Biofertilizer and Bioherbicide Potential of Microalgae-Based Wastewater and Diplotaxis harra Boiss for Sustainable Barley Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Microalgal Biomass, and Treated Wastewater Characterization
2.2. Plant Material
2.3. Pot Experiments
2.4. Field Experiments
2.5. Physiological Responses on Hordeum vulgare and Emex spinosa
2.5.1. Electrolyte Leakage
2.5.2. Lipid Peroxidation
2.5.3. Proline Content
2.5.4. DPPH Scavenging Assay
- PI: percentage inhibition.
- DOcontrol: Absorbance of the control reaction.
- DOextract: Absorbance in the presence of E. spinosa or H. vulgare extract.
2.5.5. Metabolic Activity
2.6. Statistical Analysis
3. Results
3.1. Irrigation Water Composition and Microalgae Characterization
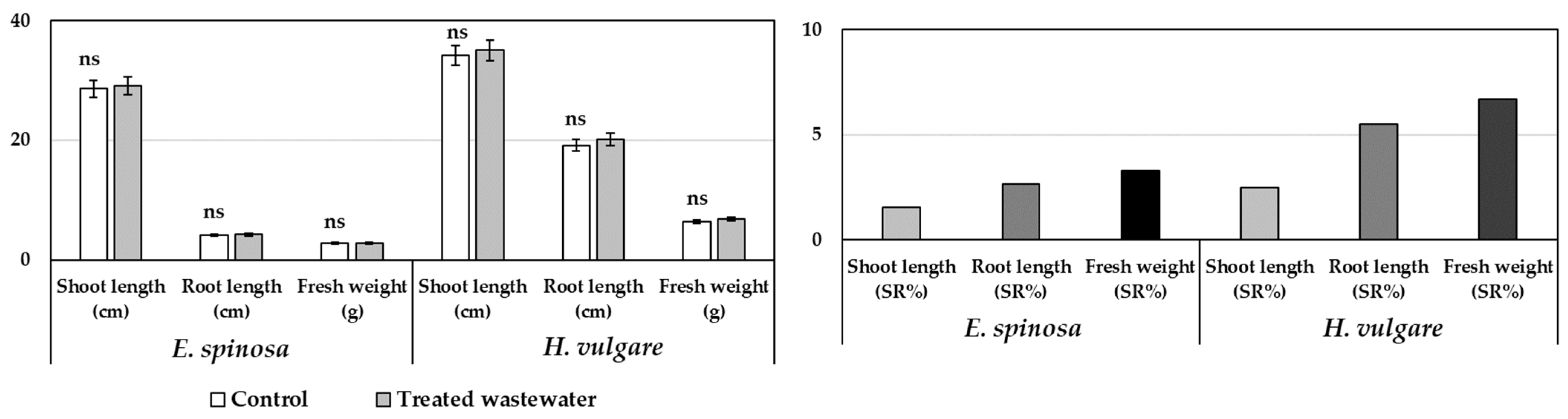
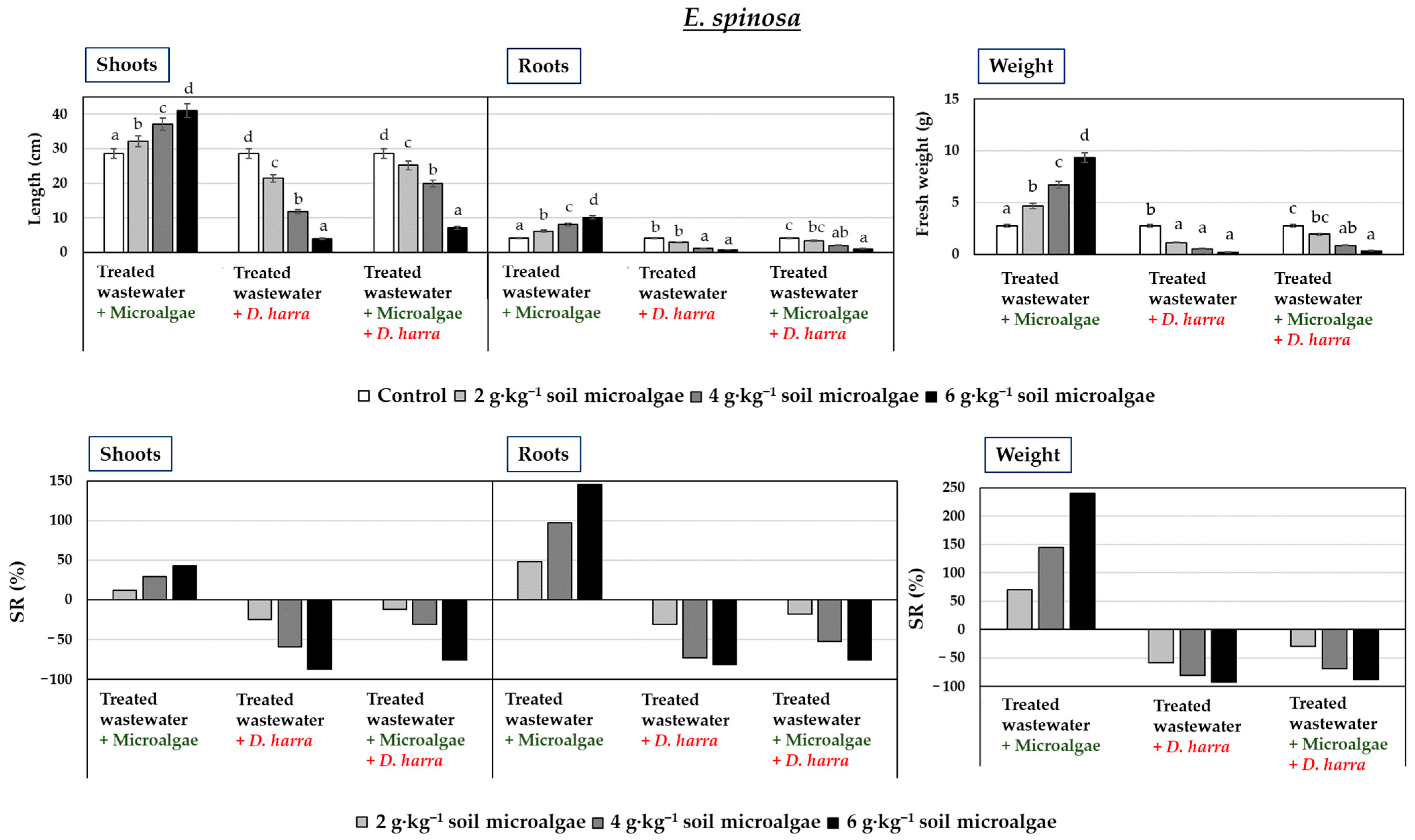
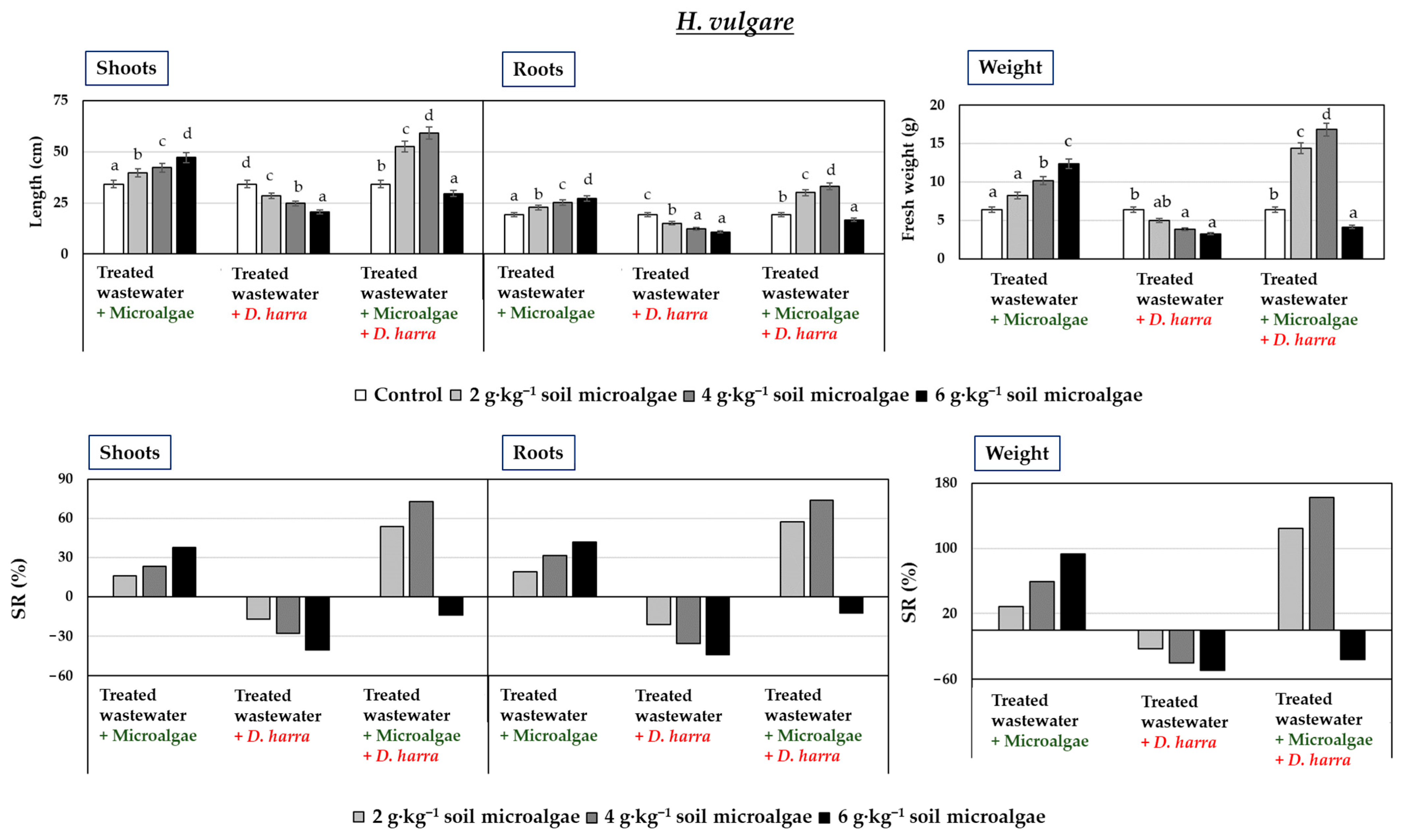
3.2. Pot Experiments
3.3. Field Experiments
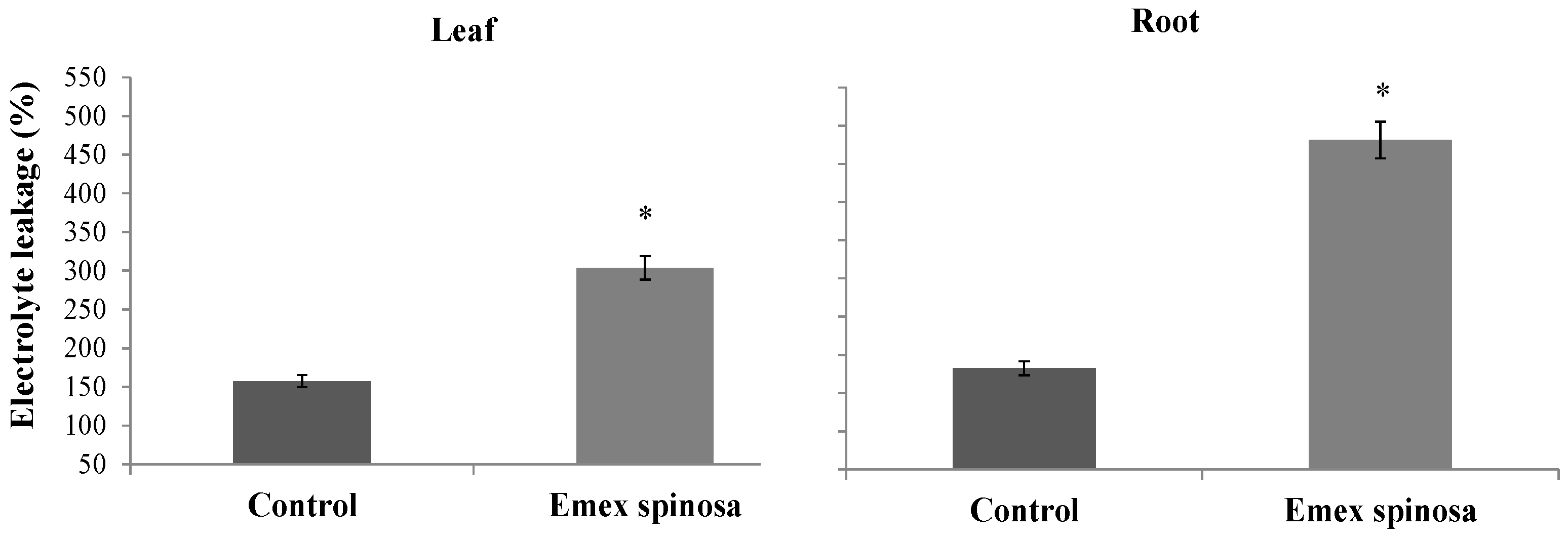
3.4. E. spinosa Physiological Response to D. harra Leaves and Microalgae Powders
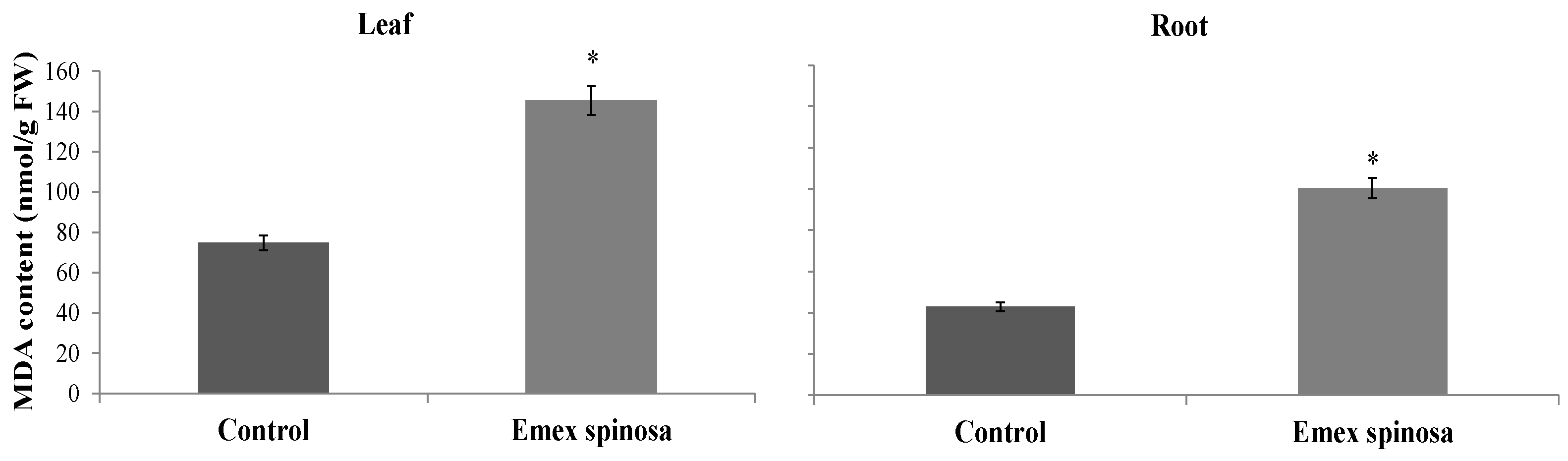
3.4.1. Electrolyte Leakage and Lipid Peroxidation
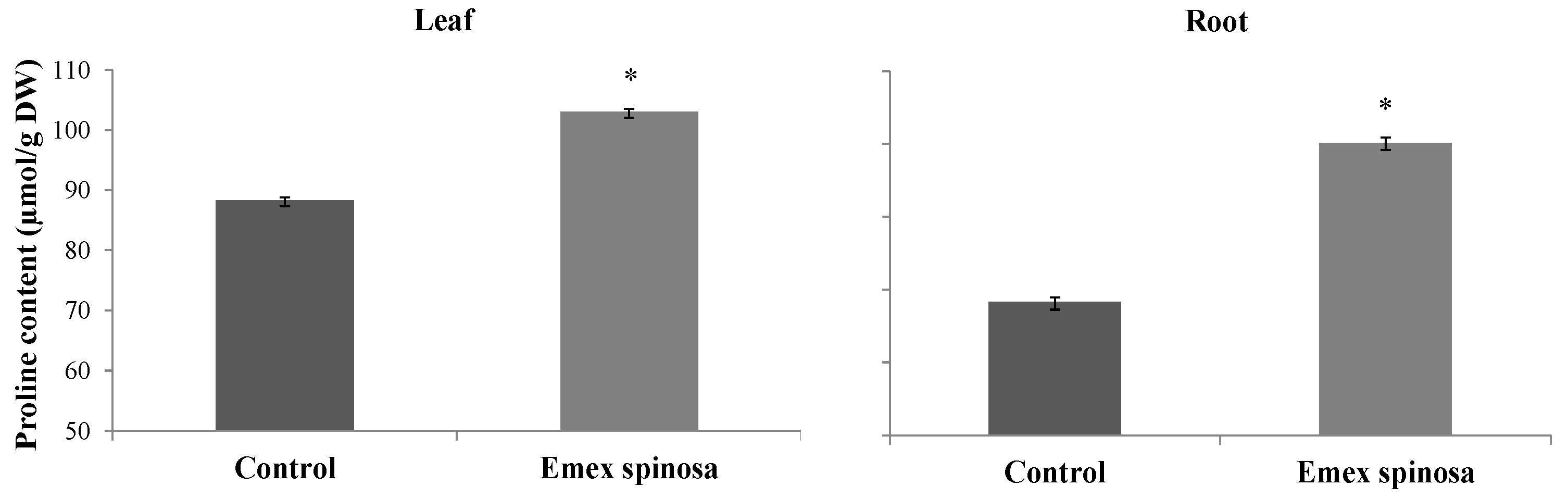
3.4.2. Content of Proline
3.4.3. DPPH Free Radical-Scavenging
3.4.4. Metabolic Activity
3.5. H. vulgare Physiological Response to D. harra Leaf and Microalgae Powders
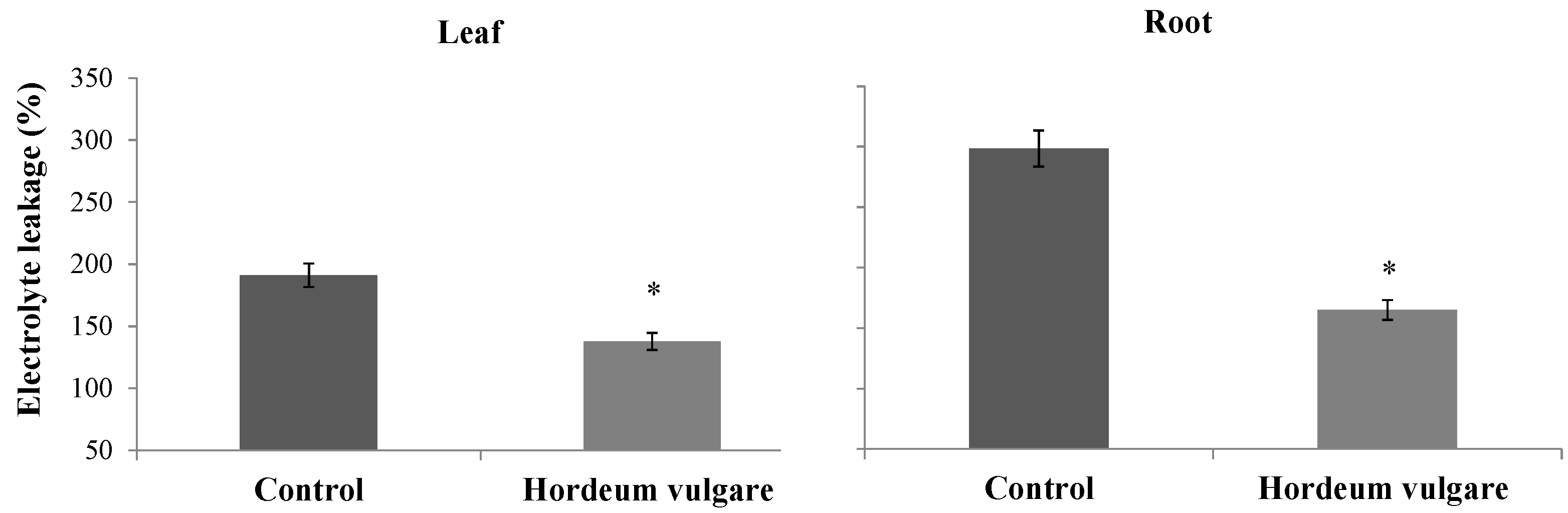
3.5.1. Electrolyte Leakage and Lipid Peroxidation
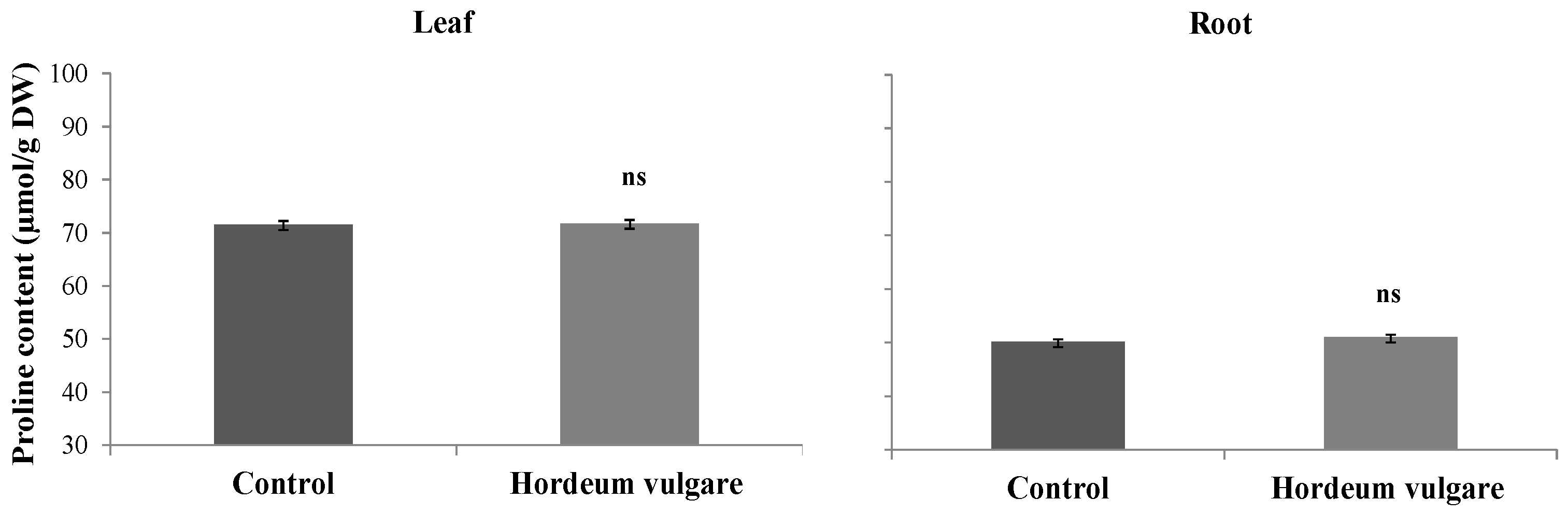
3.5.2. Content of Proline
3.5.3. DPPH Free Radical-Scavenging
3.5.4. Metabolic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BOD5 | Biochemical oxygen demand |
| COD | Chemical oxygen demand |
| DW | Dry weight |
| HRAP | High-rate algal pond |
| MDA | Malondialdehyde |
| PE | Population equivalent |
| ROS | Reactive oxygen species |
| WHO | World health organization |
| WWTPP | Pilot-scale wastewater treatment plant |
References
- Jones, E.R.; Bierkens, M.F.; van Vliet, M.T. Current and future global water scarcity intensifies when accounting for surface water quality. Nat. Clim. Change 2024, 14, 629–635. [Google Scholar] [CrossRef]
- Zorrilla, J.G.; Siciliano, A.; Petraretti, M.; Saviano, L.; Spampinato, M.; Cimmino, A.; Guida, M.; Pollio, A.; Bravi, S.; Masi, M. Ecotoxicological assessment of cyclic peptides produced by a Planktothrix rubescens bloom: Impact on aquatic model organisms. Environ. Res. 2024, 257, 119394. [Google Scholar] [CrossRef] [PubMed]
- Karimidastenaei, Z.; Avellán, T.; Sadegh, M.; Kløve, B.; Haghighi, A.T. Unconventional water resources: Global opportunities and challenges. Sci. Total Environ. 2022, 827, 154429. [Google Scholar] [CrossRef]
- Mojid, M.A.; Mainuddin, M.; Murad, K.F.I.; Mac Kirby, J. Water usage trends under intensive groundwater-irrigated agricultural development in a changing climate–Evidence from Bangladesh. Agric. Water Manag. 2021, 251, 106873. [Google Scholar] [CrossRef]
- Lopes, A.R.; Becerra-Castro, C.; Vaz-Moreira, I.; Silva, M.E.F.; Nunes, O.C.; Manaia, C.M. Irrigation with treated wastewater: Potential impacts on microbial function and diversity in agricultural soils. In Wastewater Reuse and Current Challenges, 1st ed.; Fatta-Kassinos, D., Dionysiou, D., Kümmerer, K., Eds.; The Handbook of Environmental Chemistry Series; Springer International Publishing: Cham, Switzerland, 2015; pp. 105–128. [Google Scholar] [CrossRef]
- Ortiz, A.; Díez-Montero, R.; García, J.; Khalil, N.; Uggetti, E. Advanced biokinetic and hydrodynamic modelling to support and optimize the design of full-scale high rate algal ponds. Comput. Struct. Biotechnol. J. 2022, 20, 386–398. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal. Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Akbar, R.; Sun, J.; Bo, Y.; Khattak, W.A.; Khan, A.A.; Jin, C.; Zeb, U.; Ullah, N.; Abbas, A.D.; Kiu, W.; et al. Understanding the influence of secondary metabolites in plant invasion strategies: A comprehensive review. Plants 2024, 13, 3162. [Google Scholar] [CrossRef] [PubMed]
- Jmii, G.; Khadhri, A.; Haouala, R. Thapsia garganica allelopathic potentialities explored for lettuce growth enhancement and associated weed control. Sci. Hortic. 2020, 262, 109068. [Google Scholar] [CrossRef]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Chinchilla, D.; Simonet, A.M.; Molinillo, J.M. Isolation and synthesis of allelochemicals from gramineae: Benzoxazinones and related compounds. J. Agric. Food Chem. 2006, 54, 991–1000. [Google Scholar] [CrossRef]
- Islam, A.M.; Suttiyut, T.; Anwar, M.P.; Juraimi, A.S.; Kato-Noguchi, H. Allelopathic properties of Lamiaceae species: Prospects and challenges to use in agriculture. Plants 2022, 11, 1478. [Google Scholar] [CrossRef]
- Jmii, G.; Zorrilla, J.G.; Haouala, R. Allelochemicals from Thapsia garganica leaves for Lolium perenne L. control: The magic of mixtures. Chemoecology 2022, 32, 81–87. [Google Scholar] [CrossRef]
- Tlig, T.; Gorai, M.; Neffati, M. Étude expérimentale de la compétition entre l’adventice Diplotaxis harra (Forssk.) Boiss. et l’orge (Hordeum vulgare var. Ardhaoui). Ecol. Mediterr. 2012, 38, 89–95. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Wen, Z.H.; Bakheit, A.H.; Basudan, O.A.; Ghabbour, H.A.; Al-Ahmari, A.; Feng, C.W. A major Diplotaxis harra-derived bioflavonoid glycoside as a protective agent against chemically induced neurotoxicity and parkinson’s models; in silico target prediction; and biphasic HPTLC-based quantification. Plants 2022, 11, 648. [Google Scholar] [CrossRef]
- Lukinac, J.; Jukić, M. Barley in the production of cereal-based products. Plants 2022, 11, 3519. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.M.; Tanveer, A. Germination ecology of Emex spinosa and Emex australis, invasive weeds of winter crops. Weed Res. 2014, 54, 565–575. [Google Scholar] [CrossRef]
- Keffala, C.; Jmii, G.; Mokhtar, A.; Zouhir, F.; Liady, N.D.; Tychon, B.; Jupsin, H. Diagnosis and assessment of a combined oxylag and high rate algal pond (COHRAP) for sustainable water reuse: Case study of the University Campus in Tunisia. Water 2025, 17, 1326. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- ISO 17294-2: 2023; Water Quality, Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. ISO: Geneva, Switzerland, 2003.
- USEPA. Sw-846 EPA method 3051A. Microwave assisted acid digestion of sediments, sludges, soils and oils. In Test Methods for Evaluating Solid Waste, 2nd ed.; US Environmental Protection Agency: Washington, DC, USA, 1998; pp. 1–30. [Google Scholar]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Doblinski, P.M.F.; Ferrarese, M.L.L.; Huber, D.A.; Scapim, C.A.; Braccini, A.L.; Ferrarese-Filho, O. Peroxidase and lipid peroxidation of soybean roots in response to p-coumaric and p-hydroxybenzoic acids. Braz. Arch. Biol. Technol. 2003, 46, 193–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem. Pharm. Bull 1988, 36, 2090–2097. [Google Scholar] [CrossRef]
- Sampietro, D.A.; Vattuone, M.A.; Isla, M.I. Plant growth inhibitors isolated from sugarcane (Saccharum officinarum) Straw. J. Plant Physiol. 2006, 163, 837–846. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for the Safe Use of Wastewater, Excreta and Greywater, 3rd ed.; World Health Organization: Geneva, Switzerland, 2006; Volume 2, pp. 1–196. [Google Scholar]
- Tunisian Standards NT 106.03; Reuse of Treated Wastewater in Agriculture—Physicochemical and Biological Specifications. National Institute for Standardization and Industrial Properties-INNORPI, Tunisia: Tunis, Tunisia, 1989. (In French)
- European Union. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003; European Union: Luxembourg, 2019. [Google Scholar]
- Álvarez-González, A.; Uggetti, E.; Serrano, L.; Gorchs, G.; Escolà Casas, M.; Matamoros, V.; Gonzalez-Flo, E.; Díez-Montero, R. The potential of wastewater grown microalgae for agricultural purposes: Contaminants of emerging concern, heavy metals and pathogens assessment. Environ. Pollut. 2023, 324, 121399. [Google Scholar] [CrossRef]
- Dagnaisser, L.S.; Dos Santos, M.G.B.; Rita, A.V.S.; Chaves Cardoso, J.; De Carvalho, D.F.; De Mendonça, H.V. Microalgae as bio-fertilizer: A new strategy for advancing modern agriculture, wastewater bioremediation, and atmospheric carbon mitigation. Water Air Soil Pollut. 2022, 233, 477. [Google Scholar] [CrossRef]
- Alshahrani, T.S.; Verlinden, S.; Siedel, G.E. Effects of leaf extracts of Ziziphus spina-christi and Prosopis juliflora on each others seedlings roots. Allelopath. J 2009, 23, 111–118. [Google Scholar]
- Bahloul, N.; Bellili, S.; Aazza, S.; Chérif, A.; Faleiro, M.L.; Antunes, M.D.; Miguel, M.G.; Mnif, W. Aqueous extracts from Tunisian diplotaxis: Phenol content, antioxidant and anti-acetylcholinesterase activities, and impact of exposure to simulated gastrointestinal fluids. Antioxidants 2016, 5, 12. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Khaliq, A.; Matloob, A.; Farooq, M.; Mushtaq, M.N.; Khan, M.B. Effect of crop residues applied isolated or in combination on the germination and seedling growth of horse purslane (Trianthema portulacastrum). Planta Daninha 2011, 29, 121–128. [Google Scholar] [CrossRef]
- Mamude, C.; Asfaw, Z. Allelopathic effects of Oldeania alpina (K. Schum.) Stapleton leaf aqueous extract on seed germination and initial seedling growth of two selected crops. Adv. Bamboo Sci. 2023, 4, 100034. [Google Scholar] [CrossRef]
- Mohammadrezakhani, S.; Hajilou, J.; Rezanejad, F.; Zaare-Nahandi, F. Assessment of exogenous application of proline on antioxidant compounds in three Citrus species under low temperature stress. Plant-Environ. Interact. 2019, 14, 347–358. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Kong-ngern, K.; Bunnag, S.; Theerakulpisut, P. Proline, hydrogen peroxide, membrane stability and antioxidant enzyme activity as potential indicators for salt tolerance in Rice (Oryza sativa L.). Int. J. Bot. 2012, 8, 54–65. [Google Scholar] [CrossRef]
- Van Tonder, A.; Joubert, A.M.; Cromarty, A.D. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res. Notes 2015, 8, 47. [Google Scholar] [CrossRef] [PubMed]

| Treatment Code | Description | Dosage | Experimental Aim |
|---|---|---|---|
| A | Pots irrigated with treated wastewater | - | Focus on the effects of treated wastewater |
| B | Pots irrigated with treated wastewater and microalgae powder applied to the soil at a depth of 0.5 cm | 2, 4, and 6 g·kg−1 soil (respectively, 0.63, 1.26, 1.89 t·ha−1) | Focus on microalgae effects |
| C | Pots irrigated with treated wastewater and D. harra leaf powder applied to the soil at a depth of 0.5 cm | 2, 4, and 6 g·kg−1 soil | Focus on D. harra effects |
| D | Pots irrigated with treated wastewater and 1:1 mixture of D. harra leaf and microalgae powder applied to the soil at a depth of 0.5 cm | 2, 4, and 6 g·kg−1 soil (total, both combined equally) | Focus on the effects of the combination of microalgae and D. harra |
| Parameters | Unit | Maximum Concentration (NT 106.03) | World Health Organization (WHO) | Measured Concentration |
|---|---|---|---|---|
| pH | - | 6.5–8.5 | 6.5–8.0 | 8.35 ± 0.26 |
| Conductivity | µS/cm | 7000 | 700–3000 | 1973 ± 0.42 |
| Chemical Oxygen demand (COD) | mg O2/L | 90 | 500 | 75 ± 19 |
| Biochemical Oxygen demand (BOD5) | mg O2/L | 30 | 300 | 13.5 ± 8 |
| Cadmium (Cd) | mg/L | 0.01 | <0.01 | 0.0001 ± 0.00005 |
| Chromium (Cr) | mg/L | 0.1 | 0.02 | 0.0007 ± 0.0005 |
| Copper (Cu) | mg/L | 0.5 | 0.2 | 0.008 ± 0.0009 |
| Iron (Fe) | mg/L | 5 | 0.10–1.50 | 0.179 ± 0.021 |
| Mercury (Hg) | mg/L | 0.001 | - | 0.0002 ± 0.0001 |
| Nickel (Ni) | mg/L | 0.2 | 0.2 | 0.006 ± 0.002 |
| Plumb (Pb) | mg/L | 0.001 | <5.00 | 0.0008 ± 0.0001 |
| Zinc (Zn) | mg/L | 5 | <2.00 | 0.101 ± 0.012 |
| NH4 | mg N/L | - | - | 0.9 ± 0.2 |
| NO3 | mg N/L | - | 50.00 | 2.7 ± 1 |
| PO4 | mg P/L | - | 30.00 | 1 ± 0.4 |
| Calcium (Ca) | mg/L | - | 230.00 | 134 ± 22 |
| Magnesium (Mg) | mg/L | - | 60.00 | 57 ± 19 |
| Sodium (Na) | mg/L | - | 69.00–207.00 | 101 ± 19 |
| Algal Biomass (mg/kg DW) | European Regulation [29] (mg/kg DW) | |
|---|---|---|
| Ni | 13.35 ± 1.12 | 50 |
| Cd | 0.17 ± 0.03 | 3 |
| Hg | 0.39 ± 0.08 | 1 |
| Pb | 2.36 ± 0.04 | 120 |
| Cu | 16.44 ± 1.78 | 600 |
| Zn | 96.68 ± 3.52 | 1500 |
| Fe | 1858.00 ± 12.61 | - |
| Measured Parameters | Control | Treated Seedlings |
|---|---|---|
| H. vulgare | ||
| Shoot length (cm) | 40.03 ± 5.27 | 70.72 * ± 9.42 |
| Stimulation/reduction (%) | - | +76.66 |
| Root length (cm) | 26.12 ± 2.18 | 47.13 * ± 8.34 |
| Stimulation/reduction (%) | - | +80.43 |
| Fresh weight (g) | 8.76 ± 1.21 | 23.73 * ± 1.52 |
| Stimulation/reduction (%) | - | +170.89 |
| E. spinosa | ||
| Shoot length (cm) | 31.21 ± 1.17 | 19.27 * ± 1.59 |
| Stimulation/reduction (%) | - | −38.25 |
| Root length (cm) | 6.07 ± 0.82 | 2.32 * ± 1.12 |
| Stimulation/reduction (%) | −61.77 | |
| Fresh weight (g) | 4.38 ± 1.25 | 1.21 * ± 0.34 |
| Stimulation/reduction (%) | - | −72.37 |
| IC50 (µg/mL) | ||
| Roots | Leaves | |
| E. spinosa | Control 456.76 ± 0.008 | 445.93 ± 0.007 |
| Treated 288.4 * ± 0.005 | 375.8 * ± 0.004 | |
| H. vulgare | Control 395.87 ± 0.007 | 333.57 ± 0.005 |
| Treated 300.57 * ± 0.006 | 283.68 * ± 0.009 | |
| Formazan Content (% of Control) | ||
| E. spinosa | Roots | 31.76 ± 0.95 |
| Leaves | 55.93 ± 0.49 | |
| H. vulgare | Roots | 159.05 ± 0.82 |
| Leaves | 135.86 ± 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jmii, G.; Keffala, C.; Zorrilla, J.G.; Zouhir, F.; Jupsin, H.; Mokhtar, A.; Tychon, B. Biofertilizer and Bioherbicide Potential of Microalgae-Based Wastewater and Diplotaxis harra Boiss for Sustainable Barley Production. Agronomy 2025, 15, 2020. https://doi.org/10.3390/agronomy15092020
Jmii G, Keffala C, Zorrilla JG, Zouhir F, Jupsin H, Mokhtar A, Tychon B. Biofertilizer and Bioherbicide Potential of Microalgae-Based Wastewater and Diplotaxis harra Boiss for Sustainable Barley Production. Agronomy. 2025; 15(9):2020. https://doi.org/10.3390/agronomy15092020
Chicago/Turabian StyleJmii, Ghofrane, Chema Keffala, Jesús G. Zorrilla, Fouad Zouhir, Hugues Jupsin, Ameni Mokhtar, and Bernard Tychon. 2025. "Biofertilizer and Bioherbicide Potential of Microalgae-Based Wastewater and Diplotaxis harra Boiss for Sustainable Barley Production" Agronomy 15, no. 9: 2020. https://doi.org/10.3390/agronomy15092020
APA StyleJmii, G., Keffala, C., Zorrilla, J. G., Zouhir, F., Jupsin, H., Mokhtar, A., & Tychon, B. (2025). Biofertilizer and Bioherbicide Potential of Microalgae-Based Wastewater and Diplotaxis harra Boiss for Sustainable Barley Production. Agronomy, 15(9), 2020. https://doi.org/10.3390/agronomy15092020







