Physiological and Transcriptomic Mechanisms of Exogenous Salicylic Acid-Induced Resistance to Ear Rot in Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Test Methods
2.2.1. Determination of Colony Area
2.2.2. Inoculation Methods and Field Resistance Determination
2.2.3. Sample Preparation and Enzyme Activity Determination
2.2.4. Transcriptomic Analysis
Total RNA Extraction, Library Construction
Sequence Alignment and Analysis of Differentially Expressed Genes
GO Function and KEGG Pathway Enrichment Analysis
Differentially Expressed Genes Validation by RT-qPCR
Weighted Gene Co-Expression Network Analysis (WGCNA)
2.2.5. Data Processing
3. Results
3.1. The Effect of Exogenous SA Treatment on the Occurrence of Maize Ear Rot
3.1.1. The Effect of Exogenous SA on the Mycelial Growth of Fusarium graminearum
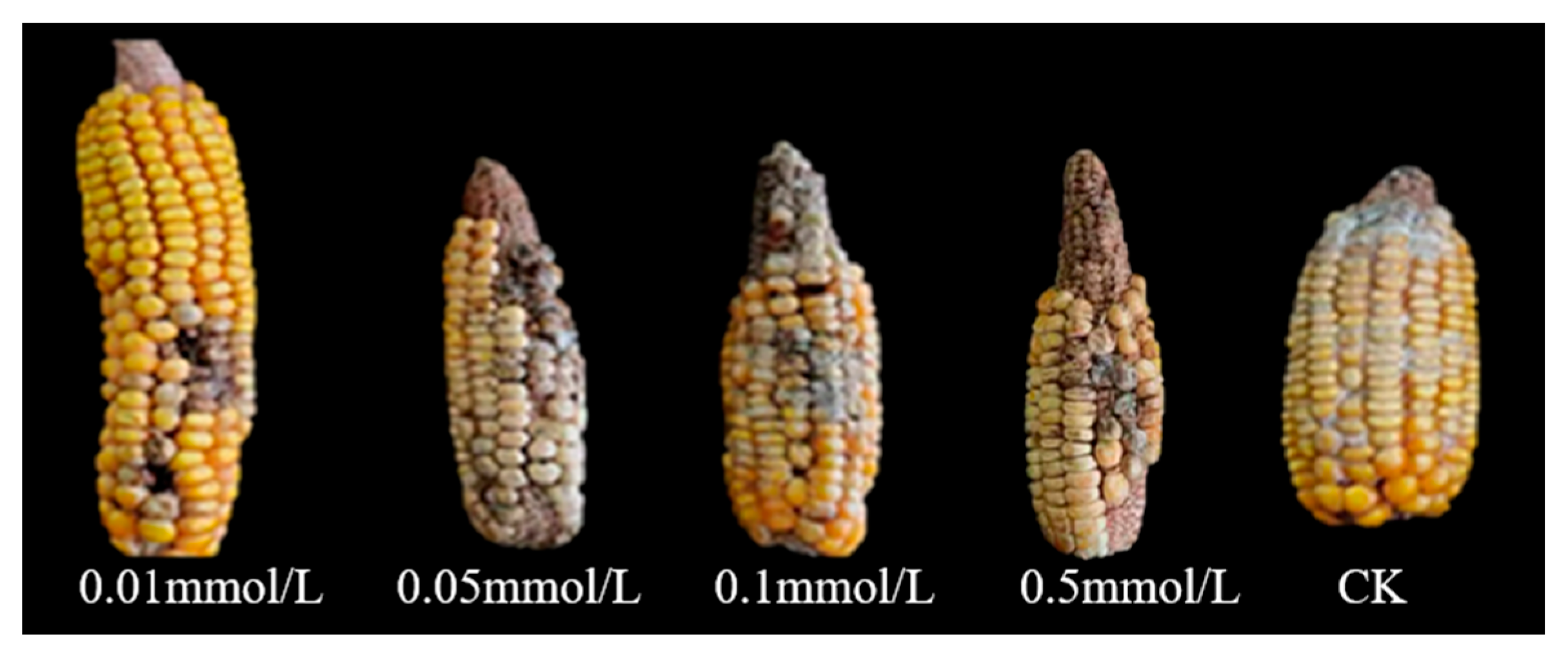
3.1.2. The Effect of Exogenous SA on Maize Ear Rot Resistance
3.1.3. Effects of SA Inhibitors on Resistance to Maize Ear Rot
3.2. The Effect of SA Treatment on Disease Resistance-Related Indicators in Maize Kernels
3.3. Transcriptome Sequencing and Analysis
3.3.1. Quality Assessment of Sequencing Data
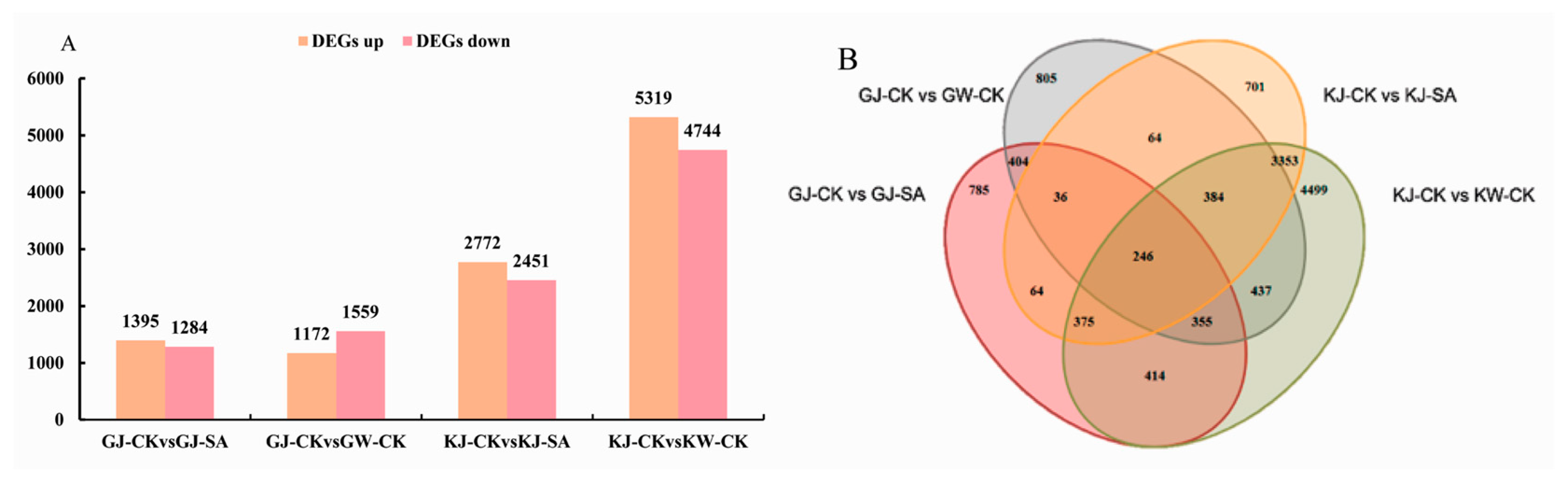
3.3.2. Analysis of Differentially Expressed Genes
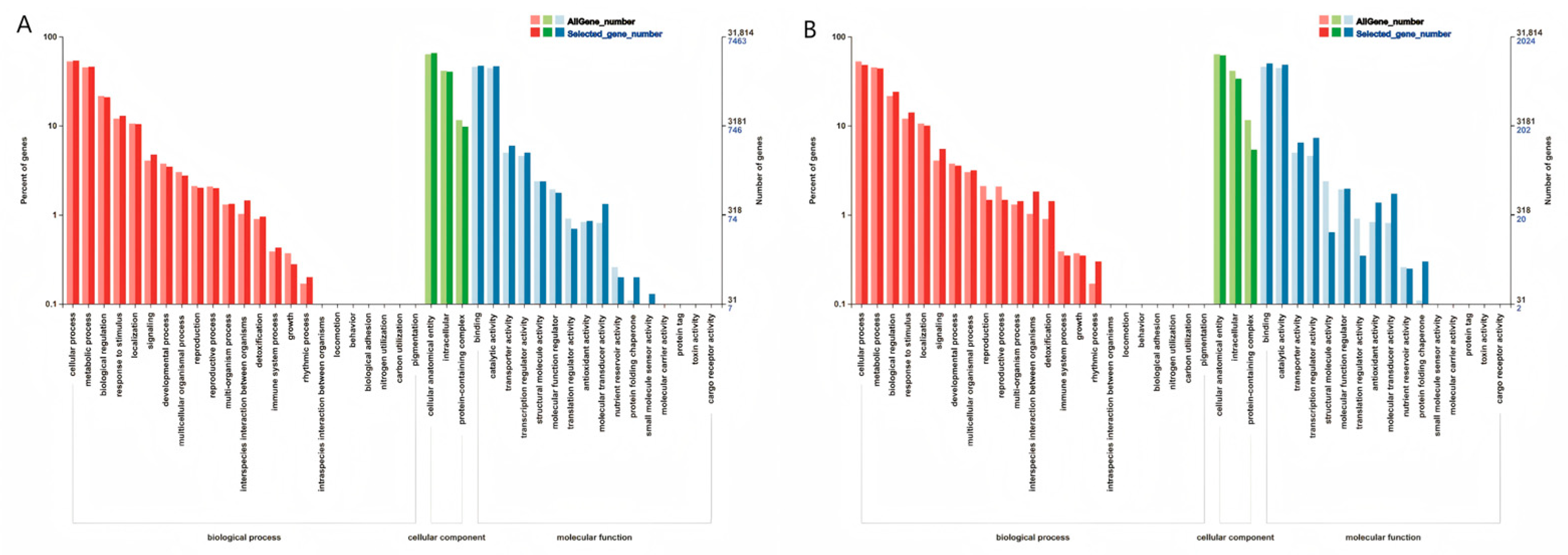
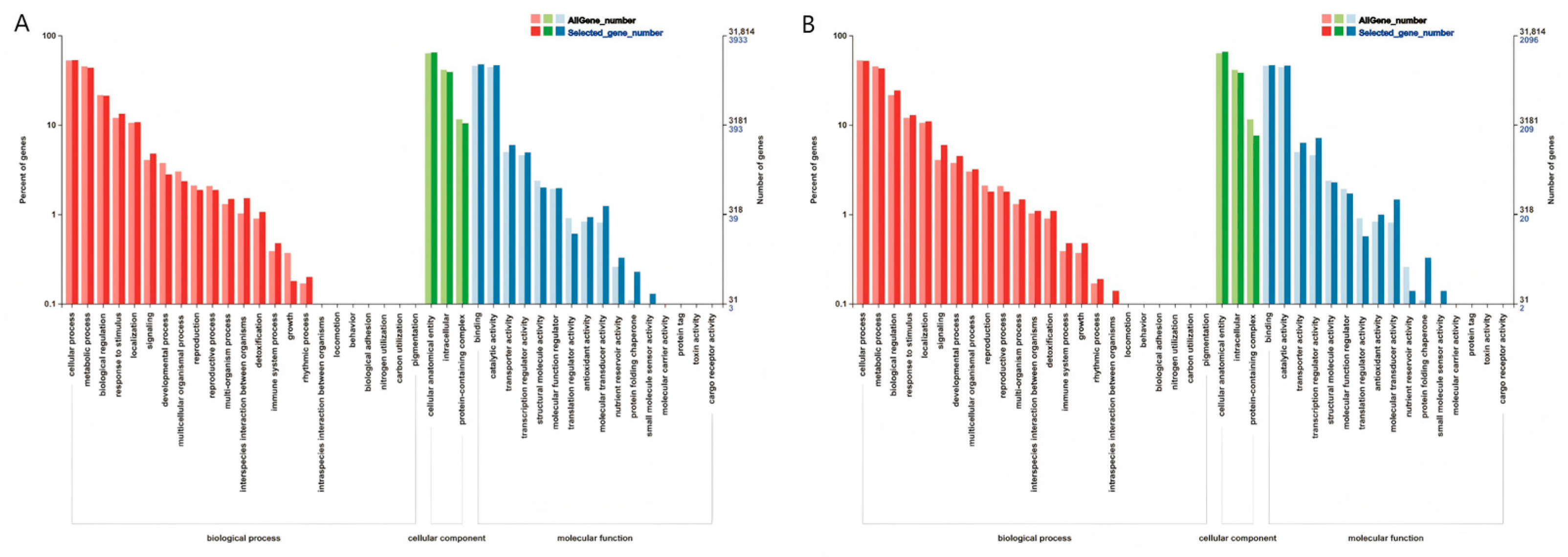
3.3.3. GO Enrichment Analysis
GO Enrichment Analysis of Two Inbred Lines Under Maize Ear Rot Stress
GO Enrichment Analysis of Two Inbred Lines Under Exogenous SA Treatment
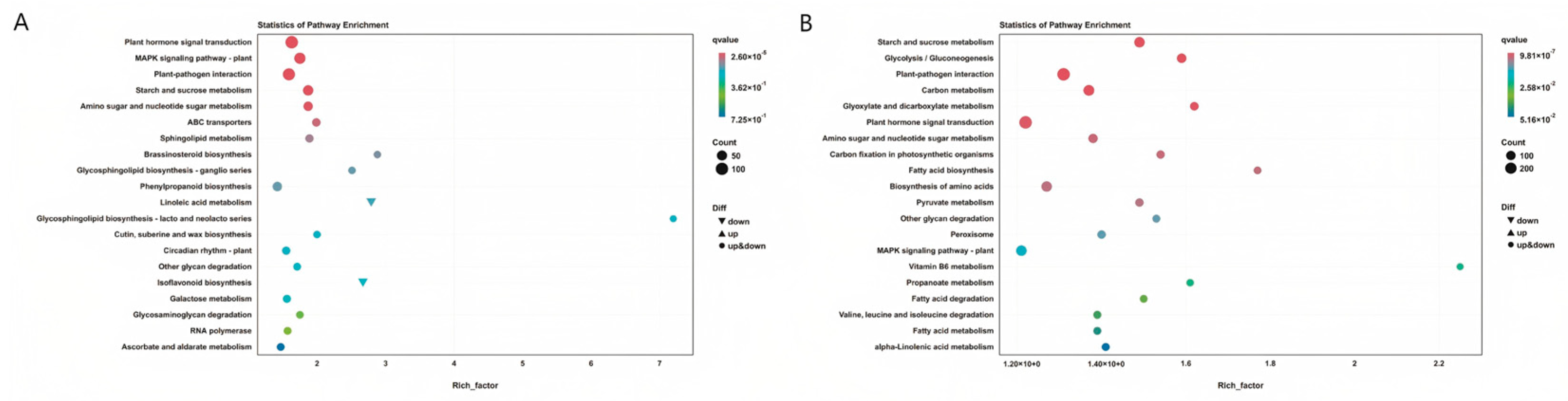
3.3.4. Enrichment Analysis of Differentially Expressed Genes KEGG
KEGG Enrichment Analysis of Two Inbred Lines Under Fusarium graminearum Stress
KEGG Enrichment Analysis of Two Inbred Lines Under Exogenous SA Treatment
3.3.5. RT-qPCR Validation
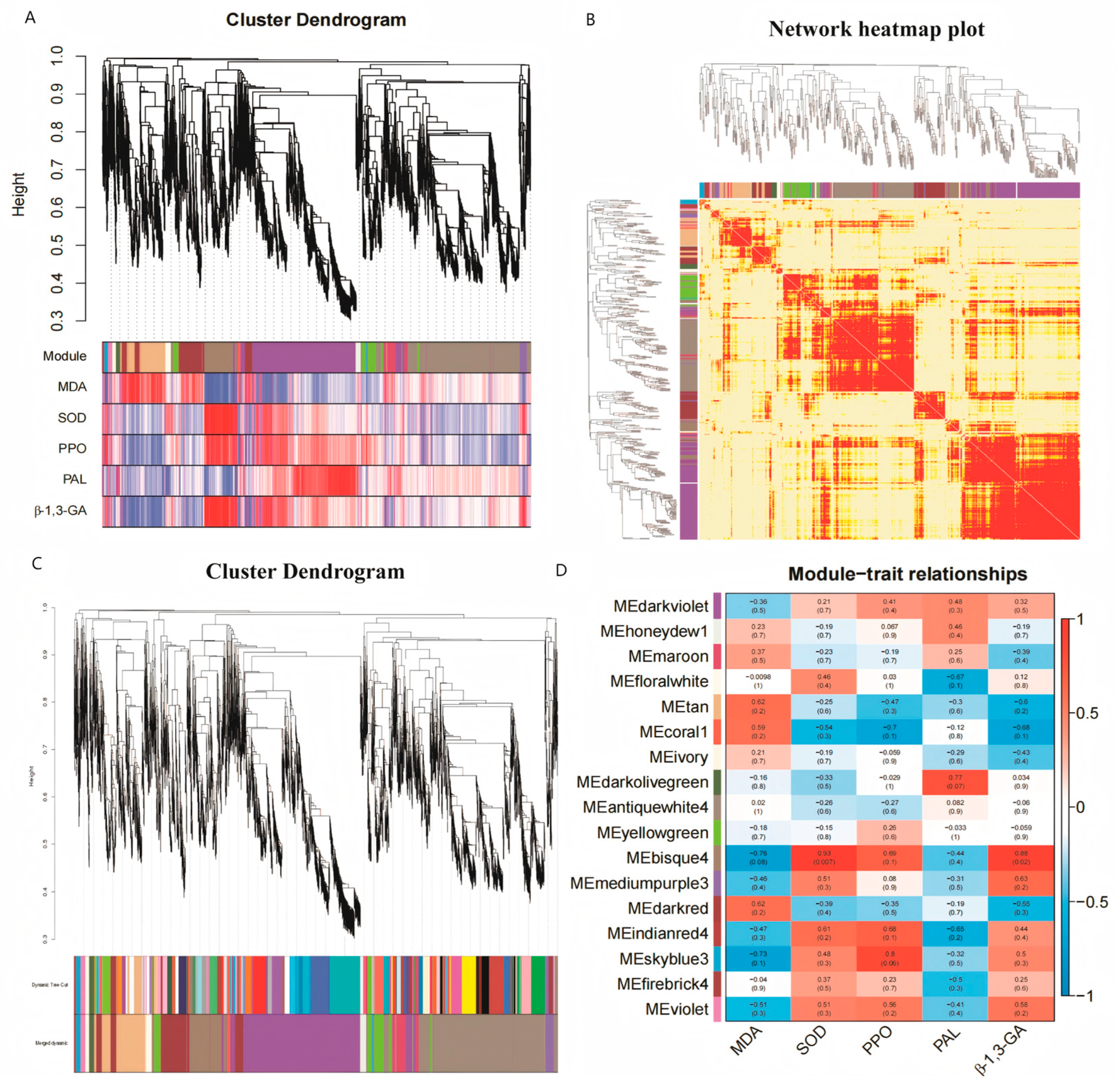
3.3.6. WGCNA Analysis
Construction of Gene Co-Expression Module
Functional Analysis of Related Gene Modules
Gene Module Core Genes and Visualization Analysis
4. Discussion
4.1. The Inhibitory Effect of Exogenous SA on Fusarium graminearum and Its Disease Resistance Effect
4.2. Regulation of Exogenous SA on the Defense Enzyme System Related to Maize Ear Rot
4.3. Analysis of Key Disease Resistant Genes Regulated by the SA Signaling Pathway
- (1)
- The defense function of the aspartate protease gene
- (2)
- The regulatory role of terpenoid metabolism genes
- (3)
- Induced expression of disease-related proteins
- (4)
- The regulatory network of signal transduction proteins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, L.R. High-yield maize planting and pest control technology. Mod. Rural Sci. Technol. 2025, 20–21. [Google Scholar]
- Ping, C.Z.; Hong, M.; Wang, W.D.; Zhao, B.Y.N.M.L.; Mei, L.; Liu, P.F.; Zhao, W.Y.G. Effect of Tillage management modes on farmland physical properties in black soil region. Chin. Agric. Sci. Bull. 2020, 36, 83–89. [Google Scholar]
- Li, M. Control measures for common pests and diseases in maize cultivation. Hebei Agric. 2024, 63–64. [Google Scholar]
- Su, Y.J.; Liu, P.L.; Gao, K.K.; Song, J.F.; Yang, M.L.; Lu, H.W.; Qin, G.W.; Zhang, X.C. Research progress on ear rot in maize. Heilongjiang Agric. Sci. 2024, 103–113. [Google Scholar]
- Ding, X.Y.; Zhang, J.; Su, B.L.; Xu, H.F. Role of salicylic acid in plant disease resistance. Chin. Bull. Bot. 2001, 163–168. [Google Scholar]
- Chen, S.Q.; Wang, Z.P.; Shi, Y.Y.; Shang, Z.Y.; Zhu, S.S.; Yang, M. Exogenous salicylic acid regulates root metabolism in Panax notoginseng and influences the incidence of root rot disease. Chin. J. Biol. Control 2025, 41, 177–185. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Xie, X.N.; Li, X.; Ouyang, Q.F. Effects of salicylic acid on the physiological function of mango seedlings under the stress of leaf blight. Shanxi Agric. Sci. 2024, 52, 130–135. [Google Scholar]
- Qu, Y.T.; Chen, P.; Gou, W.C.; Feng, X.D. Effect of exogenous salicylic acid on the activity of defense enzymes against gray mold in cucumber seedlings. Yan’an Univ. (Nat. Sci.) 2024, 43, 54–58. [Google Scholar] [CrossRef]
- Yi, C.B. Induce Resistance in Maize against Curvularia Leaf Spot Disease by Exogenous Chemicals. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2005. [Google Scholar]
- Seifert, K.A.; Aoki, T.; Baayen, R.P.; Brayford, D.; Burgess, L.W.; Chulze, S.; Gams, W.; Geiser, D.; Gruyter, J.D.; Leslie, J.F.; et al. The name Fusarium moniliforme should no longer be used. Mycol. Res. 2003, 107, 643–644. [Google Scholar] [CrossRef]
- Qin, Z.H.; Ren, X.; Jiang, K.; Wu, X.F.; Yang, Z.H.; Wang, X.M. Identification of Fusarium species and F. graminearum species complex causing maize ear rot in China. J. Plant Prot. 2014, 41, 589–596. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, J.B.; Zhang, Y.X.; Yang, D.D.; Tan, J. Research comparison of two dominant pathogens on maize ear rot. J. Yunnan Univ. (Nat. Sci. Ed.) 2022, 44, 647–654. [Google Scholar]
- Small, I.M.; Flett, B.C.; Marasas, W.F.O.; McLeod, A.; Stander, M.A.; Viljoen, A. Resistance in Maize Inbred Lines to Fusarium verticillioides and Fumonisin Accumulation in South Africa. Plant Dis. 2012, 96, 96. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.Y.; Jia, J.; Bai, X.; Meng, L.M.; Zhang, W.; Jing, R.; Wu, R.B.; Su, Q.F. Identification of pathogenic fusarium spp. Causing maize ear rot and susceptibility of some strains to fungicides in jilin province. Sci. Agric. Sin. 2023, 56, 64–78. [Google Scholar]
- Ji, W.P.; Wang, P. Investigation on the occurrence of corn ear rot disease in Heilongjiang agricultural reclamation. Bull. Agric. Sci. Technol. 2021, 49–51. [Google Scholar]
- Wang, T. Study on Resistance Mechanism of Maize to Ustilago Maydis Induced by Exogenous Jasmonic Acid and Salicylic Acid. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2022. [Google Scholar]
- NY/T 1248.8-2016; Technical Specification for Identification of Maize Resistance to Diseases and Insect Pests. China Agriculture Press: Beijing, China, 2016.
- Chen, G. Induced Resistence in Safflower against Safflower Rust Disease by Exogenous Chemicals. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2009. [Google Scholar]
- Veronese, P.; Ruiz, M.T.; Coca, M.A.; Hernandez-Lopez, A.; Lee, H.; Ibeas, J.I.; Damsz, B.; Pardo, J.M.; Hasegawa, P.M.; Bressan, R.A.; et al. In Defense against Pathogens. Both Plant Sentinels and Foot Soldiers Need to Know the Enemy. Plant Physiol. 2003, 131, 1580–1590. [Google Scholar] [CrossRef]
- Jia, H.; Gong, X.D.; Gu, S.Q.; Han, J.M.; Dong, J.G. Study on the Role of Exogenous Salicylic Acid in Inducing Resistance to Big Blotch Disease in Maiz; Chinese Plant Protection Society: Beijing, China; College of Life Sciences, Hebei Agriculeure University: Baoding, China, 2014; pp. 1–3. [Google Scholar]
- Wang, C.C. Effect of Exogenous Salicylic Acid on Soybean Resistance to Root Rot Disease. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2022. [Google Scholar]
- Wang, Y.Z.; Miller, J.D. Effects of Fusarium graminearum Metabolites on Wheat Tissue in Relation to Fusarium Head Blight Resistance. J. Phytopathol. 2010, 122, 118–125. [Google Scholar] [CrossRef]
- Liu, H.Y.; Si, E.J.; Guo, M.; Qi, T.T.; Wei, J.M.; Yao, L.R.; Wang, J.C.; Li, B.C.; Meng, Y.X.; Ma, X.L.; et al. Resistance of barley to Pyrenophora graminea induced by exogenous salicylic scid. Acta Phytopathol. Sin. 2022, 52, 658–668. [Google Scholar] [CrossRef]
- Xu, Q.Q. Effects of Salicylic Acid and Benzothiadiazole on Related Physiological Indicators for Cabbage Clubroot Resistence. Master’s Thesis, Southwest University, Chongqin, China, 2015. [Google Scholar]
- Ge, Q.D.; Zhang, S.N.; Hao, J.X.; Ma, W.H.; Lu, M.F.; Xu, L.P.; Gou, M.Y. Research progress of aspartic protease in plant immunity. Plant Physiol. 2023, 59, 1863–1869. [Google Scholar] [CrossRef]
- Guevara, G.M.; Almeida, C.; Mendieta, R.J.; Faro, C.J.; Veríssimo, P.; Pires, E.V.; Daleo, G.R. Molecular cloning of a potato leaf cDNA encoding an aspartic protease (StAsp) and its expression after P. infestans infection. Plant Physiol. Biochem. 2005, 43, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.K.Y.; Dupuis, J.H.; Dee, D.R.; Bryksa, B.C.; Yada, R.Y. Roles of Plant-Specific Inserts in Plant Defense. Trends Plant Sci. 2020, 25, 682–694. [Google Scholar] [CrossRef]
- Liu, M.L.; Yu, H.W.; Li, J.; Dong, N.; Chen, B.W.; Xu, R.; Wu, J.X.; Chang, X.W.; Wang, J.T.; Peng, H.S.; et al. Cloning, expression, and functional analysis of the full-length cdna of acetyl-coa c-acetyltransferase (AACT) genes related to terpenoid synthesis in Platycodon grandiflorus. Protein Pept. Lett. 2022, 29, 1061–1071. [Google Scholar] [CrossRef]
- Xu, R.; Wu, J.X.; Zhang, Y.Z.; Jiang, L.Y.; Yao, J.C.; Zha, L.P.; Xie, J. Isolation, characterisation, and expression profiling of DXS and DXR genes in Atractylodes lancea. Genome 2023, 66, 150–164. [Google Scholar] [CrossRef]
- Ding, Y.; Weckwerth, P.R.; Poretsky, E.; Murphy, K.M.; Sims, J.; Saldivar, E.; Christensen, S.A.; Char, S.N.; Yang, B.; Tong, A.D.; et al. Genetic elucidation of interconnected antibiotic pathways mediating maize innate immunity. Nat. Plants 2020, 6, 1375–1388. [Google Scholar] [CrossRef]
- Guan, Y.Q.; He, X.; Wen, D.; Chen, S.M.; Chen, F.D.; Chen, F.; Jiang, Y.F. Fusarium oxysporum infection on root elicit aboveground terpene production and salicylic acid accumulation in Chrysanthemum morifolium. Plant Physiol. Biochem. 2022, 190, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Jiang, L.Y.; Jin, L.M.; Wu, J.J.; Wang, J.S.; Xu, P.F.; Zhang, S.Z. Advances on Class 10 Pathogenesis-related Proteins. Soybean Sci. 2013, 32, 415–419. [Google Scholar]
- Zhao, Q.; Cui, M.J.; Han, S.Y.; Guo, T.D.; Du, P.; Liu, H.; Xu, J.; Huang, B.Y.; Dong, W.Z.; Zhang, X.Y. Plant Pathogenesis-Related Protein PR10 and Their Research Progress in Peanut. SAU 2024, 42, 1–10. [Google Scholar] [CrossRef]
- Zhao, S.Q.; Li, M.Y.; Ren, X.P.; Wang, C.Y.; Sun, X.B.; Sun, M.L.; Yu, X.M.; Wang, X.D. Enhancement of broad-spectrum disease resistance in wheat through key genes involved in systemic acquired resistance. Front. Plant Sci. 2024, 15, 1355178. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Castroverde, C.D.M. Salicylic acid: A key regulator of redox signalling and plant immunity. Plant Physiol. Biochem. 2021, 168, 381–397. [Google Scholar] [CrossRef]
- Diop, A.; Pietrangeli, P.; Nardella, C.; Pennacchietti, V.; Pagano, L.; Toto, A.; Di Felice, M.; Di Matteo, S.; Marcocci, L.; Malagrinò, F. Biophysical Characterization of the Binding Mechanism between the MATH Domain of SPOP and Its Physiological Partners. Int. J. Mol. Sci. 2023, 24, 10138. [Google Scholar] [CrossRef]
- Stogios, P.J.; Downs, G.S.; Jauhal, J.J.; Nandra, S.K.; Privé, G.G. Sequence and structural analysis of BTB domain proteins. Genome Biol. 2005, 6, R82. [Google Scholar] [CrossRef]
- Orosa, B.; He, Q.; Mesmar, J.; Gilroy, E.M.; McLellan, H.; Yang, C.W.; Craig, A.; Bailey, M.; Zhang, C.J.; Moore, J.D.; et al. BTB-BACK domain protein POB1 suppresses immune cell death by targeting ubiquitin E3 ligase PUB17 for degradation. PLoS Genet 2017, 13, e1006540. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Li, R.L.; Wang, J.L.; Shao, J.W.; Zhao, H.Y. Functional analysis of plastid phosphate transporter OsPPT family members in rice. Acta Agric. Zhejiangensis 2022, 34, 2329–2339. [Google Scholar]
- Shrader, C.W.; Foster, D.; Kharel, Y.; Huang, T.; Lynch, K.R.; Santos, W.L. Imidazole-based sphingosine-1-phosphate transporter Spns2 inhibitors. J. Med. Chem. 2023, 96, 129516. [Google Scholar] [CrossRef] [PubMed]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, F.; Lan, N.; Lu, W.; Wang, F. Physiological and Transcriptomic Mechanisms of Exogenous Salicylic Acid-Induced Resistance to Ear Rot in Maize. Agronomy 2025, 15, 2002. https://doi.org/10.3390/agronomy15082002
Jiao F, Lan N, Lu W, Wang F. Physiological and Transcriptomic Mechanisms of Exogenous Salicylic Acid-Induced Resistance to Ear Rot in Maize. Agronomy. 2025; 15(8):2002. https://doi.org/10.3390/agronomy15082002
Chicago/Turabian StyleJiao, Fangju, Ning Lan, Weijie Lu, and Fang Wang. 2025. "Physiological and Transcriptomic Mechanisms of Exogenous Salicylic Acid-Induced Resistance to Ear Rot in Maize" Agronomy 15, no. 8: 2002. https://doi.org/10.3390/agronomy15082002
APA StyleJiao, F., Lan, N., Lu, W., & Wang, F. (2025). Physiological and Transcriptomic Mechanisms of Exogenous Salicylic Acid-Induced Resistance to Ear Rot in Maize. Agronomy, 15(8), 2002. https://doi.org/10.3390/agronomy15082002




