1. Introduction
Feeding a growing global population within planetary boundaries is one of the most pressing challenges of the 21st century. The world’s population is expected to surpass 9.7 billion by 2050 [
1], which requires that significant transformations in food systems must undergo major transformations to satisfy rising demand without worsening environmental degradation. A critical component of this challenge lies in nutrient management—particularly the use of phosphorus (P), an essential and irreplaceable element for plant growth. Since the 20th century, agricultural intensification has relied heavily on synthetic fertilizers, especially those derived from phosphate rock, to sustain food production. However, this model has come under scrutiny due to concerns about finite P reserves, low use efficiency, environmental losses, and growing geopolitical dependencies [
2,
3].
Phosphorus fertilizers remain indispensable for high-yield agriculture because of their central role in energy transfer (ATP), root development, and reproductive processes in plants [
4]. However, plant uptake of P in soils is frequently restricted by its tendency to form insoluble compounds, which can limit availability to between 15 and 35% of applied inputs within the first year [
5]. The remainder accumulates in soils or is lost to aquatic systems, where it drives eutrophication—a major breach of the Earth’s biogeochemical boundaries [
6,
7]. Certain authors forecast a potential “phosphorus peak,” which could lead to subsequent supply shortages [
2], whereas others assert that mineral P reserves will persist for centuries if they are managed effectively [
8]. Thus, the primary concern is no longer imminent scarcity but rather profound inefficiencies and unsustainable externalities in the current phosphorus cycle, with most P rock stocks being concentrated in a few countries, posing a significant threat to global food security [
2].
In this context, increasing attention has turned toward circular economy strategies aimed at closing nutrient loops and reducing dependence on finite resources [
9]. Organic waste streams—such as livestock manure, food residues, and agro-industrial by-products—contain substantial quantities of recoverable P yet remain underutilized [
8]. A novel and promising approach to valorize these residues is through insect bioconversion, in which species like
Hermetia illucens (black soldier fly, BSF) and
Tenebrio molitor (yellow mealworm, TM) convert organic waste into high-value protein and a secondary by-product known as frass. This material, a mixture of insect excreta, exuviae, and undigested substrate, has emerged as a potential organic fertilizer [
10,
11].
Preliminary studies suggest that insect frass is rich in organic matter and nutrients, such as nitrogen, phosphorus, and potassium, and that they may also host beneficial microbial communities [
12,
13]. However, the agronomic performance of frass—especially its effectiveness as a P source—remains poorly understood. Questions remain regarding the lability and speciation of P in frass and its release dynamics in soil. Some studies highlight the potential of frass to enhance plant growth and soil health, while others note its variability and caution against overgeneralization [
14,
15]. Furthermore, regulatory frameworks—such as the EU Fertilizing Products Regulation—require sterilization of frass, which may reduce the viability of its microbial components, thus reducing its potential benefits [
11,
16].
Since a substantial portion of P remains in organic forms [
17], the activation of the P cycle is needed to ensure effectiveness of P fertilizers. Otherwise, organic P cannot be mineralized and therefore the uptake by plants in the short–medium term would not be guaranteed. It is hypothesized that the sterilization process has a detrimental impact on P cycling potential compared to other bio-based fertilizers, such as vermicompost, which has been proven to enhance soil P cycling [
18].
To address these knowledge gaps, the present study evaluated the agronomic potential of frass from Hermetia illucens and Tenebrio molitor as alternative P fertilizers under controlled conditions. Specifically, we (1) characterized the physicochemical properties of each frass type, (2) assessed their capacity to support lettuce (Lactuca sativa L.) growth in P-deficient soil conditions, and (3) quantified their effects on soil P availability and biochemical activity related to the P cycle.
By providing a detailed comparative analysis of insect frass as a phosphorus source, this study will contribute new insights to the emerging field of entomological bioconversion and its role in sustainable agriculture. This provides a foundation for future research aimed at maximizing the use of excrements, adapting them to specific crop management systems, and incorporating them into more comprehensive nutrient recycling frameworks that align with the goals of the circular economy and climate-resilient agriculture.
2. Materials and Methods
This experiment was performed in the ULMA greenhouse at Agricultural Engineering School (ETSIA), Universidad de Sevilla.
2.1. Fertilizer, Growth Substrate, and Basal Nutrition
2.1.1. Fertilizer Materials
A greenhouse experiment was conducted to evaluate the potential of insect rearing residues as P fertilizers. Two types of insect frass were tested: frass from black soldier fly (Hermetia illucens; BSF) and yellow mealworm (Tenebrio molitor; TM). In addition, a widely studied organic fertilizer (vermicompost) and a simple superphosphate mineral fertilizer were used for comparison.
Frass from
Hermetia illucens was kindly provided by Prof. Jesús D. Fernández Bayo (Department of Soil Science and Agricultural Chemistry, University of Granada). Larvae were reared on olive mill pomace, a by-product of the olive oil industry. The material was ground and sieved to <2 mm. A portion of the sieved frass was pasteurized at 70 °C for 60 min following EU Regulation 142/2011 [
19] and stored in sealed bags at 4 °C until use.
Frass from
Tenebrio molitor was obtained from Protiberia [
20]. Larvae were reared on wheat bran. The same post-processing was applied: sieving to <2 mm, pasteurization (70 °C for 60 min), and cold storage (4 °C) in sealed bags until the start of the experiment.
Vermicompost, used as an organic control treatment, was produced at the Agricultural Engineering School (ETSIA, University of Seville) from horse manure using red Californian earthworms (Eisenia fetida and Eisenia andrei). The composting process included a one-month precomposting stage, six months of vermicomposting, and one month of stabilization. The final product was air-dried, ground, sieved (<2 mm), and stored in sealed bags at ambient temperature.
Chemical characterization was performed to determine application rates. Frass (raw and pasteurized) and vermicompost were analyzed for moisture, total carbon (C), total nitrogen (N), total phosphorus (P), potassium (K), nitrate (NO
3−), and ammonium (NH
4+). Analyses were conducted by the Agricultural Research Service (SIA) at the Research, Technology, and Innovation Center of the University of Seville (CITIUS). Results are shown in
Table 1.
C and N were quantified via the DUMAS method using an LECO
® CNS-Trumac (Leco, St. Joseph, MI, USA) analyzer. Total P was determined by dry ashing following Kuo and Sainju (1996) [
21] and quantified colorimetrically using the method of Murphy and Riley [
22]. NO
3− and NH
4+ were extracted with 0.5 M KCl and measured colorimetrically [
23].
A positive control (+P Mineral) using dipotassium phosphate trihydrate (K2HPO4·3H2O) was included as a soluble inorganic P source (13.58% P). Despite contributing additional potassium, no K correction was applied since all treatments received a complete nutrient solution (minus P) to ensure that P was the only limiting nutrient.
2.1.2. Soil Properties and Preparation
The experiment was conducted using a calcareous Calcic Xerochrept [
24] collected from the top 30 cm of an olive grove. The soil was mixed 2:1 (
v/
v) with horticultural perlite to enhance aeration and structure, sieved to <2 mm, and 750 g dry weight was used per 1 L polypropylene pot (10.5 × 10.5 × 14 cm). The soil had low available P (P-Olsen = 8.535 mg kg
−1), below the Critical P Level (12 mg kg
−1) calculated via Recena et al. (2022) [
25]. Full characterization of the soil is represented in
Table 2. The densimeter method was used to determine the texture of the soil [
26]. Soil Organic Carbon (SOC) was determined according to the Walkley and Black (1934) procedure [
27]. Cation Exchange Capacity (CEC) was determined according to Sumner and Miller (1996) [
28]. The calcimeter method was performed to determine the total carbonates (CCE). The P availability index was assessed by the Olsen method [
29]. The content of iron oxides was determined with a sequential extraction according to Ruiz et al. (1997) involving an extraction with citrate–ascorbate and subsequently with citrate–bicarbonate dithionite [
30].
2.2. Plant Material and Transplanting
Lettuce (
Lactuca sativa L. var. Maravilla) was selected for its short growth cycle, relevance to organic production, and suitability as a model plant in frass studies [
31,
32]. Uniform seedlings (BBCH 12–13) were rinsed using tap and deionized water before transplanting to remove nursery substrate.
2.3. Experimental Design
The experiment was performed from 12 February to 31 March 2025 (48 days after transplanting). Natural light and ambient temperature/humidity were maintained. A randomized block design was used with eight treatments and four replicates (thirty-two pots total). Fertilizer treatments involved the following:
Non-fertilized control (PC).
Mineral P (K2HPO4·3H2O) (PCM).
Black soldier fly frass (PB).
Black soldier fly frass mixed with mineral P at a 1:1 ratio (PBM).
Mealworm frass (PT).
Mealworm frass mixed with mineral P at a 1:1 ratio (PTM).
Vermicompost (PV).
Vermicompost mixed with mineral P at a 1:1 ratio (PVM).
Phosphorus application was based on an estimated crop demand of 40 mg P per plant, setting target P doses of 50 mg P kg−1 soil for organic, 30 mg kg−1 for mineral, and 40 mg kg−1 for mixed treatments. Those ratios were chosen to apply similar doses of mineral P.
2.4. Cultivation and Nutrient Management
Plants were irrigated daily with deionized water, maintaining 70–80% field capacity, monitored gravimetrically. A modified Hoagland and Arnon [
33] nutrient solution (excluding P) was applied intermittently to ensure that nutrients other than P were not limiting (see
Table 3 for composition). The total volume of the nutrient solution applied was 530 mL per pot.
2.5. Non-Destructive Measurements and Harvest
SPAD chlorophyll index was measured on day 48 (DDT 48) using a SPAD-502 Plus device (Minolta Camera Co. Ltd., Osaka, Japan). Three readings per plant were averaged.
At harvest (DDT 48), plants were divided into shoots and roots, washed, dried (65 °C), and weighed to estimate shoot and root biomass. Dried samples were ground (<2 mm) for total P analysis. Rhizosphere soil was collected and frozen at −23 °C, and bulk soil was dried (34 °C), sieved, and stored for chemical analysis.
2.6. Laboratory Analyses
2.6.1. Organic Fertilizer Characterization
pH and EC were measured using standardized UNE-EN methods [
34,
35]. Inorganic P fractionation was performed using extraction: water [
36,
37] (ISO 15958:2019), NaHCO
3 [
37], neutral ammonium citrate (NAC) [
37], and H
2SO
4 [
30]. Phosphorus in the extracts was determined colorimetrically [
22]. Organic P in fertilizer was estimated as the difference between the inorganic P extracted with H
2SO
4 and the total P of the fertilizer, which was determined by calcination and acid digestion [
21]. All analyses were conducted with three replicates.
2.6.2. Plant Tissue Analysis
Total P in biomass was determined by dry ashing (550 °C, 8 h), followed by acid digestion (HCl 1 N) and colorimetric determination of P [
22] at 882 nm.
2.6.3. Post-Harvest Soil Analysis
Soil pH (1:2.5), EC (1:5), and Olsen P [
29] were determined. The total P in the bicarbonate extract used for Olsen P was determined after digestion with persulfate and sulfuric acid [
38], and organic P was calculated by difference.
β-glucosidase and alkaline phosphatase were analyzed from rhizosphere soils. β-glucosidase activity was measured following Eivazi and Tabatabai [
39], and phosphatase activity following Tabatabai and Bremner [
40], both based on p-nitrophenol colorimetric detection at 410 nm.
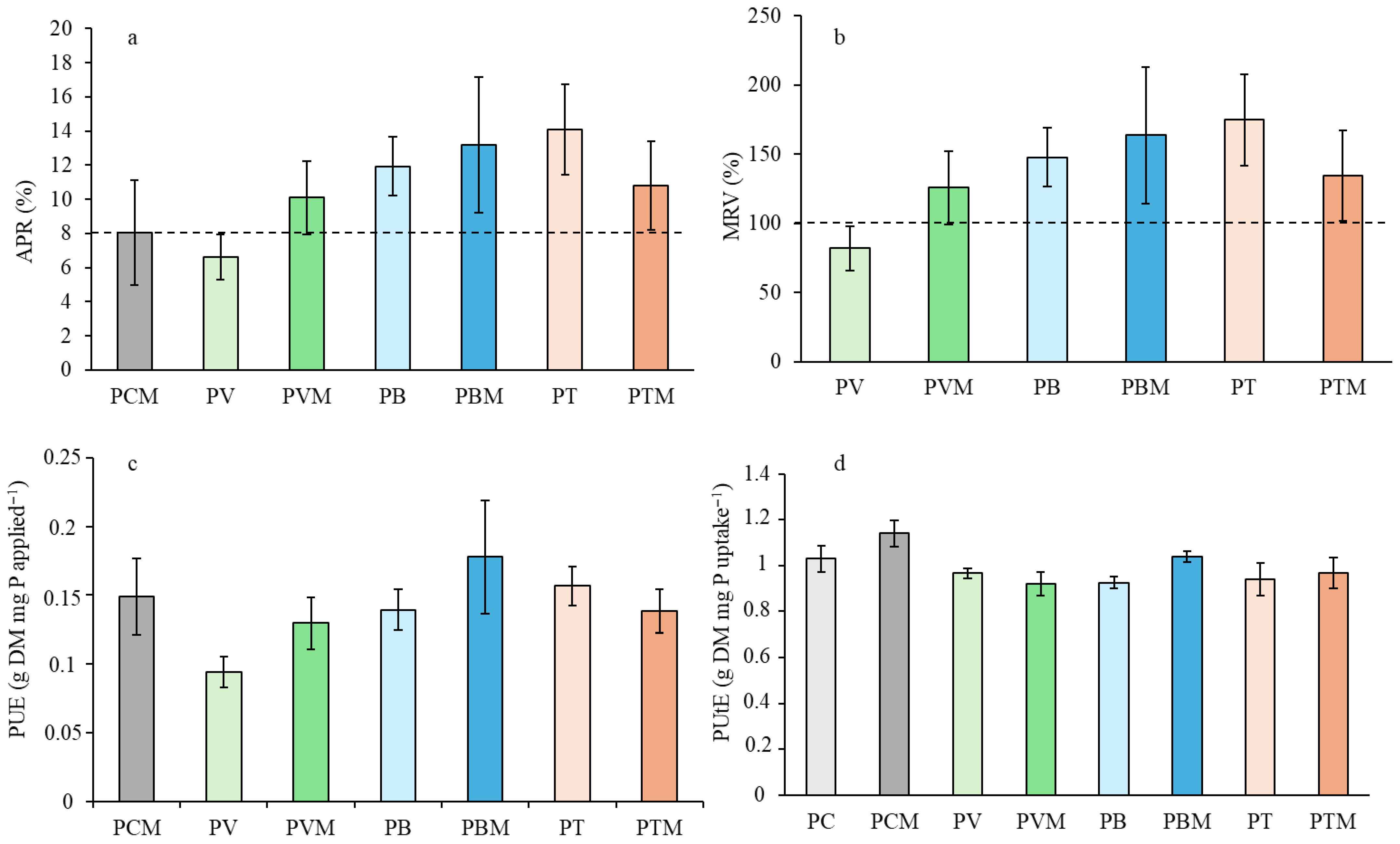
Four indexes were used to determine phosphorus use efficiency:
Total P in shoots and roots (mg pot−1), which was calculated as shoot dry weight × tissue P concentration.
Apparent P recovery (APR): % of applied P taken up by the plant above control [
41].
Mineral fertilizer replacement value (MRV): Relative effectiveness of organic/mixed treatments vs. mineral P [
41].
Phosphorus use efficiency (PUE): Shoot dry weight/P applied.
P uptake efficiency (PUtE): Shoot dry weight/P uptake [
42].
2.7. Statistical Analysis
The effects of the fertilizer treatments on studied variables were assessed by means of an analysis of variance (ANOVA). Previously, data were tested for normality and homoscedasticity (α = 0.05). If these requirements were not met, then a power transformation was performed. For ANOVA, a general linear model (GLM) was used including block as random factor. If significant, the means for each treatment were compared using Tukey’s HSD test (p < 0.05) as a post hoc analysis. Analyses were conducted using Statgraphics Centurion 18 v.16.
4. Discussion
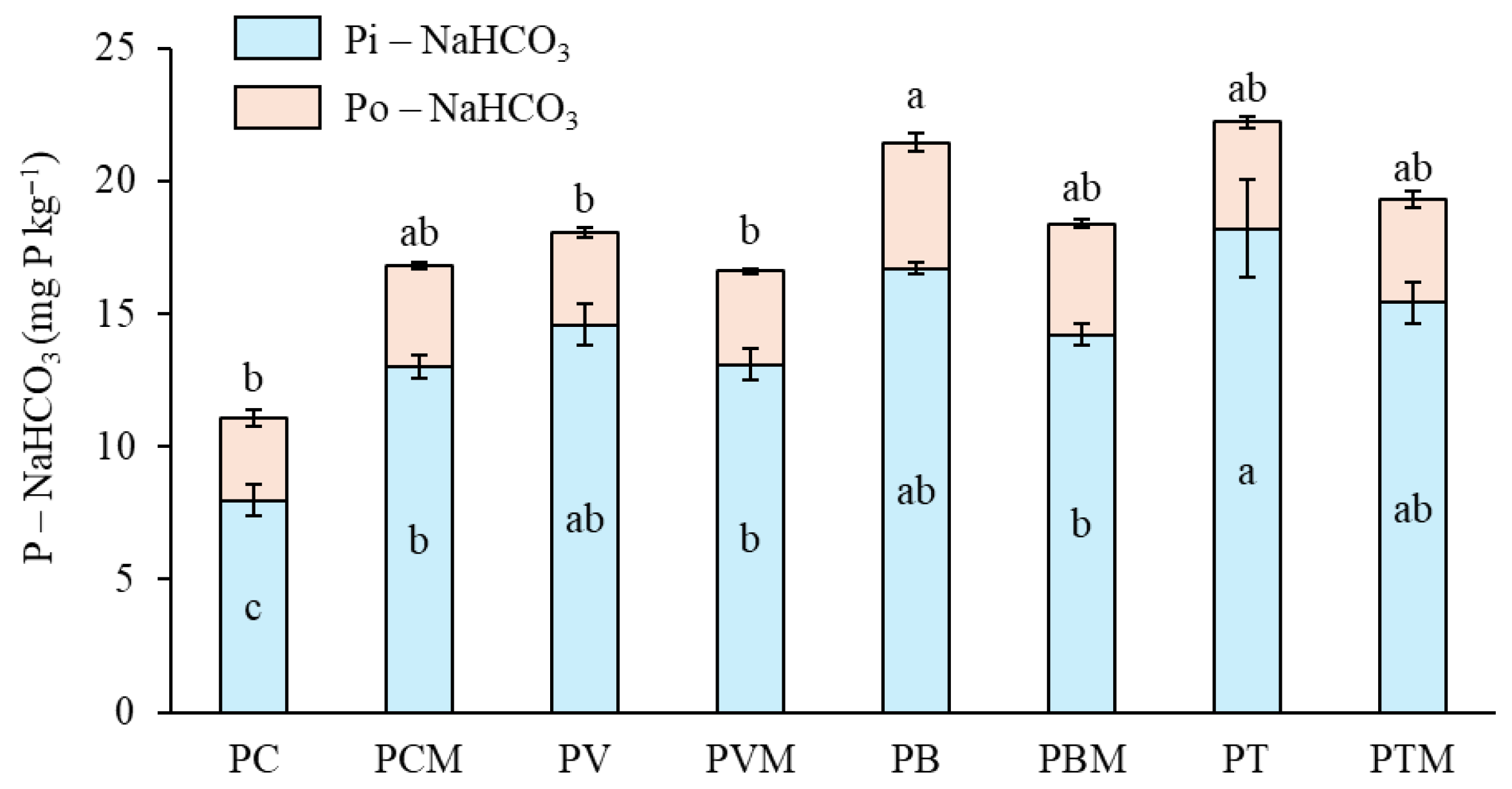
The results of the present study strongly support that frass from insects used in the production of protein-rich feed can be valorized by effectively replacing P mineral fertilizers. Overall, both TM and BSF frass outperformed mineral P fertilizer in almost all variables studied to evaluate the efficiency of these organic products as P fertilizers. However, these improvements cannot be attributed to standard parameters of organic fertilizers, such as the C/N ratio. Although TM had the lowest value (8.38), which would explain the better results for this product, the vermicompost value was lower than that of BSF and did not lead to poorer results for this frass. All evidence suggests that the main reason for the superior performance of the frass lies in the differences in phosphorus lability, which we will discuss later. A significant fraction of P in frass was soluble in water (36% and 33.7%, respectively, for TM and BSF frass). This P was assumed to be readily available to crops [
43]. Usually, organic fertilizers tend to have a low water-soluble P content [
37], as was the case with the vermicompost used in this study [
36]. According to previous studies, water-soluble P ranges from 3% to 30% depending on the raw material and production technique [
44]. Both studied frasses showed even higher values than those reported for chicken manure, whose nutrient bioavailability was relatively high [
44]. Contrasting to vermicompost, BSF and TM had practically all the same inorganic P as water-soluble P. This contributes to explaining the better performance of both frasses as P fertilizers compared to vermicompost.
It has been postulated that water and NAC extractions tend not to accurately estimate the performance of bio-based fertilizers in supplying P to crops [
45]. However, in this study, the results of the chemical extractions are strongly related to the performance of fertilizers, confirming the initial hypothesis and, therefore, highlighting the relevance of chemical extraction as a useful method to predict P bioavailability and the performance of organic fertilizers.
This study intended to apply the same amount of inorganic P since it was difficult to achieve similar efficiencies to those of soluble mineral fertilizer when part of the P supplied was organic and needed to be mineralized to become available to plants. This was necessary for practical implementation, since farmers would never apply treatments with lower efficiency than commercial mineral fertilizers, and even more so at a higher application cost, since the concentration of nutrients is usually much lower in organic fertilizers. Even with this approach, achieving similar efficiencies was not entirely expected, since the water-soluble inorganic P in the mineral fertilizer (all P was inorganic and water soluble) was much higher than in the other treatments. However, frass fertilizers outperformed mineral fertilizers in terms of apparent P recovery (APR) with mineral fertilizer replacement values (MRVs) exceeding 100%. This replacement value is in fact a ratio between the APR of the organic fertilizer and that of the soluble mineral fertilizer. This is a relevant finding because it means that, with the proposed practice, frass and even vermicompost can replace the use of mineral fertilizers. Even more, with an MRV around 180%, a successful replacement of mineral fertilizer can probably be achieved on a total P basis for calculating the organic fertilizer rates. It is also worth noting that an MRV of around 50% has generally been reported for organic-based fertilization [
46,
47]. These results regarding the effectiveness of TM and BSF frasses as P fertilizer not only lead us to consider them a solid substitute to mineral fertilization, but even a better option, especially if these products provide additional benefits on soil health indicators related to P cycling.
It is important to note that the APR is a ratio between P uptake from fertilizer and the applied P rate. Therefore, the fraction of applied P that is taken up by the crop in frass treatments is higher than in soluble mineral fertilizer. This cannot be explained in terms of the inorganic P content of fertilizers, and additional factors must contribute to this high efficiency. In frass and vermicompost, inorganic P is supplied along with organic matter. This organic matter decreases P precipitation or adsorption processes that limit the efficiency of mineral P fertilizers [
48]. This is important in the soil used in the study where precipitation of Ca phosphate due to the pH of the soil [
49,
50] or adsorption on Fe in oxides [
47] are relevant factors explaining the low efficiency of mineral P fertilizers. In addition, organic fertilizers can contain phytohormones and plant growth promoters [
51,
52] as has been observed in frasses from insect production [
14,
53] which are related to chitin degradation [
11]. This may explain the significant increase in biomass production with frass compared to mineral fertilization, since P applied with all the treatments should be enough to cover the crop needs. This is also supported by the SPAD index, which can be considered a physiological state indicator [
54].
More than 50% of the total P in frass was in organic form, which could be released over time by mineralization, contributing to the residual effect of the fertilizer. This was reflected in the increased organic P concentration in bicarbonate extracts with PB. This was not possible with mineral fertilization, since P was readily available and residual P was mostly immobilized after the first growing season. Thus, with frass treatments, there was a short-term effect, explained by the soluble inorganic P content and a decreased rate of precipitation and adsorption because of organic matter supply, and a potential long-term effect explained by the organic P content.
With frass treatments there is not only an effective P supply to crops, but also an enhancement of soil health indicators related to P cycling, i.e., an increase in phosphatase activity. This activity is important in the organic P cycle as a relevant mechanism to supply P to crops transforming organic P to available P [
46,
49] This contradicts our second hypothesis, proving that the stabilization of the material, which is a mandatory procedure, does not negatively affect the organic P cycle in soil and, therefore, the mineralization of the organic forms it contains. Furthermore, it has been shown to exceed the enzymatic potential of vermicompost, which is known to increase the enzymatic activity of soils [
45]. PB led to higher phosphatase activity than PT. The latter had a higher P content. Thus, the amount of frass applied, and consequently organic matter, was higher with PB than with PT. A higher dose of organic matter could have boosted microbial activity and, therefore, the phosphatase activity ascribed to microorganisms.
Other factors are relevant when evaluating fertilizers as an alternative to mineral soluble fertilizers. Soil CE was significantly affected, but changes were not significant in terms of affecting P dynamics or crop development.
This study provides a knowledge base to promote the use of insect frasses as effective alternatives to mineral fertilization and to predict their efficiency based on different P extractions. However, further research involving long-term field experiments and products of different origins, in particular, frasses obtained from insects under different diets, is required to provide general management recommendations to improve the use of these promising fertilizers.