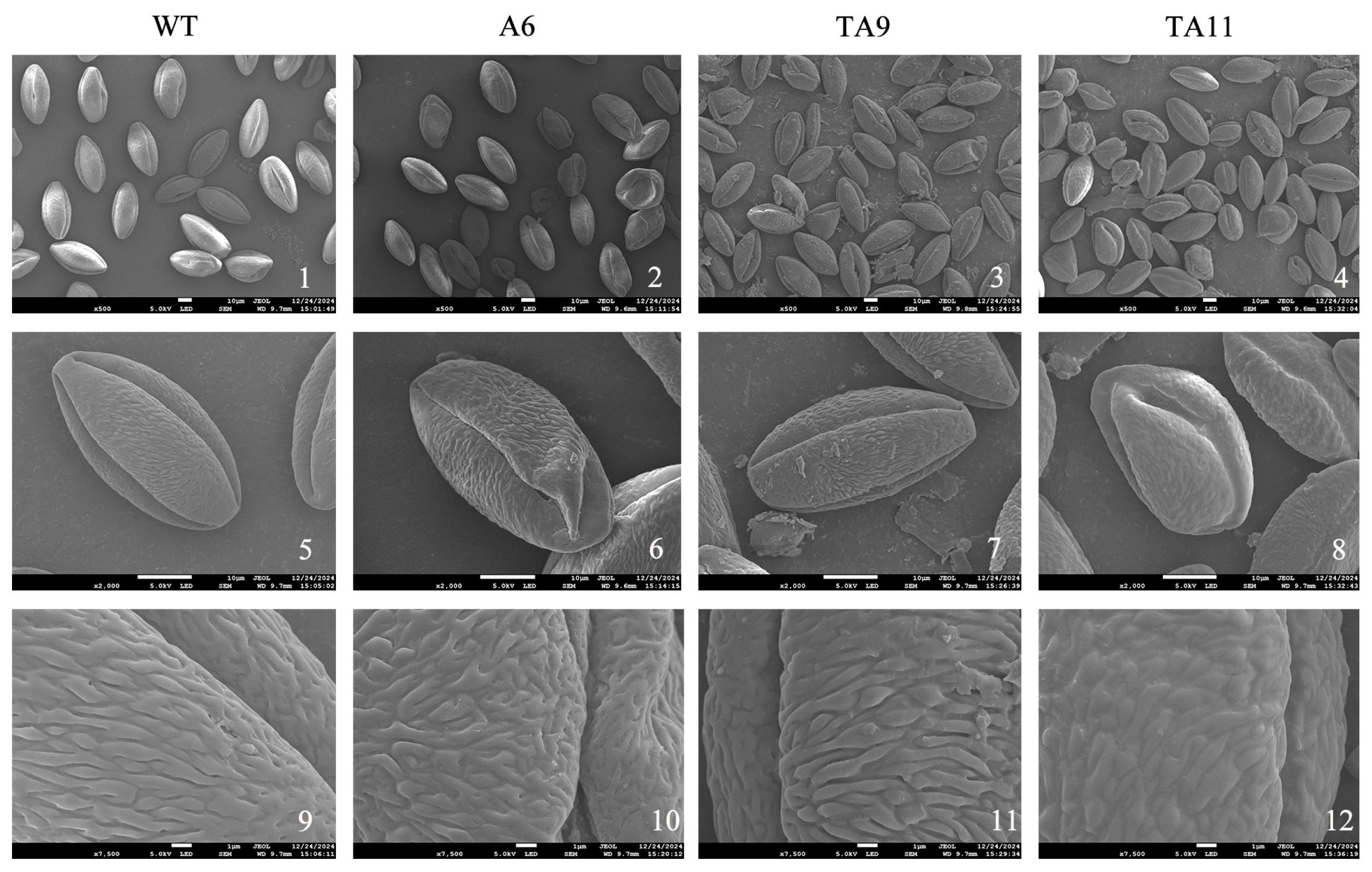
3.12. Pollen Morphology of pTRV-TeAMS N. benthamiana
4. Discussion
4.1. TeAMS Is a Key Factor in Regulating Marigold Male Fertility
4.2. Overexpression of TeAMS Influenced the Development of Pollen and Fertility of Tobacco
4.3. Accurate Regulation of Silent TeAMS Is Essential for Fertility
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Burlec, A.F.; Pecio, Ł.; Kozachok, S.; Mircea, C.; Corciovă, A.; Vereștiuc, L.; Cioancă, O.; Oleszek, W.; Hăncianu, M. Phytochemical Profile, Antioxidant Activity, and Cytotoxicity Assessment of Tagetes erecta L. Flowers. Molecules 2021, 26, 1201. [Google Scholar] [CrossRef] [PubMed]
- Zafar, J.; Aqeel, A.; Shah, F.I.; Ehsan, N.; Gohar, U.F.; Moga, M.A.; Festila, D.; Ciurea, C.; Irimie, M.; Chicea, R. Biochemical and Immunological Implications of Lutein and Zeaxanthin. Int. J. Mol. Sci. 2021, 22, 10910. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration-Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef]
- Du, M.; Zhou, K.; Liu, Y.; Deng, L.; Zhang, X.; Lin, L.; Zhou, M.; Zhao, W.; Wen, C.; Xing, J.; et al. A Biotechnology-Based Male-Sterility System for Hybrid Seed Production in Tomato. Plant J. 2020, 102, 1090–1100. [Google Scholar] [CrossRef]
- Luo, H.; Liu, Z.; Zhu, J.; Li, Z.; Qiu, X.; Wang, W.; Gao, C.; Qi, J.; Bao, M.; Liu, Y. Identification and Expression of Laccase Gene Family in Potato (Solanum tuberosum). Agronomy 2025, 15, 585. [Google Scholar] [CrossRef]
- He, Y.-H.; Ning, G.-G.; Sun, Y.-L.; Hu, Y.; Zhao, X.-Y.; Bao, M.-Z. Cytological and Mapping Analysis of a Novel Male Sterile Type Resulting from Spontaneous Floral Organ Homeotic Conversion in Marigold (Tagetes erecta L.). Mol. Breed. 2010, 26, 19–29. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Xu, X.; Tan, Y.-P.; Li, S.-Q.; Hu, J.; Huang, J.-Y.; Yang, D.-C.; Li, Y.-S.; Zhu, Y.-G. Inheritance and Molecular Mapping of Two Fertility-Restoring Loci for Honglian Gametophytic Cytoplasmic Male Sterility in Rice (Oryza sativa L.). Mol. Genet. Genom. 2004, 271, 586–594. [Google Scholar] [CrossRef]
- Sumalatha, A. Genetic Inheritance and Identification of Molecular Markers Linked to Male Sterility in African Marigold (Tagetes erecta L.). arXiv 2025. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, D.; Wang, P.; Zhao, J.; Pan, B.; Zhang, H. Rapd Markers Developed for Identifying Male Sterility in Tagetes erecta. Acta Hortic. 2010, 88, 289–294. [Google Scholar] [CrossRef]
- Cholin, S. Validation of SCAR Marker Linked to Genic Male Sterility in Marigold: As a Forward Step towards Marker Assisted Breeding Programme. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 3373–3383. [Google Scholar] [CrossRef]
- Tang, N.; Liu, W.; Zhang, W.; Tang, D. Integrative Analysis of Transcriptomic and Proteomic Changes Related to Male Sterility in Tagetes erecta. Physiol. Mol. Biol. Plants 2020, 26, 2061–2074. [Google Scholar] [CrossRef]
- Sanders, P.M.; Bui, A.Q.; Weterings, K.; McIntire, K.N.; Hsu, Y.-C.; Lee, P.Y.; Truong, M.T.; Beals, T.P.; Goldberg, R.B. Anther Developmental Defects in Arabidopsis thaliana Male-Sterile Mutants. Sex. Plant Reprod. 1999, 11, 297–322. [Google Scholar] [CrossRef]
- Szymkowiak, E.J.; Sussex, I.M. What chimeras can tell us about plant development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 351–376. [Google Scholar] [CrossRef]
- Berger, F.; Haseloff, J.; Schiefelbein, J.; Dolan, L. Positional Information in Root Epidermis Is Defined during Embryogenesis and Acts in Domains with Strict Boundaries. Curr. Biol. 1998, 8, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, A.; Kröber, S.; Unte, U.S.; Huijser, P.; Dekker, K.; Saedler, H. The Arabidopsis ABORTED MICROSPORES (AMS) Gene Encodes a MYC Class Transcription Factor. Plant J. 2003, 33, 413–423. [Google Scholar] [CrossRef]
- Xu, J.; Ding, Z.; Vizcay-Barrena, G.; Shi, J.; Liang, W.; Yuan, Z.; Werck-Reichhart, D.; Schreiber, L.; Wilson, Z.A.; Zhang, D. ABORTED MICROSPORES Acts as a Master Regulator of Pollen Wall Formation in Arabidopsis. Plant Cell 2014, 26, 1544–1556. [Google Scholar] [CrossRef]
- Guo, J.; Liu, C.; Wang, P.; Cheng, Q.; Sun, L.; Yang, W.; Shen, H. The Aborted Microspores (AMS)-like Gene Is Required for Anther and Microspore Development in Pepper (Capsicum annuum L.). Int. J. Mol. Sci. 2018, 19, 1341. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Ding, Y.; Yang, F.; Zhang, J.; Xie, J.; Zhao, C.; Du, K.; Zeng, Y.; Zhao, K.; Li, Z.; et al. Gene Silencing, Knockout and over-Expression of a Transcription Factor ABORTED MICROSPORES (SlAMS) Strongly Affects Pollen Viability in Tomato (Solanum lycopersicum). BMC Genom. 2022, 23, 346. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, W.; Chen, L.; Wang, Y.; Tang, D. Reference Gene Selection for Real-Time Quantitative Reverse-Transcription Polymerase Chain Reaction in Flower Buds of Marigold. J. Am. Soc. Hortic. Sci. 2021, 146, 363–373. [Google Scholar] [CrossRef]
- Weigel, D.; Glazebrook, J. Transformation of Agrobacterium Using the Freeze-Thaw Method. CSH Protoc. 2006, 2006, pdb.prot4666. [Google Scholar] [CrossRef]
- Gietz, R.D.; Woods, R.A. Yeast Transformation by the LiAc/SS Carrier DNA/PEG Method. In Yeast Protocol; Xiao, W., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 107–120. ISBN 978-1-59259-958-5. [Google Scholar]
- Sevilleno, S.S.; An, H.R.; Cabahug-Braza, R.A.M.; Ahn, Y.-J.; Hwang, Y.-J. Cytogenetic Study and Pollen Viability of Phalaenopsis Queen Beer ‘Mantefon’. Plants 2023, 12, 2828. [Google Scholar] [CrossRef]
- Bouatta, A.M.; Anzenberger, F.; Riederauer, L.; Lepper, A.; Denninger, P. Polarized Subcellular Activation of Rho Proteins by Specific ROPGEFs Drives Pollen Germination in Arabidopsis thaliana. PLoS Biol. 2025, 23, e3003139. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Sun, H.; Luo, H.; Zhao, L.; Dong, Z.; Yan, S.; Zhao, C.; Liu, R.; Xu, C.; et al. IRREGULAR POLLEN EXINE1 Is a Novel Factor in Anther Cuticle and Pollen Exine Formation. Plant Physiol. 2016, 173, 307–325. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, Y.; Jiao, S.; Jin, Y.; Ji, P.; Luan, F. Mapping and Preliminary Analysis of ABORTED MICROSPORES (AMS) as the Candidate Gene Underlying the Male Sterility (MS-5) Mutant in Melon (Cucumis melo L.). Front. Plant Sci. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Zhou, H.-S.; Han, Y.; Zeng, Q.-Y.; Zhu, J.; Yang, Z.-N. Positive Regulation of AMS by TDF1 and the Formation of a TDF1–AMS Complex Are Required for Anther Development in Arabidopsis thaliana. New Phytol. 2018, 217, 378–391. [Google Scholar] [CrossRef]
- Thorstensen, T.; Grini, P.E.; Mercy, I.S.; Alm, V.; Erdal, S.; Aasland, R.; Aalen, R.B. The Arabidopsis SET-Domain Protein ASHR3 Is Involved in Stamen Development and Interacts with the bHLH Transcription Factor ABORTED MICROSPORES (AMS). Plant Mol. Biol. 2008, 66, 47–59. [Google Scholar] [CrossRef]
- Lou, Y.; Xu, X.-F.; Zhu, J.; Gu, J.-N.; Blackmore, S.; Yang, Z.-N. The Tapetal AHL Family Protein TEK Determines Nexine Formation in the Pollen Wall. Nat. Commun. 2014, 5, 3855. [Google Scholar] [CrossRef]
- Chen, X.; Yang, S.; Zhang, Y.; Zhu, X.; Yang, X.; Zhang, C.; Li, H.; Feng, X. Generation of Male-Sterile Soybean Lines with the CRISPR/Cas9 System. Crop J. 2021, 9, 1270–1277. [Google Scholar] [CrossRef]
- Xu, J.; Yang, C.; Yuan, Z.; Zhang, D.; Gondwe, M.Y.; Ding, Z.; Liang, W.; Zhang, D.; Wilson, Z.A. The ABORTED MICROSPORES Regulatory Network Is Required for Postmeiotic Male Reproductive Development in Arabidopsis thaliana. Plant Cell 2010, 22, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.C.; Pearce, S.; Band, L.R.; Yang, C.; Ferjentsikova, I.; King, J.; Yuan, Z.; Zhang, D.; Wilson, Z.A. Biphasic Regulation of the Transcription Factor ABORTED MICROSPORES (AMS) Is Essential for Tapetum and Pollen Development in Arabidopsis. New Phytol. 2017, 213, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Seol, Y.-J.; Oh, J.-H.; Bae, S.-H.; Lee, J.; Jeong, H.-W.; Ahn, I.P.; Kim, E.; Kim, S.M.; Park, J. CRISPR/Cas9-Mediated Knockout of Transcription Factor MS1-like and AMS-like Genes Causes Male Sterility in Tomatoes. Plant Biotechnol. Rep. 2025, 19, 301–310. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Tian, J.; Tang, D.; Liang, Q.; Tang, N. Expression and Functional Analysis of the ABORTED MICROSPORES (AMS) Gene in Marigold (Tagetes erecta L.). Agronomy 2025, 15, 2058. https://doi.org/10.3390/agronomy15092058
Ma X, Tian J, Tang D, Liang Q, Tang N. Expression and Functional Analysis of the ABORTED MICROSPORES (AMS) Gene in Marigold (Tagetes erecta L.). Agronomy. 2025; 15(9):2058. https://doi.org/10.3390/agronomy15092058
Chicago/Turabian StyleMa, Xuejing, Jinhua Tian, Daocheng Tang, Qiuyue Liang, and Nan Tang. 2025. "Expression and Functional Analysis of the ABORTED MICROSPORES (AMS) Gene in Marigold (Tagetes erecta L.)" Agronomy 15, no. 9: 2058. https://doi.org/10.3390/agronomy15092058
APA StyleMa, X., Tian, J., Tang, D., Liang, Q., & Tang, N. (2025). Expression and Functional Analysis of the ABORTED MICROSPORES (AMS) Gene in Marigold (Tagetes erecta L.). Agronomy, 15(9), 2058. https://doi.org/10.3390/agronomy15092058




