Planting Diversification Enhances Phosphorus Availability and Reshapes Fungal Community Structure in the Maize Rhizosphere
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.2. Soil Samples Collection and Biochemical Measurement
2.3. DNA Extraction and Fungal Community Measurement
2.4. Bioinformatic Analysis of High-Throughput Sequencing Data
2.5. Statistical Analysis
3. Results
3.1. Variations in Soil Properties and Crop Yields Under Different Planting Patterns
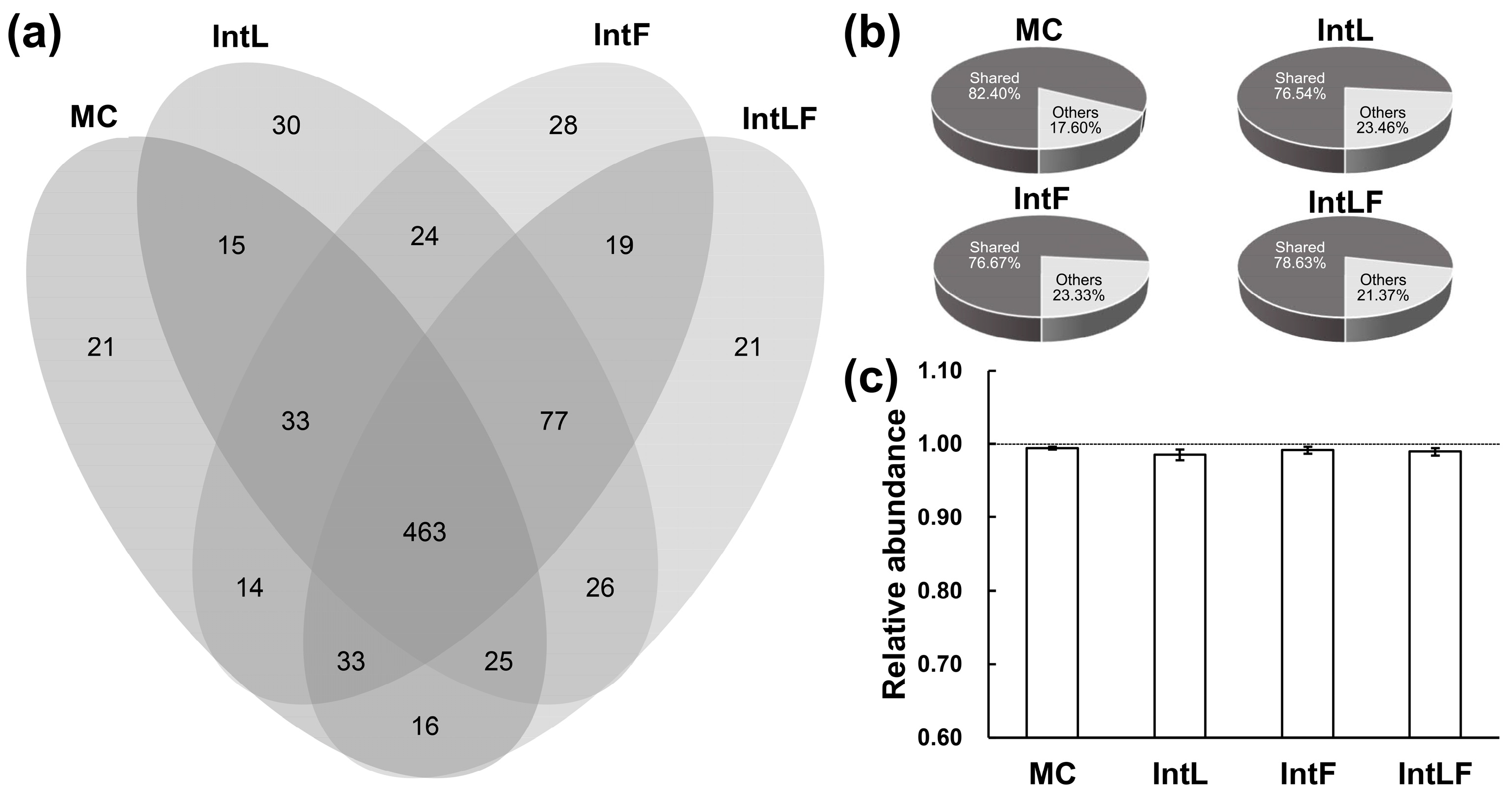
3.2. Different Cropping Patterns Shaped Distinct Fungal Communities in the Maize Rhizosphere
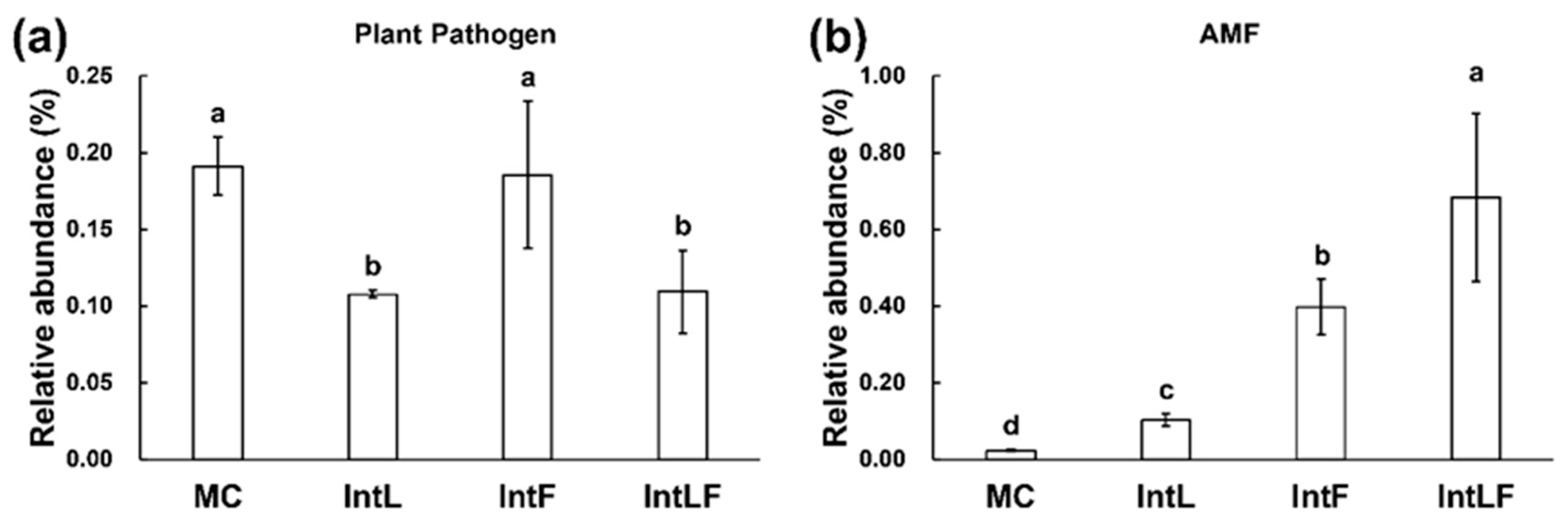
3.3. Planting Diversification Modified the Functional Profiles of the Rhizosphere Fungal Community
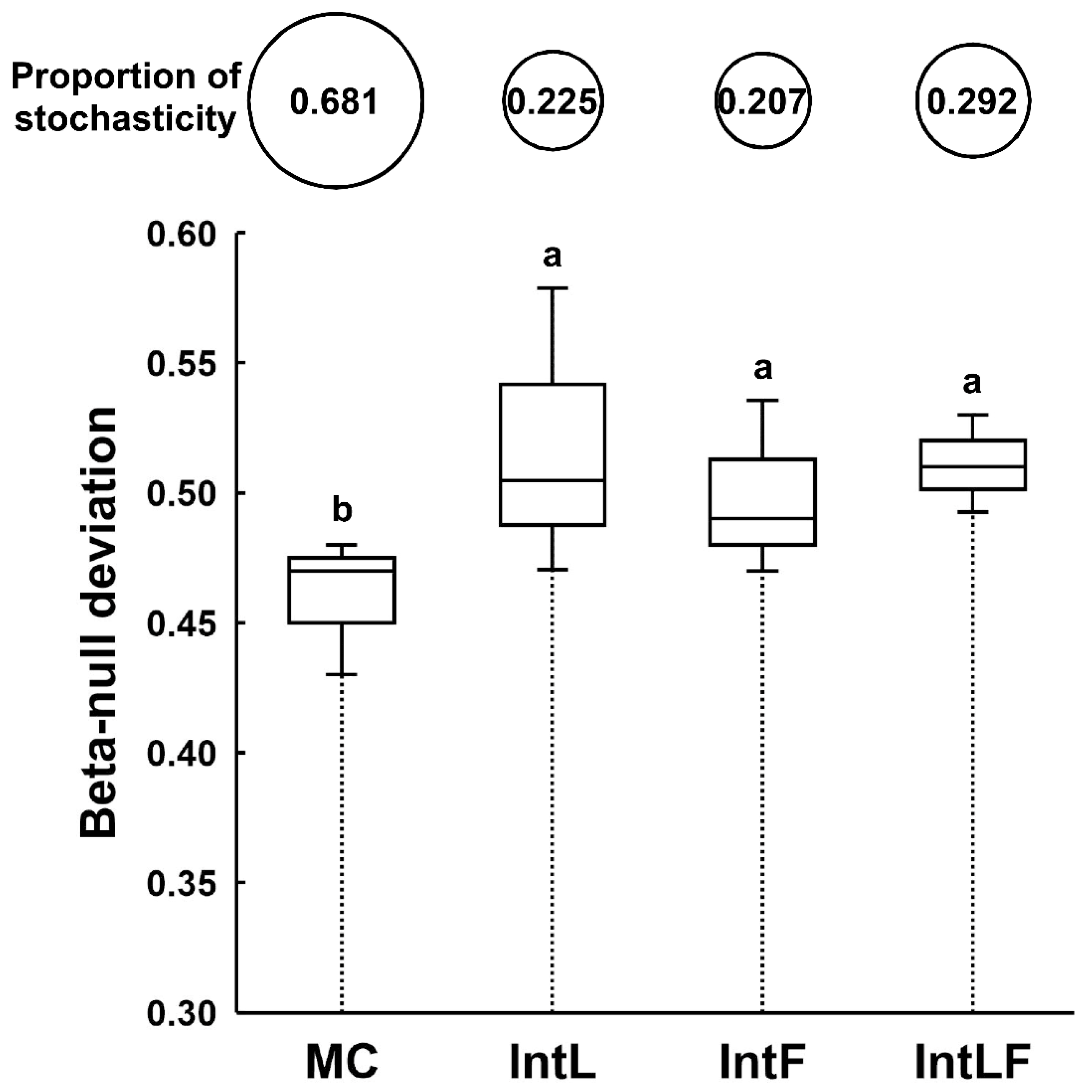
3.4. Planting Diversification Increased the Contribution of Deterministic Processes to Rhizosphere Fungal Community Assembly
3.5. Correlation Analysis of Fungal Community Against Soil Properties and Crop Yields
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, R.B.; Zhang, X.X.; Guo, X.S.; Wang, D.Z.; Chu, H.Y. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Sun, R.B.; Li, W.Y.; Hu, C.S.; Liu, B.B. Long-term urea fertilization alters the composition and increases the abundance of soil ureolytic bacterial communities in an upland soil. FEMS Microbiol. Ecol. 2019, 95, fiz044. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, W.; Wang, X.; Wang, F.; Dong, W.; Hu, C.; Liu, B.; Sun, R. Nitrogen Leaching Greatly Impacts Bacterial Community and Denitrifiers Abundance in Subsoil under Long-Term Fertilization. Agric. Ecosyst. Environ. 2020, 294, 106885. [Google Scholar] [CrossRef]
- Bijay, S.; Craswell, E. Fertilizers and nitrate pollution of surface and ground water: An increasingly pervasive global problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Wu, W.; Zhai, F.; Liu, Z.; Liu, C.; Gu, Y.; Li, P. The Spatial and Seasonal Variability of Nutrient Status in the Seaward Rivers of China Shaped by the Human Activities. Ecol. Indic. 2023, 157, 111223. [Google Scholar] [CrossRef]
- Kiran, B.R.; Prasad, M.N.V.; Mohan, S.V. Chapter 2—Farm to Fork: Sustainable Agrifood Systems. In Sustainable and Circular Management of Resources and Waste Towards a Green Deal; Vara Prasad, M.N., Smol, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 25–38. ISBN 978-0-323-95278-1. [Google Scholar]
- Wang, Z.; Tang, K.; Struik, P.C.; Ashraf, M.N.; Zhang, T.; Zhao, Y.; Wu, R.; Jin, K.; Li, Y. Alteration of Microbial Carbon and Nitrogen Metabolism within the Soil Metagenome with Grazing Intensity at Semiarid Steppe. J. Environ. Manag. 2023, 347, 119078. [Google Scholar] [CrossRef]
- Mahlayeye, M.; Darvishzadeh, R.; Nelson, A. Cropping Patterns of Annual Crops: A Remote Sensing Review. Remote Sens-Basel 2022, 14, 2404. [Google Scholar] [CrossRef]
- Toker, P.; Canci, H.; Turhan, I.; Isci, A.; Scherzinger, M.; Kordrostami, M.; Yol, E. The advantages of intercropping to improve productivity in food and forage production—A review. Plant Prod. Sci. 2024, 27, 155–169. [Google Scholar] [CrossRef]
- Brooker, R.W.; Bennett, A.E.; Cong, W.-F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.M.; Jones, H.G.; Karley, A.J.; et al. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, P.; Zhang, X.; Du, Q.; Zheng, B.; Yang, H.; Luo, K.; Lin, P.; Li, Y.; Pu, T.; et al. Maize-legume intercropping achieves yield advantages by improving leaf functions and dry matter partition. BMC Plant Biol. 2023, 23, 438. [Google Scholar] [CrossRef]
- Peixoto, L.; Olesen, J.E.; Elsgaard, L.; Enggrob, K.L.; Banfield, C.C.; Dippold, M.A.; Nicolaisen, M.H.; Bak, F.; Zang, H.; Dresbøll, D.B.; et al. Deep-rooted perennial crops differ in capacity to stabilize C inputs in deep soil layers. Sci. Rep. 2022, 12, 5952. [Google Scholar] [CrossRef]
- Homulle, Z.; George, T.S.; Karley, A.J. Root traits with team benefits: Understanding belowground interactions in intercropping systems. Plant Soil 2022, 471, 1–26. [Google Scholar] [CrossRef]
- Raseduzzaman, M.; Dong, W.; Gaudel, G.; Aluoch, S.O.; Timilsina, A.; Li, X.; Hu, C. Maize-soybean intercropping reduces greenhouse gas emissions from the fertilized soil in the North China Plain. J. Soils Sediments 2024, 24, 3115–3131. [Google Scholar] [CrossRef]
- Long, G.; Li, L.; Wang, D.; Zhao, P.; Tang, L.; Zhou, Y.; Yin, X. Nitrogen levels regulate intercropping-related mitigation of potential nitrate leaching. Agric. Ecosyst. Environ. 2021, 319, 107540. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, J.; Wang, D.; Yang, S.; Cen, Z.; Dong, Y. Faba bean-wheat intercropping controls the occurrence of faba bean Fusarium wilt by improving the microecological environment of rhizosphere soil. Eur. J. Soil Biol. 2024, 123, 103685. [Google Scholar] [CrossRef]
- Sun, R.B.; Guo, X.S.; Wang, D.Z.; Chu, H.Y. Effects of long-term application of chemical and organic fertilizers on the abundance of microbial communities involved in the nitrogen cycle. Appl. Soil Ecol. 2015, 95, 171–178. [Google Scholar] [CrossRef]
- Li, W.Y.; Xiao, Q.; Hu, C.S.; Liu, B.B.; Sun, R.B. A comparison of the efficiency of different urease inhibitors and their effects on soil prokaryotic community in a short-term incubation experiment. Geoderma 2019, 354, 113877. [Google Scholar] [CrossRef]
- Sun, R.B.; Wang, F.H.; Hu, C.S.; Liu, B.B. Metagenomics reveals taxon-specific responses of the nitrogen-cycling microbial community to long-term nitrogen fertilization. Soil Biol. Biochem. 2021, 156, 108214. [Google Scholar] [CrossRef]
- Wang, X.L.; Chi, Y.K.; Song, S.Z. Important soil microbiota’s effects on plants and soils: A comprehensive 30-year systematic literature review. Front. Microbiol. 2024, 15, 1347745. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Ding, J.; Li, H.; Wang, X.; Li, W.; Li, K.; Ye, X.; Sun, S. Mitigating nitrate leaching in cropland by enhancing microbial nitrate transformation through the addition of liquid biogas slurry. Agric. Ecosyst. Environ. 2023, 345, 108324. [Google Scholar] [CrossRef]
- Sun, R.; Dsouza, M.; Gilbert, J.A.; Guo, X.; Wang, D.; Guo, Z.; Ni, Y.; Chu, H. Fungal community composition in soils subjected to long-term chemical fertilization is most influenced by the type of organic matter. Environ. Microbiol. 2016, 18, 5137–5150. [Google Scholar] [CrossRef]
- Sun, R.B.; Chen, Y.; Han, W.; Dong, W.; Zhang, Y.; Hu, C.; Liu, B.; Wang, F. Different contribution of species sorting and exogenous species immigration from manure to soil fungal diversity and community assemblage under long-term fertilization. Soil Biol. Biochem. 2020, 151, 108049. [Google Scholar] [CrossRef]
- Frac, M.; Hannula, S.E.; Belka, M.; Jedryczka, M. Fungal Biodiversity and Their Role in Soil Health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef]
- Liu, P.F.; Zhao, Y.K.; Ma, J.N.; Cao, Y.; Zhang, M.X.; Yu, J.; Guan, H.B.; Xing, Y.S.; Wang, X.Q.; Jia, X. Impact of various intercropping modes on soil quality, microbial communities, yield and quality of Platycodon grandiflorum (Jacq.) A. DC. BMC Plant Biol. 2025, 25, 503. [Google Scholar] [CrossRef]
- Xun, W.; Li, W.; Xiong, W.; Ren, Y.; Liu, Y.; Miao, Y.; Xu, Z.; Zhang, N.; Shen, Q.; Zhang, R. Diversity-Triggered Deterministic Bacterial Assembly Constrains Community Functions. Nat. Commun. 2019, 10, 3833. [Google Scholar] [CrossRef]
- Guo, X.; Hou, Z.; Wu, X.; Liu, W.; Cai, J.; An, S. Long-Term Intercropping Shaped Soil Bacterial Microbiome Composition and Structure of Maize Fields in a Semiarid Region. Soil Tillage Res. 2025, 247, 106383. [Google Scholar] [CrossRef]
- Lee, H.; Bloxham, B.; Gore, J. Resource Competition Can Explain Simplicity in Microbial Community Assembly. Proc. Natl. Acad. Sci. USA 2023, 120, e2212113120. [Google Scholar] [CrossRef]
- Ge, S.; Chen, Y.; Wang, Z.; Li, Z.; Shen, C.; Zhang, T.; Wang, J. Deep insights into the diversified cropping and their impact on arbuscular mycorrhizal fungi: A global meta-analysis. Agric. Ecosyst. Environ. 2025, 383, 109537. [Google Scholar] [CrossRef]
- Zhou, G.; Li, G.; Liang, H.; Liu, R.; Ma, Z.; Gao, S.; Chang, D.; Liu, J.; Chadwick, D.R.; Jones, D.L.; et al. Green Manure Coupled With Straw Returning Increases Soil Organic Carbon via Decreased Priming Effect and Enhanced Microbial Carbon Pump. Glob. Change Biol. 2025, 31, e70232. [Google Scholar] [CrossRef]
- Li, H.; Fan, Z.; Wang, Q.; Wang, G.; Yin, W.; Zhao, C.; Yu, A.; Cao, W.; Chai, Q.; Hu, F. Green manure and maize intercropping with reduced chemical N enhances productivity and carbon mitigation of farmland in arid areas. Eur. J. Agron. 2023, 145, 126788. [Google Scholar] [CrossRef]
- Zhang, T.; Zuo, Q.; Shi, J.; Fan, Y.; Zhang, S.; Wu, X.; Ben-Gal, A. Evaluation and Improvement of Crop Root-Water-Uptake and Transpiration in AquaCrop. Agric. Water Manag. 2025, 318, 109686. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, H.; Chang, D.; Zhang, J.; Bao, X.; Cui, H.; Cao, W. Maize-Green Manure Intercropping Improves Maize Yield and P Uptake by Shaping the Responses of Roots and Soil. J. Integr. Agric. 2025, in press. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, Y.; Zhang, Y.; Fei, J.; Rong, X.; Peng, J.; Yin, L.; Luo, G. Intercropping Enhances Maize Growth and Nutrient Uptake by Driving the Link between Rhizosphere Metabolites and Microbiomes. New Phytol. 2024, 243, 1506–1521. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, W.; Liu, Y.; Yun, W.; Luo, B.; Chai, R.; Zhang, C.; Xiang, X.; Su, X. Changes in phosphorus mobilization and community assembly of bacterial and fungal communities in rice rhizosphere under phosphate deficiency. Front. Microbiol. 2022, 13, 953340. [Google Scholar] [CrossRef]
- Sun, R.; Li, W.; Dong, W.; Tian, Y.; Hu, C.; Liu, B. Tillage Changes Vertical Distribution of Soil Bacterial and Fungal Communities. Front. Microbiol. 2018, 9, 699. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, J.; Lu, X.; Zhao, Z.; Li, K.; Wang, F.; Zhang, C.; Sun, R. Dosage effects of organic manure on bacterial community assemblage and phosphorus transformation profiles in greenhouse soil. Front. Microbiol. 2023, 14, 1188167. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Sun, R.; Wang, X.; Hamoud, Y.A.; Lu, M.; Shaghaleh, H.; Zhang, W.; Zhang, C.; Ma, C. Dynamic variation of bacterial community assemblage and functional profiles during rice straw degradation. Front. Microbiol. 2023, 14, 1173442. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, R.; Sun, T.; Chen, P.; Yu, Z.; Ding, L.; Jiang, Y.; Wang, X.; Dai, C.; Sun, B. Evidence for involvement of keystone fungal taxa in organic phosphorus mineralization in subtropical soil and the impact of labile carbon. Soil Biol. Biochem. 2020, 148, 107900. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Xiang, X.; Wu, Y.; Zhang, F.; Kuang, Y.; Li, C.; Sun, R.; Hui, C. Evidence for cross transmission of pathogens between wild hooded cranes and domestic geese. J. Avian Biol. 2023, 2023, e03083. [Google Scholar] [CrossRef]
- Sun, R.; Wang, D.; Guo, Z.; Hua, K.; Guo, X.; Chen, Y.; Liu, B.; Chu, H. Combined application of organic manure and chemical fertilizers stabilizes soil N-cycling microflora. Soil Ecol. Lett. 2023, 5, 220165. [Google Scholar] [CrossRef]
- Tucker, C.M.; Shoemaker, L.G.; Davies, K.F.; Nemergut, D.R.; Melbourne, B.A. Differentiating between niche and neutral assembly in metacommunities using null models of beta-diversity. Oikos 2016, 125, 778–789. [Google Scholar] [CrossRef]
- Sun, R.B.; Wang, X.; Tian, Y.; Guo, K.; Feng, X.; Sun, H.; Liu, X.; Liu, B. Long-Term Amelioration Practices Reshape the Soil Microbiome in a Coastal Saline Soil and Alter the Richness and Vertical Distribution Differently Among Bacterial, Archaeal, and Fungal Communities. Front. Microbiol. 2022, 12, 768203. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.; Deng, Y.; Tiedje, J.M.; Zhou, J. A general framework for quantitatively assessing ecological stochasticity. Proc. Natl. Acad. Sci. USA 2019, 116, 16892–16898. [Google Scholar] [CrossRef]
- Mihrete, T.B.; Mihretu, F.B. Crop Diversification for Ensuring Sustainable Agriculture, Risk Management and Food Security. Glob. Chall. 2025, 9, 2400267. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus Acquisition and Utilization in Plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Li, M.; Hu, J.; Lin, X. The roles and performance of arbuscular mycorrhizal fungi in intercropping systems. Soil Ecol. Lett. 2022, 4, 319–327. [Google Scholar] [CrossRef]
- Weber, S.E.; Bascompte, J.; Kahmen, A.; Niklaus, P.A. AMF Diversity Promotes Plant Community Phosphorus Acquisition and Reduces Carbon Costs per Unit of Phosphorus. New Phytol. 2025, nph.70161. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, C.; Wang, G. Inoculation with Arbuscular Mycorrhizal Fungi Improves Plant Biomass and Nitrogen and Phosphorus Nutrients: A Meta-Analysis. BMC Plant Biol. 2024, 24, 960. [Google Scholar] [CrossRef]
- Chaudhary, A.; Poudyal, S.; Kaundal, A. Role of Arbuscular Mycorrhizal Fungi in Maintaining Sustainable Agroecosystems. Appl. Microbiol. 2025, 5, 6. [Google Scholar] [CrossRef]
- Kwon, W.; Kim, D.; Kim, H.-Y.; Jeong, S.W.; Lee, S.-G.; Kim, H.-C.; Lee, Y.-J.; Kwon, M.K.; Hwang, J.-S.; Han, J.E.; et al. Microglial Phagocytosis of Polystyrene Microplastics Results in Immune Alteration and Apoptosis in Vitro and in Vivo. Sci. Total. Environ. 2022, 807, 150817. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-X.; Wang, X.; Sun, Y.; Cheng, Y.; Liu, S.; Chen, X.; Feng, G.; Kuyper, T.W. Arbuscular Mycorrhizal Fungi Negatively Affect Nitrogen Acquisition and Grain Yield of Maize in a N Deficient Soil. Front. Microbiol. 2018, 9, 418. [Google Scholar] [CrossRef]
- Hodge, A.; Storer, K. Arbuscular Mycorrhiza and Nitrogen: Implications for Individual Plants through to Ecosystems. Plant Soil 2015, 386, 1–19. [Google Scholar] [CrossRef]
- Zhu, S.; Morel, J.-B. Molecular Mechanisms Underlying Microbial Disease Control in Intercropping. Mol. Plant-Microbe Interact. 2019, 32, 20–24. [Google Scholar] [CrossRef]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Chen, H.; Gleixner, G. Increased Soil Carbon Storage through Plant Diversity Strengthens with Time and Extends into the Subsoil. Glob. Change Biol. 2023, 29, 2627–2639. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, K.; Wurzburger, N.; Zhang, J. Relationships between plant diversity and soil microbial diversity vary across taxonomic groups and spatial scales. Ecosphere 2022, 11, e02999. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.Y.H.; Chen, X.; Huang, Z. Meta-analysis shows positive effects of plant diversity on microbial biomass and respiration. Nat. Commun. 2019, 10, 1332. [Google Scholar] [CrossRef]
- Zhou, T.; Liang, G.; Reich, P.B.; Delgado-Baquerizo, M.; Wang, C.; Zhou, Z. Promoting effect of plant diversity on soil microbial functionality is amplified over time. One Earth 2024, 7, 2139–2148. [Google Scholar] [CrossRef]
- Jayaramaiah, R.H.; Martins, C.S.; Egidi, E.; Macdonald, C.A.; Wang, J.-T.; Liu, H.; Reich, P.B.; Delgado-Baquerizo, M.; Singh, B.K. Soil function-microbial diversity relationship is impacted by plant functional groups under climate change. Soil Biol. Biochem. 2025, 200, 109623. [Google Scholar] [CrossRef]
- Qiao, M.; Sun, R.; Wang, Z.; Dumack, K.; Xie, X.; Dai, C.; Wang, E.; Zhou, J.; Sun, B.; Peng, X.; et al. Legume Rhizodeposition Promotes Nitrogen Fixation by Soil Microbiota under Crop Diversification. Nat. Commun. 2024, 15, 2924. [Google Scholar] [CrossRef] [PubMed]


| Treatment | TN (g·kg−1) | AN (mg·kg−1) | NH4+-N (mg·kg−1) | NO3−-N (mg·kg−1) | SOM (g·kg−1) | AP (mg·kg−1) | Maize Yield (kg·hm−2) | |
|---|---|---|---|---|---|---|---|---|
| 2022 | 2023 | |||||||
| MC | 1.42a | 146.32a | 3.30b | 16.64a | 25.77a | 19.31c | 5975c | 6665c |
| IntL | 1.44a | 147.86a | 3.40b | 17.53a | 26.44a | 20.10bc | 7186a | 8624b |
| IntF | 1.35a | 136.31a | 4.72a | 9.40b | 24.07a | 23.15b | 6617b | 8954b |
| IntLF | 1.44a | 140.16a | 3.75b | 9.02b | 25.34a | 42.32a | 7584a | 9202a |
| Factors | Fungal Community | Plant Pathogens | AMF | |||
|---|---|---|---|---|---|---|
| R | p Value | R | p Value | R | p Value | |
| TN | 0.047 | 0.349 | −0.390 | 0.210 | −0.284 | 0.372 |
| AN | 0.277 | 0.067 | −0.489 | 0.106 | −0.773 | 0.003 |
| NH4+-N | −0.073 | 0.658 | 0.308 | 0.331 | 0.510 | 0.094 |
| NO3−-N | −0.018 | 0.513 | −0.308 | 0.331 | −0.685 | 0.017 |
| SOM | 0.136 | 0.225 | −0.447 | 0.145 | −0.794 | 0.002 |
| AP | 0.430 | 0.005 | 0.177 | 0.581 | 0.745 | 0.005 |
| Crop yield | 0.308 | 0.213 | −0.503 | 0.002 | 0.493 | 0.103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, Y.; Li, X.; Li, H.; Dong, W.; Qin, S.; Liu, X.; Zhang, L.; Hu, C.; He, H.; et al. Planting Diversification Enhances Phosphorus Availability and Reshapes Fungal Community Structure in the Maize Rhizosphere. Agronomy 2025, 15, 1993. https://doi.org/10.3390/agronomy15081993
Li Y, Zhang Y, Li X, Li H, Dong W, Qin S, Liu X, Zhang L, Hu C, He H, et al. Planting Diversification Enhances Phosphorus Availability and Reshapes Fungal Community Structure in the Maize Rhizosphere. Agronomy. 2025; 15(8):1993. https://doi.org/10.3390/agronomy15081993
Chicago/Turabian StyleLi, Yannan, Yuming Zhang, Xiaoxin Li, Hongjun Li, Wenxu Dong, Shuping Qin, Xiuping Liu, Lijuan Zhang, Chunsheng Hu, Hongbo He, and et al. 2025. "Planting Diversification Enhances Phosphorus Availability and Reshapes Fungal Community Structure in the Maize Rhizosphere" Agronomy 15, no. 8: 1993. https://doi.org/10.3390/agronomy15081993
APA StyleLi, Y., Zhang, Y., Li, X., Li, H., Dong, W., Qin, S., Liu, X., Zhang, L., Hu, C., He, H., Zheng, P., & Zhao, J. (2025). Planting Diversification Enhances Phosphorus Availability and Reshapes Fungal Community Structure in the Maize Rhizosphere. Agronomy, 15(8), 1993. https://doi.org/10.3390/agronomy15081993








