Effects of Glucose and Its Derivatives on Growth and Nutrient Absorption in Wheat
Abstract
1. Introduction
2. Materials and Methods
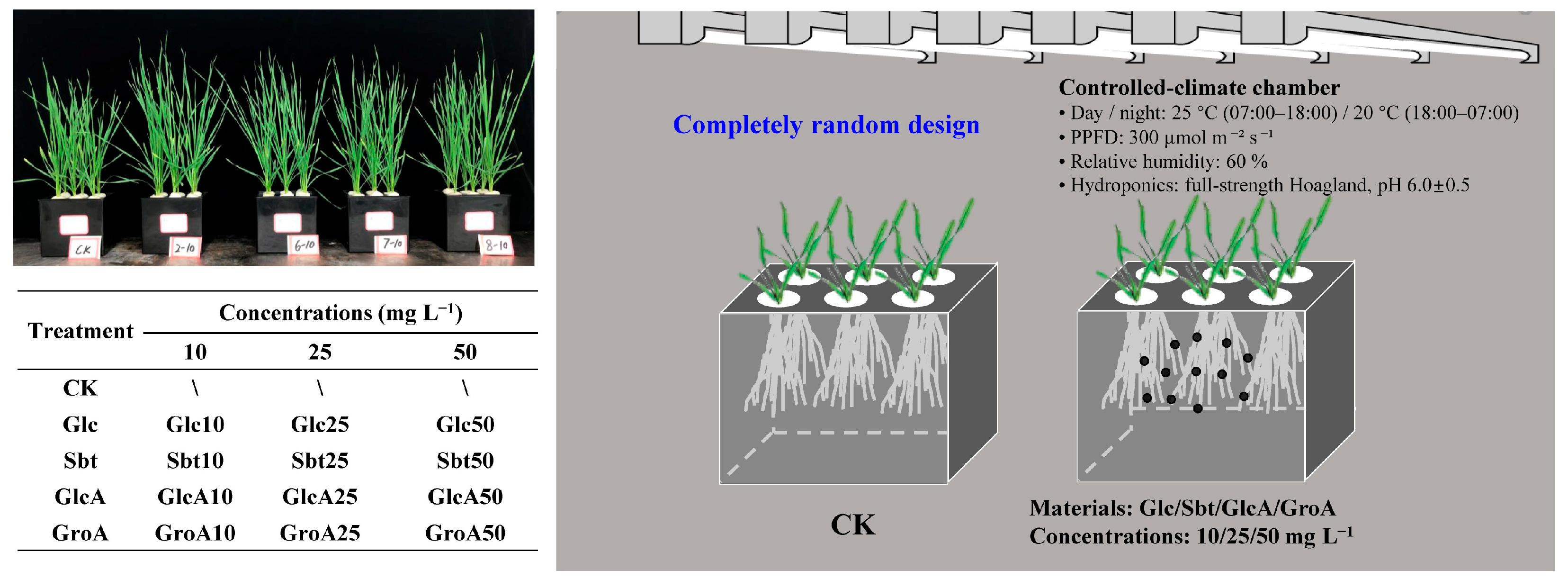
2.1. Plant Growth and Experimental Design
2.2. Laboratory Analysis
2.3. Statistical Analysis
3. Results
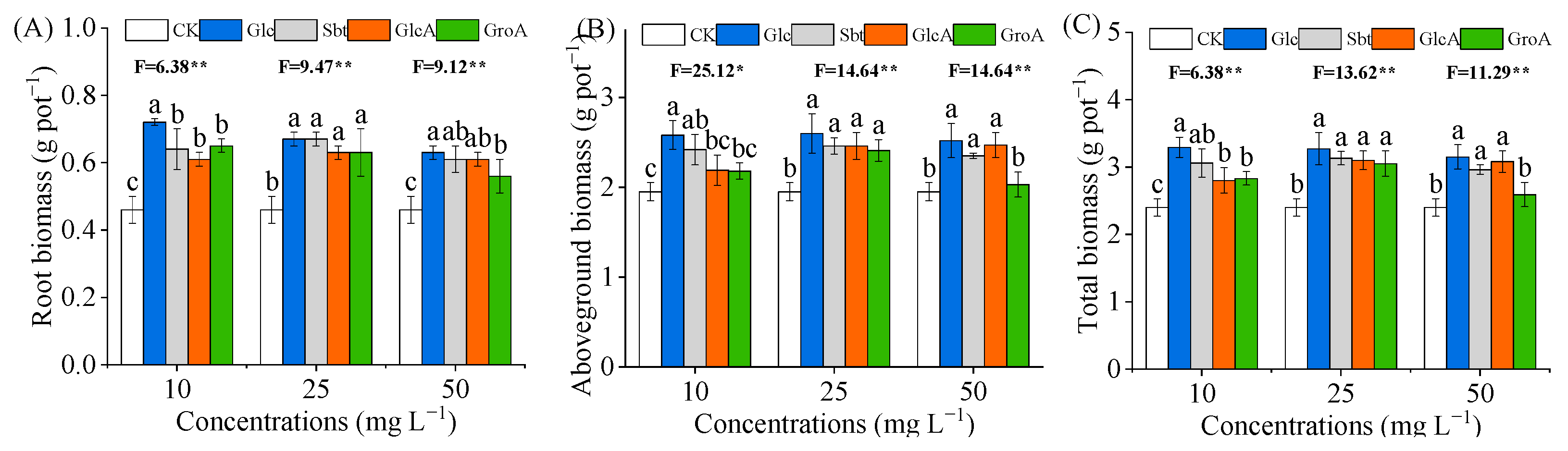
3.1. Effect of Glucose and Its Derivatives on Wheat Biomass
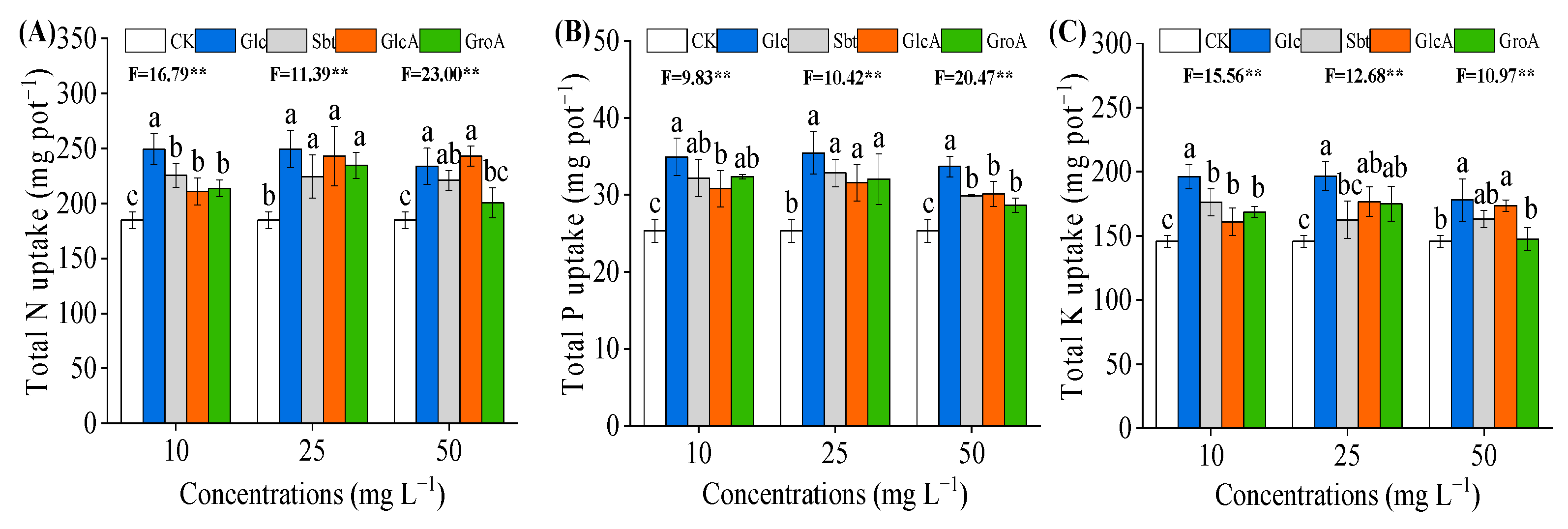
3.2. Effect of Glucose on Nutrient Uptake of Wheat Seedlings Was Greater than Its Derivatives
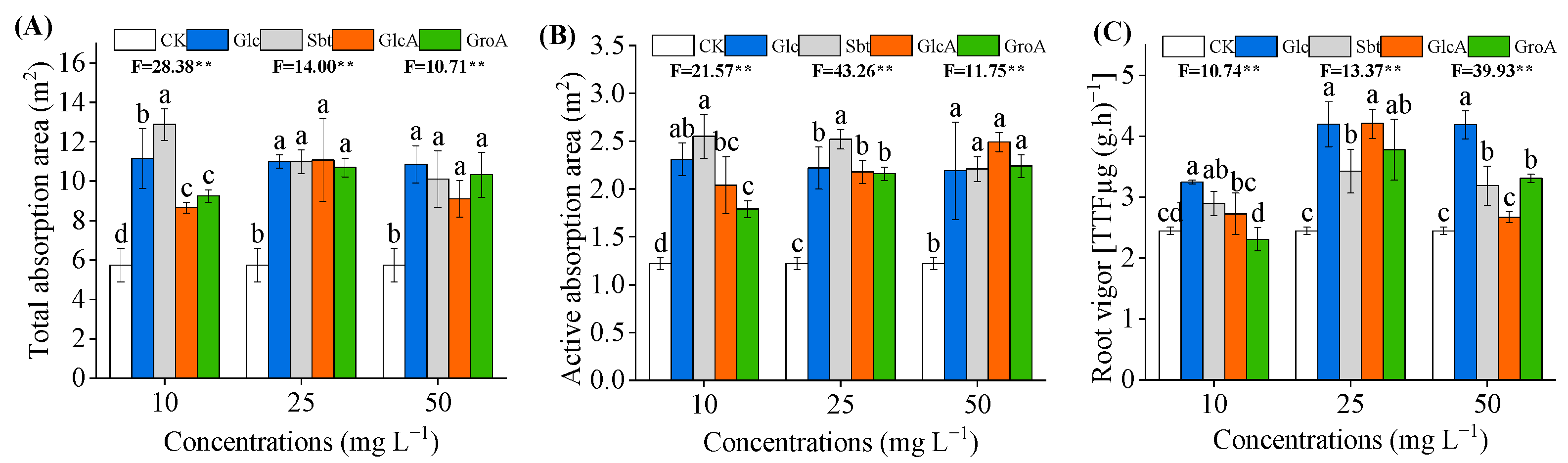
3.3. Effect of Glucose and Its Derivatives on Root Morphology and Root Activity of Wheat
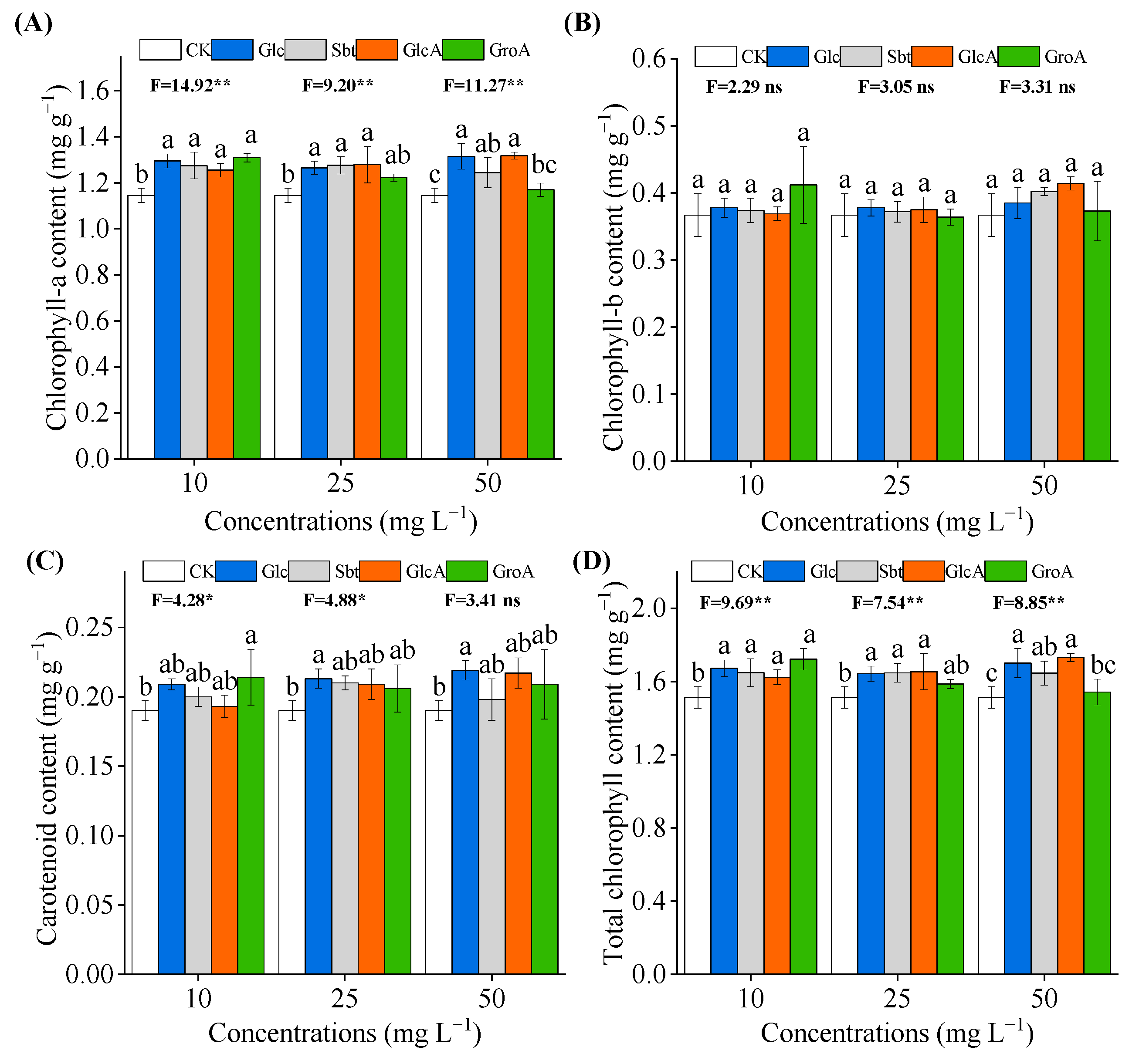
3.4. Effect of Glucose and Its Derivatives on Physiological Indicators of Wheat Leaves
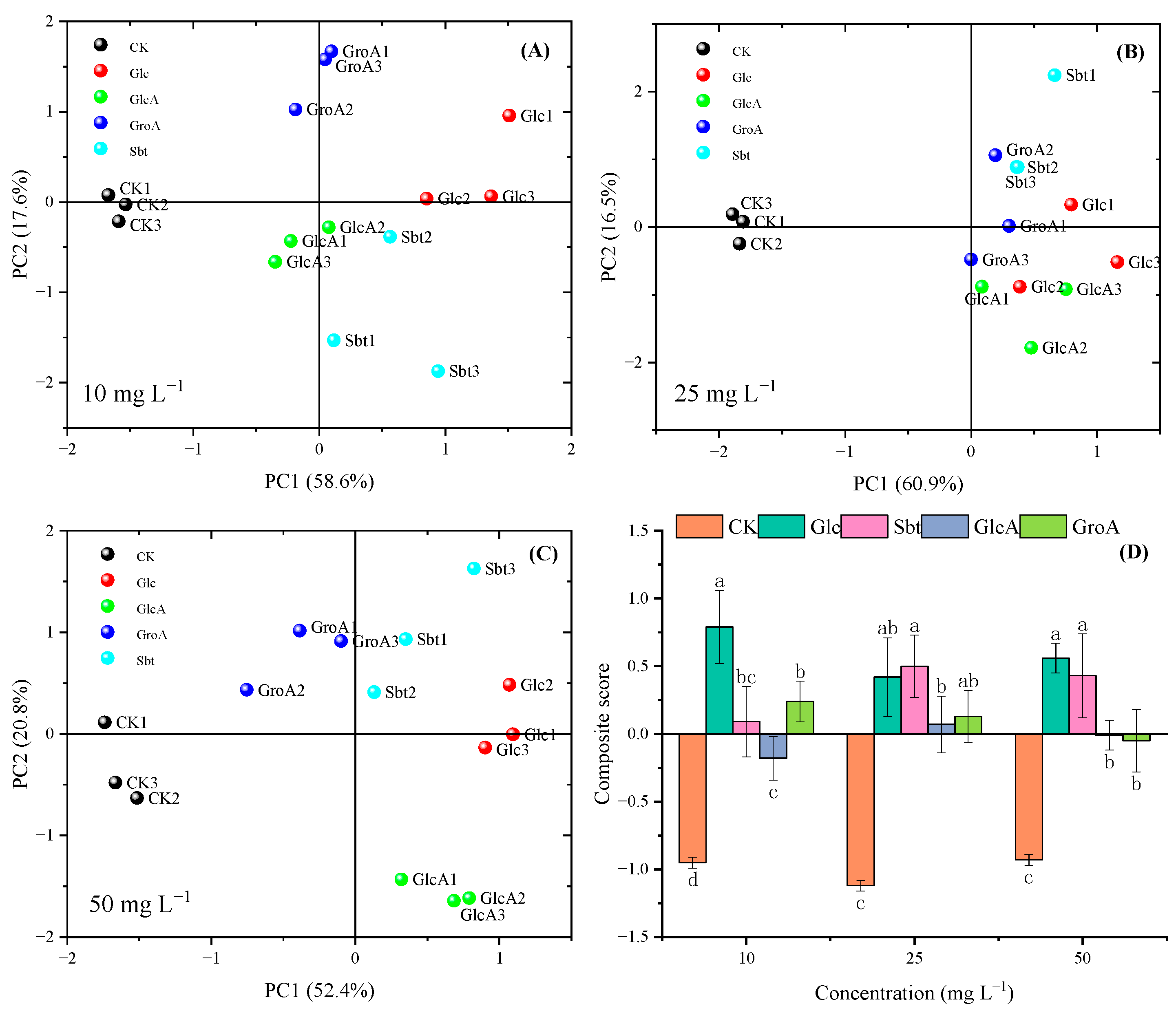
3.5. Principal Component Analysis of Glucose and Its Derivatives on Wheat Growth and Nutrient Uptake
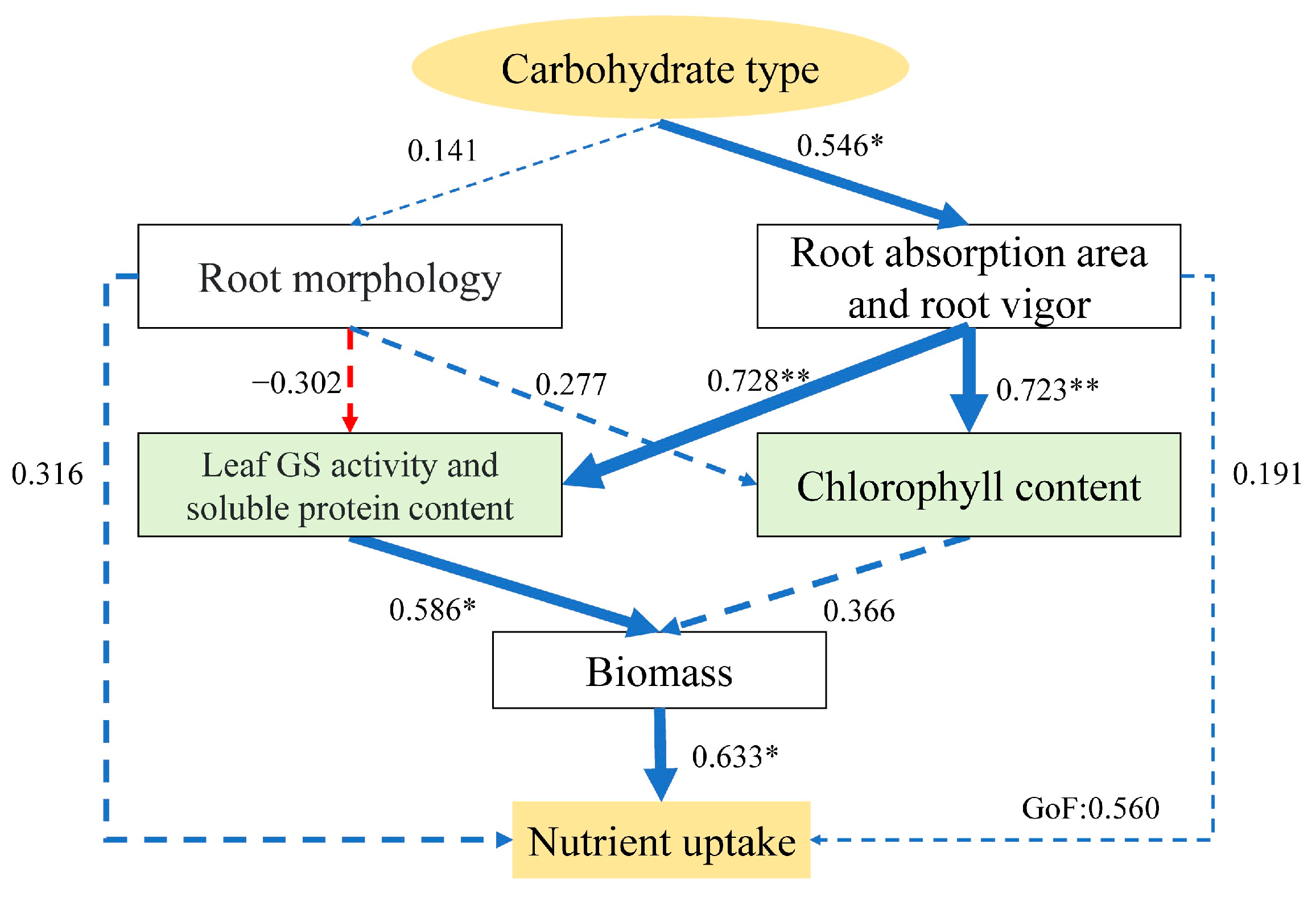
3.6. The Partial Least Squares Path Model (PLS-PM) Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, S.I. Control of Plant Development and Gene Expression by Sugar Signaling. Curr. Opin. Plant Biol. 2005, 8, 93–102. [Google Scholar] [CrossRef]
- Wang, L.H.; Li, G.L.; Wei, S.; Li, L.J.; Zuo, S.Y.; Liu, X.; Gu, W.R.; Li, J. Effects of Exogenous Glucose and Sucrose on Photosynthesis in Triticale Seedlings under Salt Stress. Photosynthetica 2019, 57, 286–294. [Google Scholar] [CrossRef]
- Qi, X.; Li, Q.; Shen, J.; Qian, C.; Xu, X.; Xu, Q.; Chen, X. Sugar Enhances Waterlogging-induced Adventitious Root Formation in Cucumber by Promoting Auxin Transport and Signalling. Plant Cell Environ. 2020, 43, 1545–1557. [Google Scholar] [CrossRef]
- Kosar, F.; Alshallash, K.S.; Akram, N.A.; Sadiq, M.; Ashraf, M.; Alkhalifah, D.H.M.; Abdel Latef, A.A.H.; Elkelish, A. Trehalose-Induced Regulations in Nutrient Status and Secondary Metabolites of Drought-Stressed Sunflower (Helianthus annuus L.) Plants. Plants 2022, 11, 2780. [Google Scholar] [CrossRef]
- Mishra, B.S.; Singh, M.; Aggrawal, P.; Laxmi, A. Glucose and Auxin Signaling Interaction in Controlling Arabidopsis Thaliana Seedlings Root Growth and Development. PLoS ONE 2009, 4, e4502. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wang, L.; Dong, Q.; Chang, S.; Wen, K.; Jia, S.; Chu, Z.; Wang, H.; Gao, P.; Zhao, H.; et al. FRET-Based Glucose Imaging Identifies Glucose Signalling in Response to Biotic and Abiotic Stresses in Rice Roots. J. Plant Physiol. 2017, 215, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Malinova, I.; Kössler, S.; Orawetz, T.; Matthes, U.; Orzechowski, S.; Koch, A.; Fettke, J. Identification of Two Arabidopsis Thaliana Plasma Membrane Transporters Able to Transport Glucose 1-Phosphate. Plant Cell Physiol. 2020, 61, 381–392. [Google Scholar] [CrossRef]
- Hu, M.; Shi, Z.; Zhang, Z.; Zhang, Y.; Li, H. Effects of Exogenous Glucose on Seed Germination and Antioxidant Capacity in Wheat Seedlings under Salt Stress. Plant Growth Regul. 2012, 68, 177–188. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, L.; Tan, W.; Gu, G.; Guo, Z. Novel 1,2,3-Triazolium-Functionalized Inulin Derivatives: Synthesis, Free Radical-Scavenging Activity, and Antifungal Activity. RSC Adv. 2017, 7, 42225–42232. [Google Scholar] [CrossRef]
- Synytsya, A. Structural Diversity of Fungal Glucans. Carbohydr. Polym. 2013, 92, 792–809. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, Z.; Huang, Y.; He, D.; Lu, L.; Wei, M.; Lin, S.; Luo, W.; Liao, X.; Jin, S.; et al. WRKY45 Positively Regulates Salinity and Osmotic Stress Responses in Arabidopsis. Plant Physiol. Biochem. 2025, 219, 109408. [Google Scholar] [CrossRef]
- Mehtiö, T.; Toivari, M.; Wiebe, M.G.; Harlin, A.; Penttilä, M.; Koivula, A. Production and Applications of Carbohydrate-Derived Sugar Acids as Generic Biobased Chemicals. Crit. Rev. Biotechnol. 2016, 36, 904–916. [Google Scholar] [CrossRef]
- Ma, Q.; Wu, Z.; Pan, F.; Wang, J.; Zhou, H.; Jiang, C.; Xu, Y.; Yu, W. Effect of Glucose Addition on the Fate of Urea-N-15 in Fixed Ammonium and Soil Microbial Biomass N Pools. Eur. J. Soil Biol. 2016, 75, 168–173. [Google Scholar] [CrossRef]
- Dongmei, L.; Zitan, Z.; Sijun, Q.; Deguo, L. Root Architecture and Nitrogen Metabolism in Roots of Apple Rootstock Respond to Exogenous Glucose Supply in Low Carbon Soil. Plant Soil Environ. 2018, 64, 240–246. [Google Scholar] [CrossRef]
- Debnath, S.C. Effects of Carbon Source and Concentration on Development of Lingonberry (Vaccinium vitis-idaea L.) Shoots Cultivated in Vitro from Nodal Explants. Vitr. Cell. Dev. Biol.-Plant 2005, 41, 145–150. [Google Scholar] [CrossRef]
- Wen, R.; Zhu, M.; Yu, J.; Kou, L.; Ahmad, S.; Wei, X.; Jiao, G.; Hu, S.; Sheng, Z.; Zhao, F.; et al. Photosynthesis Regulates Tillering Bud Elongation and Nitrogen-use Efficiency via Sugar-induced NGR5 in Rice. New Phytol. 2024, 243, 1440–1454. [Google Scholar] [CrossRef]
- Zhao, J.; Missihoun, T.D.; Bartels, D. The Role of Arabidopsis Aldehyde Dehydrogenase Genes in Response to High Temperature and Stress Combinations. J. Exp. Bot. 2017, 68, 4295–4308. [Google Scholar] [CrossRef]
- Sauerland, M.; Mertes, R.; Morozzi, C.; Eggler, A.L.; Gamon, L.F.; Davies, M.J. Kinetic Assessment of Michael Addition Reactions of Alpha, Beta-Unsaturated Carbonyl Compounds to Amino Acid and Protein Thiols. Free Radic. Biol. Med. 2021, 169, 1–11. [Google Scholar] [CrossRef]
- Zhou, X.; Joshi, S.; Patil, S.; Khare, T.; Kumar, V. Reactive Oxygen, Nitrogen, Carbonyl and Sulfur Species and Their Roles in Plant Abiotic Stress Responses and Tolerance. J. Plant Growth Regul. 2022, 41, 119–142. [Google Scholar] [CrossRef]
- Dumschott, K.; Richter, A.; Loescher, W.; Merchant, A. Post Photosynthetic Carbon Partitioning to Sugar Alcohols and Consequences for Plant Growth. Phytochemistry 2017, 144, 243–252. [Google Scholar] [CrossRef]
- Tian, G.; Liu, C.; Xu, X.; Xing, Y.; Liu, J.; Lyu, M.; Feng, Z.; Zhang, X.; Qin, H.; Jiang, H.; et al. Effects of Magnesium on Nitrate Uptake and Sorbitol Synthesis and Translocation in Apple Seedlings. Plant Physiol. Biochem. 2023, 196, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Zhang, S.; Yuan, L.; Li, Y.; Chen, C.; Zhao, B. Humic Acid Modified by Being Incorporated Into Phosphate Fertilizer Increases Its Potency in Stimulating Maize Growth and Nutrient Absorption. Front. Plant Sci. 2022, 13, 885156. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Raha, P.; Dubey, A.N.; Rani, M.; Paul, A.; Patel, R. Differential Responses of Rice (Oryza sativa L.) to Foliar Fertil. Org. Potassium Salts. J. Plant Nutr. 2020, 44, 1330–1348. [Google Scholar] [CrossRef]
- Qian, L.; Song, F.; Xia, J.; Wang, R. A Glucuronic Acid-Producing Endophyte Pseudomonas Sp. MCS15 Reduces Cadmium Uptake in Rice by Inhibition of Ethylene Biosynthesis. Front. Plant Sci. 2022, 13, 876545. [Google Scholar] [CrossRef]
- Ma, Q.; Cao, X.; Xie, Y.; Xiao, H.; Tan, X.; Wu, L. Effects of Glucose on the Uptake and Metabolism of Glycine in Pakchoi (Brassica chinensis L.) Expo. Var. Nitrogen Sources. BMC Plant Biol. 2017, 17, 58. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of Sugars under Abiotic Stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- El-Mahdy, M.T.; Youssef, M. Genetic Homogeneity and High Shoot Proliferation in Banana (Musa acuminata Colla) by Altering Medium Thiamine Level and Sugar Type. Vitr. Cell. Dev. Biol.-Plant 2019, 55, 668–677. [Google Scholar] [CrossRef]
- Afzal, S.; Chaudhary, N.; Singh, N.K. Role of Soluble Sugars in Metabolism and Sensing Under Abiotic Stress. In Plant Growth Regulators; Aftab, T., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 305–334. ISBN 978-3-030-61152-1. [Google Scholar]
- Zahid, M.; Iqbal, N.; Muhammad, S.; Faisal, S.; Mahboob, W.; Hussain, M.; Khan, Z.U.D. Efficacy of Exogenous Applications of Glucose in Improving Wheat Crop (Triticum aestivum L.) Performance under Drought Stress. Pak. J. Agric. Res. 2018, 31, 202–290. [Google Scholar] [CrossRef]
- KALRA Handbook of Reference Methods for Plant Analysis. Available online: https://www.webofscience.com/wos/alldb/full-record/CABI:19990701167 (accessed on 18 November 2022).
- Ju, H.; Liu, J.; Gao, J.; Fang, Y.; Bian, M.; Yang, Z. Potassium Uptake Characteristics of Two Maize Hybrids and Their Parents Under the Stress from a Low Amount of Potassium. J. Plant Nutr. 2014, 37, 123–135. [Google Scholar] [CrossRef]
- Chen, C.W.; Yang, Y.W.; Lur, H.S.; Tsai, Y.G.; Chang, M.C. A Novel Function of Abscisic Acid in the Regulation of Rice (Oryza sativa L.) Root Growth and Development. Plant Cell Physiol. 2006, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Macias-Sanchez, M.D.; Mantell Serrano, C.; Rodriguez Rodriguez, M.; de la Ossa, E.M.; Lubian, L.M.; Montero, O. Extraction of Carotenoids and Chlorophyll from Microalgae with Supercritical Carbon Dioxide and Ethanol as Cosolvent. J. Sep. Sci. 2008, 31, 1352–1362. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and Novel Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Takehisa, H.; Sato, Y.; Igarashi, M.; Abiko, T.; Antonio, B.A.; Kamatsuki, K.; Minami, H.; Namiki, N.; Inukai, Y.; Nakazono, M.; et al. Genome-Wide Transcriptome Dissection of the Rice Root System: Implications for Developmental and Physiological Functions: Transcriptome Dissection of the Rice Root. Plant J. 2012, 69, 126–140. [Google Scholar] [CrossRef]
- Wang, L.; Ruan, Y.-L. Shoot–Root Carbon Allocation, Sugar Signalling and Their Coupling with Nitrogen Uptake and Assimilation. Funct. Plant Biol. 2016, 43, 105. [Google Scholar] [CrossRef]
- Giehl, R.F.H.; Gruber, B.D.; von Wiren, N. Its Time to Make Changes: Modulation of Root System Architecture by Nutrient Signals. J. Exp. Bot. 2014, 65, 769–778. [Google Scholar] [CrossRef]
- Yuan, T.-T.; Xu, H.-H.; Zhang, K.-X.; Guo, T.-T.; Lu, Y.-T. Glucose Inhibits Root Meristem Growth via ABA INSENSITIVE 5, Which Represses PIN1 Accumulation and Auxin Activity in Arabidopsis. Plant Cell Environ. 2014, 37, 1338–1350. [Google Scholar] [CrossRef]
- Chen, F.; Zheng, G.; Qu, M.; Wang, Y.; Lyu, M.-J.A.; Zhu, X.-G. Knocking out negative regulator of photosynthesis 1 Increases Rice Leaf Photosynthesis and Biomass Production in the Field. J. Exp. Bot. 2021, 72, 1836–1849. [Google Scholar] [CrossRef] [PubMed]
- Cha-um, S.; Charoenpanich, A.; Roytrakul, S.; Kirdmanee, C. Sugar Accumulation, Photosynthesis and Growth of Two Indica Rice Varieties in Response to Salt Stress. Acta Physiol. Plant. 2009, 31, 477–486. [Google Scholar] [CrossRef]
- Stadnichuk, I.; Rakhimberdieva, M.G.; Bolychevtseva, Y.V.; Yurina, N.P.; Karapetyan, N.V.; Selyakh, I.O. Inhibition by Glucose of Chlorophyll a and Phycocyanobilin Biosynthesis in the Unicellular Red Alga Galdieria Partita at the Stage of Coproporphyrinogen III Formation. Plant Sci. 1998, 136, 11–23. [Google Scholar] [CrossRef]
- Ruffel, S.; Gojon, A.; Lejay, L. Signal Interactions in the Regulation of Root Nitrate Uptake. J. Exp. Bot. 2014, 65, 5509–5517. [Google Scholar] [CrossRef]
- Rossato, L.; Laine, P.; Ourry, A. Nitrogen Storage and Remobilization in Brassica Napus L. During the Growth Cycle: Nitrogen. Fluxes Within Plant Changes Soluble Protein Patterns. J. Exp. Bot. 2001, 52, 1655–1663. [Google Scholar] [CrossRef]
- Sun, Z. Synthesis, Characterization, and Antimicrobial Activities of Sulfonated Chitosan. Carbohydr. Polym. 2017, 155, 321–328. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. A Review and Experimental Verification of Using Chitosan and Its Derivatives as Adsorbents for Selected Heavy Metals. J. Environ. Manag. 2010, 91, 798–806. [Google Scholar] [CrossRef]
- Rolland, F.; Winderickx, J.; Thevelein, J.M. Glucose-Sensing Mechanisms in Eukaryotic Cells. Trends Biochem. Sci. 2001, 26, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Dillaway, D.N.; Stringer, J.W.; Rieske, L.K. Light Availability Influences Root Carbohydrates, and Potentially Vigor, in White Oak Advance Regeneration. For. Ecol. Manag. 2007, 250, 227–233. [Google Scholar] [CrossRef]
- Dumas, C.; Petrig, J.; Frei, L.; Spingler, B.; Schibli, R. Functionalization of Glucose at Position C-3 for Transition Metal Coordination: Organo-Rhenium Complexes with Carbohydrate Skeletons. Bioconjugat. Chem. 2005, 16, 421–428. [Google Scholar] [CrossRef]
- Zhou, L.; Jang, J.; Jones, T.L.; Sheen, J. Glucose and Ethylene Signal Transduction Crosstalk Revealed by an Arabidopsis Glucose-Insensitive Mutant. Proc. Natl. Acad. Sci. USA 1998, 95, 10294–10299. [Google Scholar] [CrossRef] [PubMed]
- Lejay, L.; Gansel, X.; Cerezo, M.; Tillard, P.; Müller, C.; Krapp, A.; von Wirén, N.; Daniel-Vedele, F.; Gojon, A. Regulation of Root Ion Transporters by Photosynthesis: Functional Importance and Relation with Hexokinase. Plant Cell 2003, 15, 2218–2232. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Yang, Y. The Molecular Mechanism of Plasma Membrane H+-ATPases in Plant Responses to Abiotic Stress. J. Genet. Genom. 2022, 49, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Xu, P.; Yu, Y.; Xiong, Y. Glucose-TOR Signaling Regulates PIN2 Stability to Orchestrate Auxin Gradient and Cell Expansion in Arabidopsis Root. Proc. Natl. Acad. Sci. USA 2020, 117, 32223–32225. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Zhang, S.; Xu, M.; Xu, J.; Li, Y.; Zhao, B.; Yuan, L. Effects of Glucose and Its Derivatives on Growth and Nutrient Absorption in Wheat. Agronomy 2025, 15, 2054. https://doi.org/10.3390/agronomy15092054
Yan Y, Zhang S, Xu M, Xu J, Li Y, Zhao B, Yuan L. Effects of Glucose and Its Derivatives on Growth and Nutrient Absorption in Wheat. Agronomy. 2025; 15(9):2054. https://doi.org/10.3390/agronomy15092054
Chicago/Turabian StyleYan, Yan’ge, Shuiqin Zhang, Meng Xu, Jiukai Xu, Yanting Li, Bingqiang Zhao, and Liang Yuan. 2025. "Effects of Glucose and Its Derivatives on Growth and Nutrient Absorption in Wheat" Agronomy 15, no. 9: 2054. https://doi.org/10.3390/agronomy15092054
APA StyleYan, Y., Zhang, S., Xu, M., Xu, J., Li, Y., Zhao, B., & Yuan, L. (2025). Effects of Glucose and Its Derivatives on Growth and Nutrient Absorption in Wheat. Agronomy, 15(9), 2054. https://doi.org/10.3390/agronomy15092054






