Intercropping Green Manure Species with Tea Plants Enhances Soil Fertility and Enzyme Activity and Improves Microbial Community Structure and Diversity in Tea Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Materials
2.3. Data Collection
2.3.1. Measurement of Tea Yield and Agronomic Characteristics
2.3.2. Measurement of Soil Physicochemical Properties
2.3.3. Measurement of Soil Enzyme Activities
2.3.4. Measurement of Soil Microbial Community Structure
2.4. Data Processing
3. Results and Analysis
3.1. Effects of Green Manure Intercropping on Tea Yield and Agronomic Traits
3.2. Effects of Green Manure Intercropping with Tea Plants on Soil Chemical Properties and Enzyme Activities
3.2.1. Chemical Properties of Tea Plantation Soil
3.2.2. Enzyme Activities in Tea Plantation Soil
3.3. Effects of Intercropping Tea Plants with Green Manure on the Soil Bacterial and Fungal Community Structure in Tea Plantations
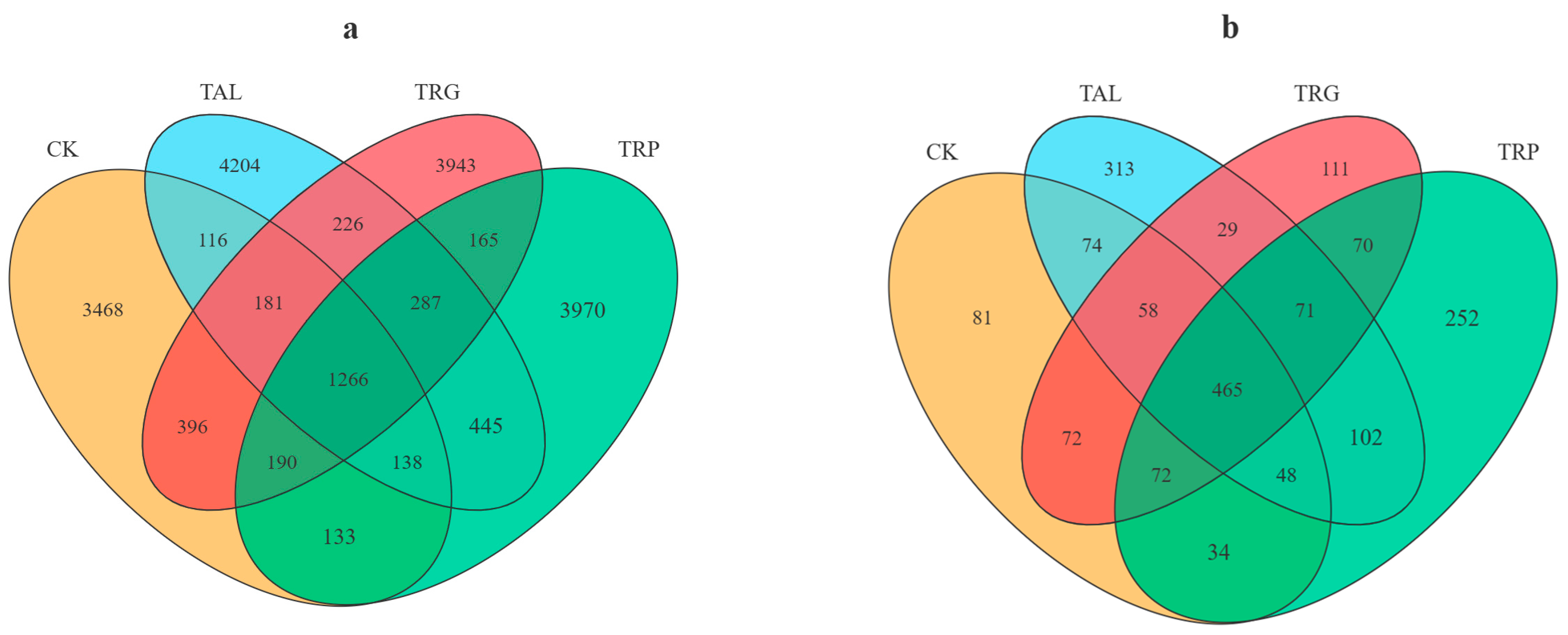
3.3.1. Composition of Soil Bacterial and Fungal Communities
3.3.2. Soil Bacterial and Fungal Community Diversity
3.4. Correlation Analysis Between Soil Environmental Factors and the Structures of Soil Bacterial and Fungal Communities
3.5. Microbial Metabolic Functions
4. Discussion
4.1. The Effect of Green Manure Intercropping on Tea Yield
4.2. The Regulatory Mechanisms of Green Manure on the Physicochemical Properties of Tea Garden Soil
4.3. The Promotive Effect of Green Manure on Soil Enzyme Activities
4.4. The Effects of Green Manure on Soil Microbial Community Structure and Diversity
4.5. Linkages Between Soil Physicochemical Properties, Microbial Community Structure, and Predicted Microbial Functions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ye, J.H.; Wang, Y.H.; Kang, J.Q.; Chen, Y.L.; Hong, L.; Li, M.J.; Jia, Y.; Wang, Y.C.; Jia, X.L.; Wu, Z.Y.; et al. Effects of long-term use of organic fertilizer with different dosages on soil improvement, nitrogen transformation, tea yield and quality in acidified tea plantations. Plants 2022, 12, 122. [Google Scholar] [CrossRef]
- Ye, J.H.; Wang, Y.H.; Wang, Y.C.; Hong, L.; Jia, X.; Kang, J.Q.; Lin, S.X.; Wu, Z.Y.; Wang, H.B. Improvement of soil acidification in tea plantations by long-term use of organic fertilizers and its effect on tea yield and quality. Front. Plant Sci. 2022, 13, 1055900. [Google Scholar] [CrossRef]
- Lei Yu Ding, D.; Duan, J.H.; Luo, Y.; Huang, F.H.; Kang, Y.K.; Chen, Y.Y.; Li, S.J. Soil microbial community characteristics and their effect on tea quality under different fertilization treatments in two tea plantations. Genes 2024, 15, 610. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Bernas, J.; Konvalina, P. Testing Biochar’s Ability to Moderate Extremely Acidic Soils in Tea-Growing Areas. Agronomy 2024, 14, 533. [Google Scholar] [CrossRef]
- Nakhli, S.A.A.; Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Application of zeolites for sustainable agriculture: A review on water and nutrient retention. Water Air Soil Pollut. 2017, 228, 464. [Google Scholar] [CrossRef]
- Das, S.; Jeong, S.T.; Das, S.; Kim, P.J. Composted cattle manure increases microbial activity and soil fertility more than composted swine manure in a submerged rice paddy. Front. Microbiol. 2017, 8, 1702. [Google Scholar] [CrossRef]
- Lei, B.N.; Juan, W.; Yao, H.Y. Ecological and environmental benefits of planting green manure in paddy fields. Agriculture 2022, 12, 223. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.L.; Zhao, Y.Y.; Hong, Z.S. Effects of green manuring on chemical characteristics and microecology of tobacco-growing soil in central henan. BMC Microbiol. 2025, 25, 42. [Google Scholar] [CrossRef]
- Pu, J.Y.; Li, Z.Y.; Tang, H.Q.; Zhou, G.P.; Wei, C.H.; Dong, W.B.; Jing, Z.J.; He, T.G. Response of soil microbial communities and rice yield to nitrogen reduction with green manure application in karst paddy areas. Front. Microbiol. 2023, 13, 1070876. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, J.; Bonet-Garcia, N.; Villagrasa, E.; Solé, A.; Liao, X.; Palet, C. Insights of microorganisms role in rice and rapeseed wastes as potential sorbents for metal removal. Int. J. Environ. Sci. Technol. 2023, 20, 801–814. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.Q.; Sun, S.G.; Liu, W.T.; Zhu, L.; Yan, X.B. Different forms and proportions of exogenous nitrogen promote the growth of alfalfa by increasing soil enzyme activity. Plants 2022, 11, 1057. [Google Scholar] [CrossRef]
- Carlos, F.S.; Schaffer, N.; Mariot, R.F.; Fernandes, R.S.; Boechat, C.L.; Roesch, L.F.W.; de Oliveira Camargo, F.A. Soybean crop incorporation in irrigated rice cultivation improves nitrogen availability, soil microbial diversity and activity, and growth of ryegrass. Appl. Soil Ecol. 2022, 170, 104313. [Google Scholar] [CrossRef]
- Timofeeva, T.A.; Chebotar, V.K.; Demidov, D.V.; Gaidukova, S.E.; Yakovleva, I.V.; Kamionskaya, A.M. Effects of apatite concentrate in combination with phosphate-solubilizing microorganisms on the yield of ryegrass cultivar Izorskiy. Agronomy 2023, 13, 1568. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Lock, S.S.M.; Yap, P.S.; Cheah, K.W.; Chan, Y.H.; Yiin, C.L.; Chai, Y.H. Immobilized enzyme/microorganism complexes for degradation of microplastics: A review of recent advances, feasibility and future prospects. Sci. Total Environ. 2022, 832, 154868. [Google Scholar] [CrossRef]
- Akbay, F.; Günaydin, T.; Arikan, S.; Kizilsimsek, M. Performance of new lactic acid bacteria strains as inoculants on the microorganism composition during fermentation of alfalfa silage containing different dry matter content. Black Sea J. Agric. 2023, 6, 402–410. [Google Scholar] [CrossRef]
- Jibola-Shittu, M.Y.; Heng, Z.; Keyhani, N.O.; Dang, Y.; Chen, R.; Liu, S.; Qiu, J. Understanding and exploring the diversity of soil microorganisms in tea (Camellia sinensis) gardens: Toward sustainable tea production. Front. Microbiol. 2024, 15, 1379879. [Google Scholar] [CrossRef]
- Bai, Y.C.; Li, B.X.; Xu, C.Y.; Raza, M.; Wang, Q.; Wang, Q.Z.; Xu, Y.J. Intercropping walnut and tea: Effects on soil nutrients, enzyme activity, and microbial communities. Front. Microbiol. 2022, 13, 852342. [Google Scholar] [CrossRef]
- Kjeldgaard, B.; Neves, A.R.; Fonseca, C.; Kovács, Á.T.; Domínguez-Cuevas, P.; Atanasova, L. Quantitative high-throughput screening methods designed for identification of bacterial biocontrol strains with antifungal properties. Microbiol. Spectr. 2022, 10, e0143321. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Wei, K.; Zhao, J.; Sun, Y.; López, I.F.; Ma, C.; Zhang, Q. Optimizing nitrogen and phosphorus application to improve soil organic carbon and alfalfa hay yield in alfalfa fields. Front. Plant Sci. 2024, 14, 1276580. [Google Scholar] [CrossRef]
- Brambilla, S.; Liebrenz, K.; Odorizzi, A.; Arolfo, V.; Maguire, V.; Moreno, V.; Ruiz, O.; Stritzler, M.; Serantes, L.; Soto, G.; et al. Exploring the cooperation between nitrogen-fixing and non-fixing alfalfa rhizobia. Symbiosis 2024, 93, 281–284. [Google Scholar] [CrossRef]
- Güler, B.; Gürel, A. A research on the production, storage and germination of synthetic seeds in tea plant (Camellia sinensis [L.] O. Kuntze). J. Sci. Rep. A 2023, 57, 68–80. [Google Scholar] [CrossRef]
- El Idrissi, A.; Tayi, F.; Dardari, O.; Essamlali, Y.; Jioui, I.; Ayouch, I.; Zahouily, M. Urea-rich sodium alginate-based hydrogel fertilizer as a water reservoir and slow-release N carrier for tomato cultivation under different water-deficit levels. Int. J. Biol. Macromol. 2024, 272, 132814. [Google Scholar] [CrossRef]
- Jia, Q.; Zheng, H.; Shi, Z.; Liu, X.; Sun, D.; Zhang, J. Effects of Straw and Green Manure Addition on Crop Yield, Soil Properties and CH4 Emissions: A Meta-Analysis. Agronomy 2024, 14, 2724. [Google Scholar] [CrossRef]
- Wei, K.; Liu, M.; Shi, Y.; Zhang, H.; Ruan, J.; Zhang, Q.; Cao, M. Metabolomics reveal that the high application of phosphorus and potassium in tea plantation inhibited amino-acid accumulation but promoted metabolism of flavonoid. Agronomy 2022, 12, 1086. [Google Scholar] [CrossRef]
- Ahmed, L.Q.; Escobar-Gutiérrez, A.J. Unexpected intraspecific variability of perennial ryegrass (Lolium perenne L.) in response to constant temperature during germination and initial heterotrophic growth. Front. Plant Sci. 2022, 13, 856099. [Google Scholar] [CrossRef]
- Liang, K.; Wang, X.; Du, Y.; Li, G.; Wei, Y.; Liu, Y.; Li, Z.; Wei, X. Effect of legume green manure on yield increases of three major crops in china: A Meta-Analysis. Agronomy 2022, 12, 1753. [Google Scholar] [CrossRef]
- Qu, J.; Li, L.; Zhao, P.; Han, D.; Zhao, X.; Zhang, Y.; Han, L.; Wang, Y. Impact of phosphorous fertilization on rape and common vetch intercropped fodder and soil phosphorus dynamics in North China. Agriculture 2022, 12, 1949. [Google Scholar] [CrossRef]
- Schimicoscki, R.S.; Oliveira, K.D.; Mendes, G.d.O.; Neto, C.N.d.Á. Production of Potassium Fertilizer from Verdete: A Review. J. Eng. Exact Sci. 2025, 11, 21569. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.G. Green manure improves humification and aggregate stability in paddy soils. Soil Biol. Biochem. 2025, 206, 109796. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, Z.; Zhang, G.; Chen, D.; Zang, L.; Liu, Q.; Guo, Y.; Xie, P.; Chen, H.; He, Y. Variability of soil enzyme activities and nutrients with forest gap renewal interacting with soil depths in degraded karst forests. Ecol. Indic. 2024, 166, 112332. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, H.; Lu, J.; Liang, Y.; Li, Y.; Xu, L.; Ye, J.; Yang, R.; Li, P.; Jiao, J.; et al. Green manuring with balanced fertilization improves soil ecosystem multifunctionality by enhancing soil quality and enzyme activities in a smooth vetch-maize rotation system. Agric. Ecosyst. Environ. 2025, 387, 109632. [Google Scholar] [CrossRef]
- Wang, L.; Luo, N.; Shi, Q.; Sheng, M. Responses of soil labile organic carbon fractions and enzyme activities to long-term vegetation restorations in the karst ecosystems, Southwest China. Ecol. Eng. 2023, 194, 107034. [Google Scholar] [CrossRef]
- Hu, A.; Huang, R.; Liu, G.D.; Huang, D.F.; Huan, H.F. Effects of green manure combined with phosphate fertilizer on movement of soil organic carbon fractions in tropical sown pasture. Agronomy 2022, 12, 1101. [Google Scholar] [CrossRef]
- Kucerik, J.; Brtnicky, M.; Mustafa, A.; Hammerschmiedt, T.; Kintl, A.; Sobotkova, J.; Alamri, S.; Baltazar, T.; Latal, O.; Naveed, M.; et al. Utilization of diversified cover crops as green manure-enhanced soil organic carbon, nutrient transformation, microbial activity, and maize growth. Agronomy 2024, 14, 2001. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhong, L.; Liu, J.H.; Liu, X.Y.; Xue, W.; Liu, X.B.; Yan, H.; Shen, T.X.; Li, J.L.; Sun, Z.G. Systematic analysis of the effects of different green manure crop rotations on soil nutrient dynamics and bacterial community structure in the Taihu Lake Region, Jiangsu. Agriculture 2024, 14, 1017. [Google Scholar] [CrossRef]
- Godinho, O.; Devos, D.P.; Quinteira, S.; Lage, O.M. The influence of the phylum Planctomycetota in the environmental resistome. Res. Microbiol. 2024, 175, 104196. [Google Scholar] [CrossRef]
- Asghar, W.; Kataoka, R. Green manure incorporation accelerates enzyme activity, plant growth, and changes in the fungal community of soil. Arch. Microbiol. 2022, 204, 7. [Google Scholar] [CrossRef]
- Tan, Y.; Du, H.; Zhang, H.; Fang, C.; Jin, G.; Chen, S.; Wu, Q.; Zhang, Y.; Zhang, M.; Xu, Y.; et al. Geographically associated fungus-bacterium interactions contribute to the formation of geography-dependent flavor during high-complexity spontaneous fermentation. Microbiol. Spectr. 2022, 10, e0184422. [Google Scholar] [CrossRef]
- Yu, L.H.; Li, D.; Zhang, Y.F.; Wang, Y.F.; Yao, Q.; Yang, K.Y. An optimal combined slow-release nitrogen fertilizer and urea can enhance the decomposition rate of straw and the yield of maize by improving soil bacterial community and structure under full straw returning system. Front. Microbiol. 2024, 15, 1358582. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.L.; Qiu, Q.Y.; Zhou, Y.; You, W.B. Changes in soil properties and enzyme stoichiometry in three different forest types changed to tea plantations. Forests 2023, 14, 2043. [Google Scholar] [CrossRef]
- Juang, K.W.; Chen, C.P. Changes in soil organic carbon and nitrogen stocks in organic farming practice and abandoned tea plantation. Bot. Stud. 2023, 64, 28. [Google Scholar] [CrossRef]
- Embacher, J.; Zeilinger, S.; Kirchmair, M.; Rodriguez-R, L.M.; Neuhauser, S. Wood decay fungi and their bacterial interaction partners in the built environment—A systematic review on fungal bacteria interactions in dead wood and timber. Fungal Biol. Rev. 2023, 45, 100305. [Google Scholar] [CrossRef]
- Zhao, P.X.; Ma, Y.F.; Li, Z.H.; Chen, J.Y.; Guo, F.T. Responses of soil trace elements, bacterial communities, and enzymes to forest fire smoke deposition. Acta Ecol. Sin. 2025, 45, 6973–6984. [Google Scholar]
- Shi, Z.L.; Pei, W.H.; Shi, Z.F.; Pu, T.; Liu, X.; Wang, Y.; He, F.F.; Yang, P.W. Effects of increased application of decomposed cattle manure on soil microbial community diversity and function in rapeseed. J. South. Agric. 2025, 1–17. Available online: https://link.cnki.net/urlid/45.1381.S.20250716.1653.004 (accessed on 25 June 2025).
- Wang, L.M.; Huang, D.F.; He, C.M.; Liu, C.L.; Li, Q.H.; Huang, Y.B.; Wang, F. Effects of Chinese milk vetch incorporation on soil physicochemical and microbial properties of yellow clayey paddy soil and rice yield. Acta Ecol. Sin. 2023, 43, 4782–4797. [Google Scholar]
- Chen, J.X.; Chen, Z.Y.; Zhao, Y.J.; Wang-Pruski, G.; Guo, R.F. Identification, analysis, and expression of the MAM gene family members in Chinese kale. Chin. J. Appl. Environ. Biol. 2022, 28, 491–497. [Google Scholar]




| Properties | TK (g·kg−1) | TP (g·kg−1) | Ca (mg·kg−1) | Mg (mg·kg−1) | TN (g·kg−1) | TC (g·kg−1) |
|---|---|---|---|---|---|---|
| Ryegrass | 32.45 | 4.93 | 0.63 | 2.70 | 45.33 | 376.11 |
| Rapeseed | 15.09 | 4.78 | 2.29 | 3.95 | 40.64 | 389.31 |
| Alfalfa | 12.06 | 4.47 | 1.90 | 3.77 | 57.43 | 394.03 |
| Measurement Items | Determination Method |
|---|---|
| pH | pH Meter Method |
| Soil organic matter (SOM) | Potassium Dichromate Method (Concentrated H2SO4 Heating) |
| Total nitrogen (TN) | Semi-micro Kjeldahl Method |
| Total phosphorus (TP) | Molybdenum Blue Colorimetric Method |
| Total potassium (TK) | Flame Photometry |
| Available Nitrogen (AN) | Alkali-hydrolysis Diffusion Method |
| Available Phosphorus (AP) | Sodium Bicarbonate Extraction |
| Available Potassium (AK) | Ammonium Acetate Extraction Followed by Flame Photometry |
| Period | Treatment | Bud Density (Buds·m−2) | Hundred-Bud Weight (g) | Yield (kg·hm−2) |
|---|---|---|---|---|
| Spring tea | CK | 918.00 ± 15.59 b | 27.93 ± 0.70 c | 601.00 ± 51.42 c |
| TAL | 1110.00 ± 65.11 a | 32.03 ± 0.93 a | 899.17 ± 97.16 a | |
| TRG | 1128.00 ± 36.37 a | 30.27 ± 0.65 b | 784.13 ± 11.78 b | |
| TRP | 1134.00 ± 47.62 a | 31.93 ± 0.76 a | 853.43 ± 25.42 ab | |
| Summer tea | CK | 825.00 ± 27.50 b | 25.50 ± 0.72 c | 532.90 ± 32.26 c |
| TAL | 1050.00 ± 93.67 a | 28.63 ± 0.50 ab | 750.77 ± 46.57 a | |
| TRG | 1038.00 ± 63.21 a | 29.60 ± 0.61 a | 673.93 ± 23.99 b | |
| TRP | 1032.00 ± 93.67 a | 28.27 ± 0.76 b | 703.50 ± 25.06 ab | |
| Autumn tea | CK | 639.00 ± 9.00 b | 24.93 ± 1.41 b | 429.77 ± 25.39 b |
| TAL | 948.00 ± 22.65 a | 26.60 ± 0.50 a | 616.23 ± 50.67 a | |
| TRG | 882.00 ± 86.79 a | 27.63 ± 0.64 a | 601.40 ± 36.32 a | |
| TRP | 951.00 ± 68.15 a | 26.60 ± 0.20 a | 593.33 ± 23.53 a |
| Treatment | pH | SOM g·kg−1 | TN g·kg−1 | TP g·kg−1 | TK g·kg−1 | AN mg·kg−1 | AP mg·kg−1 | AK mg·kg−1 |
|---|---|---|---|---|---|---|---|---|
| CK | 4.43 ± 0.05 b | 28.05 ± 2.05 b | 2.17 ± 0.02 c | 2.12 ± 0.14 b | 4.02 ± 0.82 b | 144.02 ± 12.18 c | 99.22 ± 3.25 d | 147.88 ± 0.29 c |
| TAL | 4.52 ± 0.08 ab | 36.19 ± 0.22 a | 2.51 ± 0.02 a | 2.34 ± 0.29 ab | 5.02 ± 0.26 a | 185.62 ± 7.30 a | 179.31 ± 2.37 b | 167.30 ± 4.45 b |
| TRG | 4.50 ± 0.03 ab | 34.47 ± 1.71 a | 2.22 ± 0.04 b | 2.29 ± 0.01 ab | 5.47 ± 0.13 a | 164.17 ± 2.93 b | 119.10 ± 4.52 c | 161.02 ± 4.76 b |
| TRP | 4.51 ± 0.07 a | 34.75 ± 1.21 a | 2.23 ± 0.19 b | 2.63 ± 0.19 a | 5.92 ± 0.50 a | 170.35 ± 6.75 b | 283.88 ± 7.78 a | 176.16 ± 4.80 a |
| Treatment | Amylase mg·(g·d)−1 | Catalase (U·g−1) | Urease mg·(g·h)−1 | Invertase (U·g−1) | Acid Phosphatase (U·g−1) | Alkaline Phosphatase (U·g−1) |
|---|---|---|---|---|---|---|
| CK | 1.64 ± 0.13 b | 2.50 ± 0.38 a | 427.04 ± 98.25 b | 6.78 ± 0.50 b | 53,706.46 ± 3032.75 a | 7231.22 ± 470.17 a |
| TAL | 2.51 ± 0.88 b | 2.87 ± 0.39 a | 819.06 ± 53.86 a | 7.80 ± 0.52 b | 58,341.26 ± 3152.41 a | 7508.64 ± 190.68 a |
| TRG | 1.67 ± 0.89 b | 3.06 ± 0.08 a | 467.89 ± 50.35 b | 7.69 ± 0.24 b | 56,013.15 ± 5074.06 a | 7074.17 ± 697.94 a |
| TRP | 9.67 ± 3.45 a | 3.10 ± 0.38 a | 930.31 ± 125.37 a | 12.06 ± 0.80 a | 58,134.01 ± 2179.15 a | 7237.27 ± 406.38 a |
| Treatment | Simpson Index | Shannon Index | Chao Index | ACE Index | ||||
|---|---|---|---|---|---|---|---|---|
| Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | |
| CK | 0.9838 a | 0.9125 a | 9.07 b | 5.30 a | 5647 a | 940 b | 5915 a | 924 b |
| TAL | 0.9921 a | 0.9309 a | 10.35 a | 5.54 a | 6190 a | 1062 a | 6791 a | 1063 a |
| TRG | 0.9947 a | 0.9345 a | 9.77 ab | 5.63 a | 6230 a | 1010 ab | 6506 a | 1001 ab |
| TRP | 0.9960 a | 0.9558 a | 10.09 a | 6.07 a | 6503 a | 1106 a | 6453 a | 1079 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, Q.; Chang, P.; Zhang, J.; Li, C.; Shuang, Q.; Zhang, C.; Jiang, X. Intercropping Green Manure Species with Tea Plants Enhances Soil Fertility and Enzyme Activity and Improves Microbial Community Structure and Diversity in Tea Plantations. Agronomy 2025, 15, 2055. https://doi.org/10.3390/agronomy15092055
Wang L, Liu Q, Chang P, Zhang J, Li C, Shuang Q, Zhang C, Jiang X. Intercropping Green Manure Species with Tea Plants Enhances Soil Fertility and Enzyme Activity and Improves Microbial Community Structure and Diversity in Tea Plantations. Agronomy. 2025; 15(9):2055. https://doi.org/10.3390/agronomy15092055
Chicago/Turabian StyleWang, Lixian, Qin Liu, Peiyu Chang, Jiangen Zhang, Chen Li, Qiaoyun Shuang, Chunyun Zhang, and Xinfeng Jiang. 2025. "Intercropping Green Manure Species with Tea Plants Enhances Soil Fertility and Enzyme Activity and Improves Microbial Community Structure and Diversity in Tea Plantations" Agronomy 15, no. 9: 2055. https://doi.org/10.3390/agronomy15092055
APA StyleWang, L., Liu, Q., Chang, P., Zhang, J., Li, C., Shuang, Q., Zhang, C., & Jiang, X. (2025). Intercropping Green Manure Species with Tea Plants Enhances Soil Fertility and Enzyme Activity and Improves Microbial Community Structure and Diversity in Tea Plantations. Agronomy, 15(9), 2055. https://doi.org/10.3390/agronomy15092055






